Electrophysiological Responses to Emotional Facial Expressions Following a Mild Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
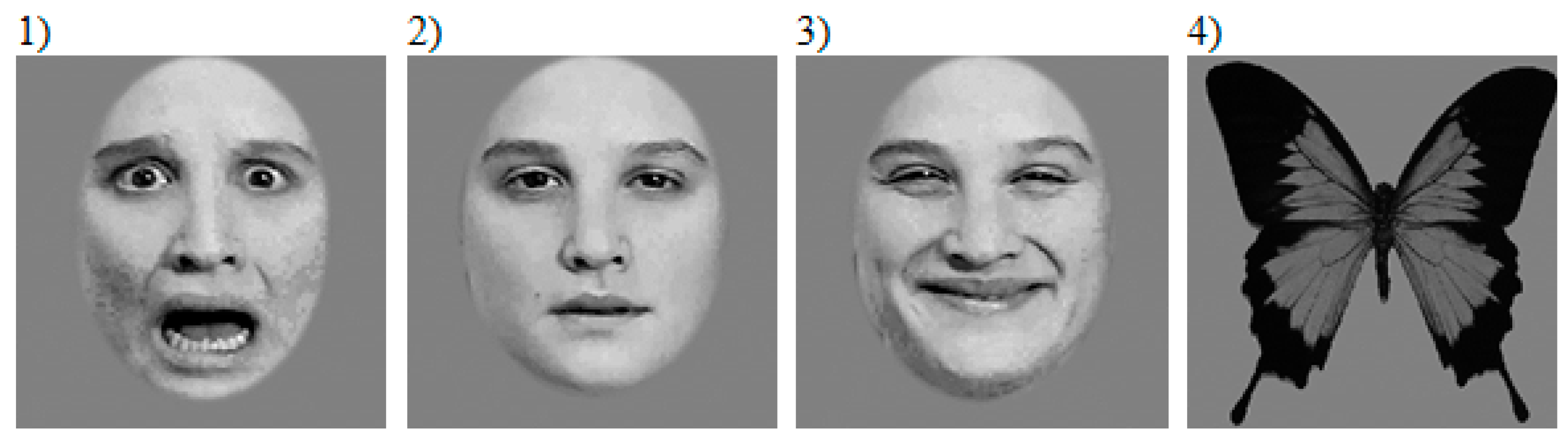
2.2. Stimuli
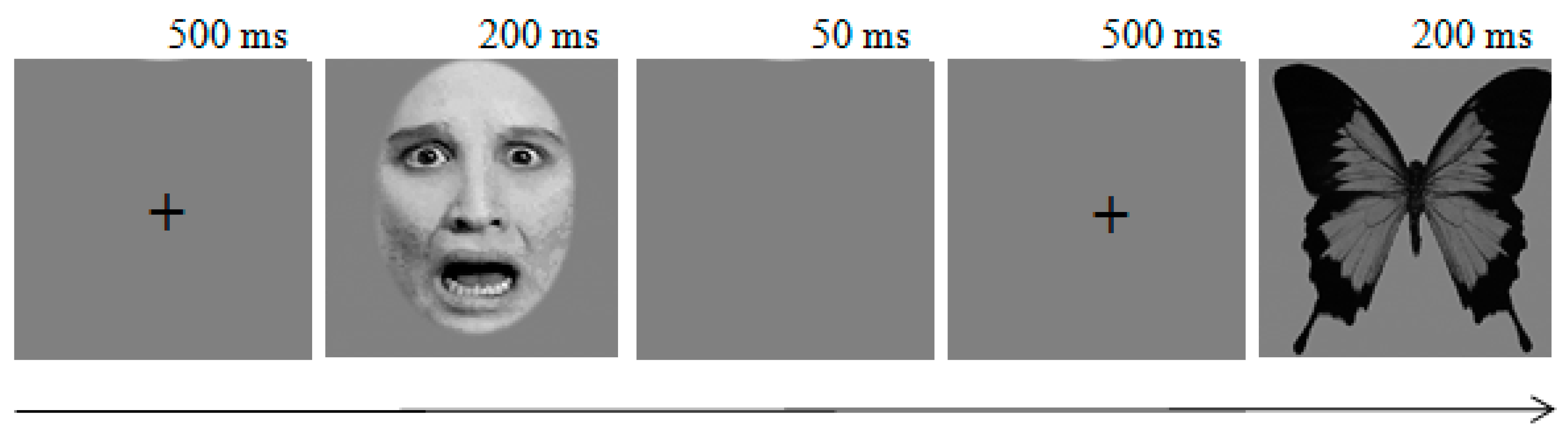
2.3. Task and Procedure
2.4. ERP Data Recording and Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical and Demographic Data
3.2. Behavioral Responses
3.3. Electrophysiological Responses to Emotional Facial Expressions
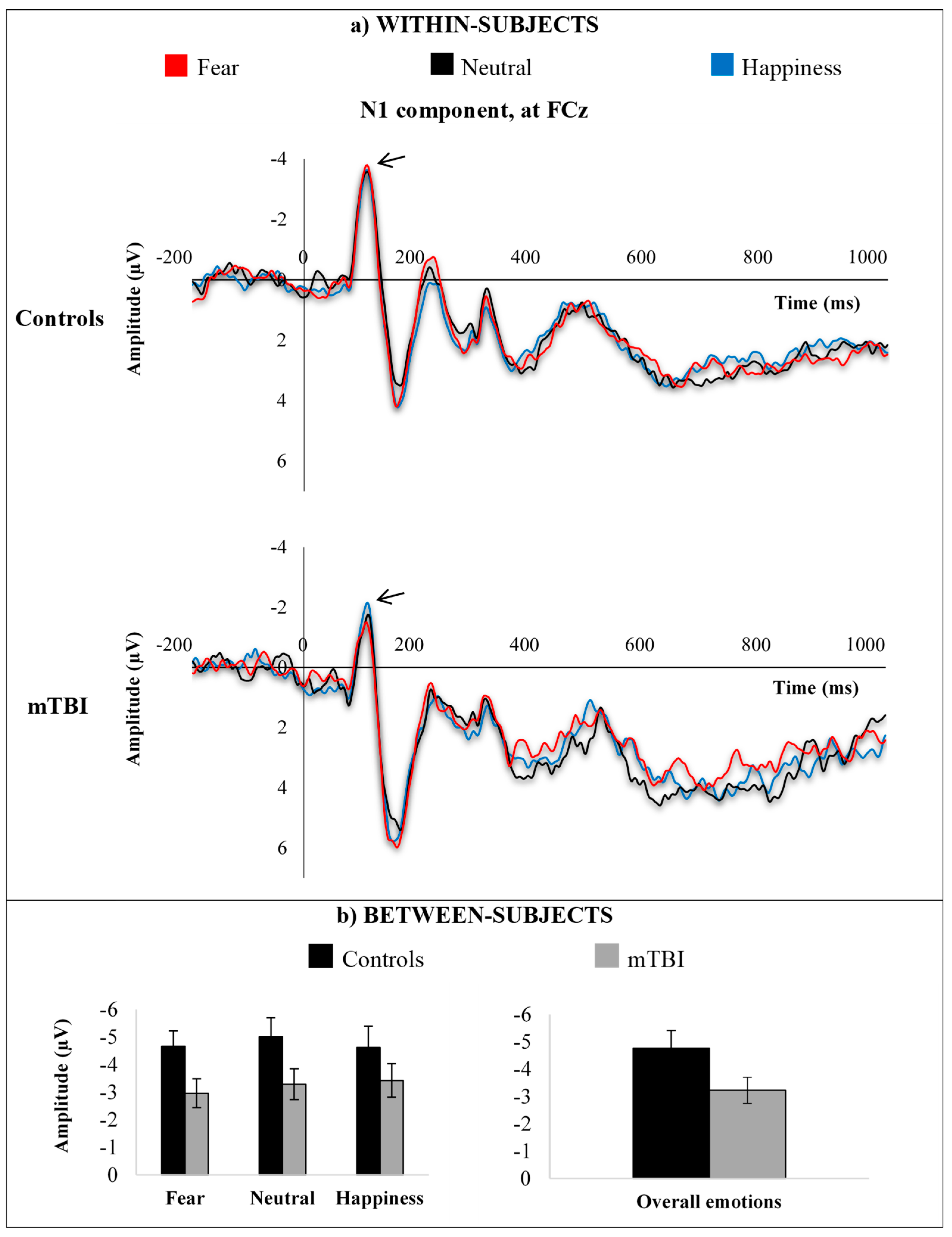
3.3.1. N1
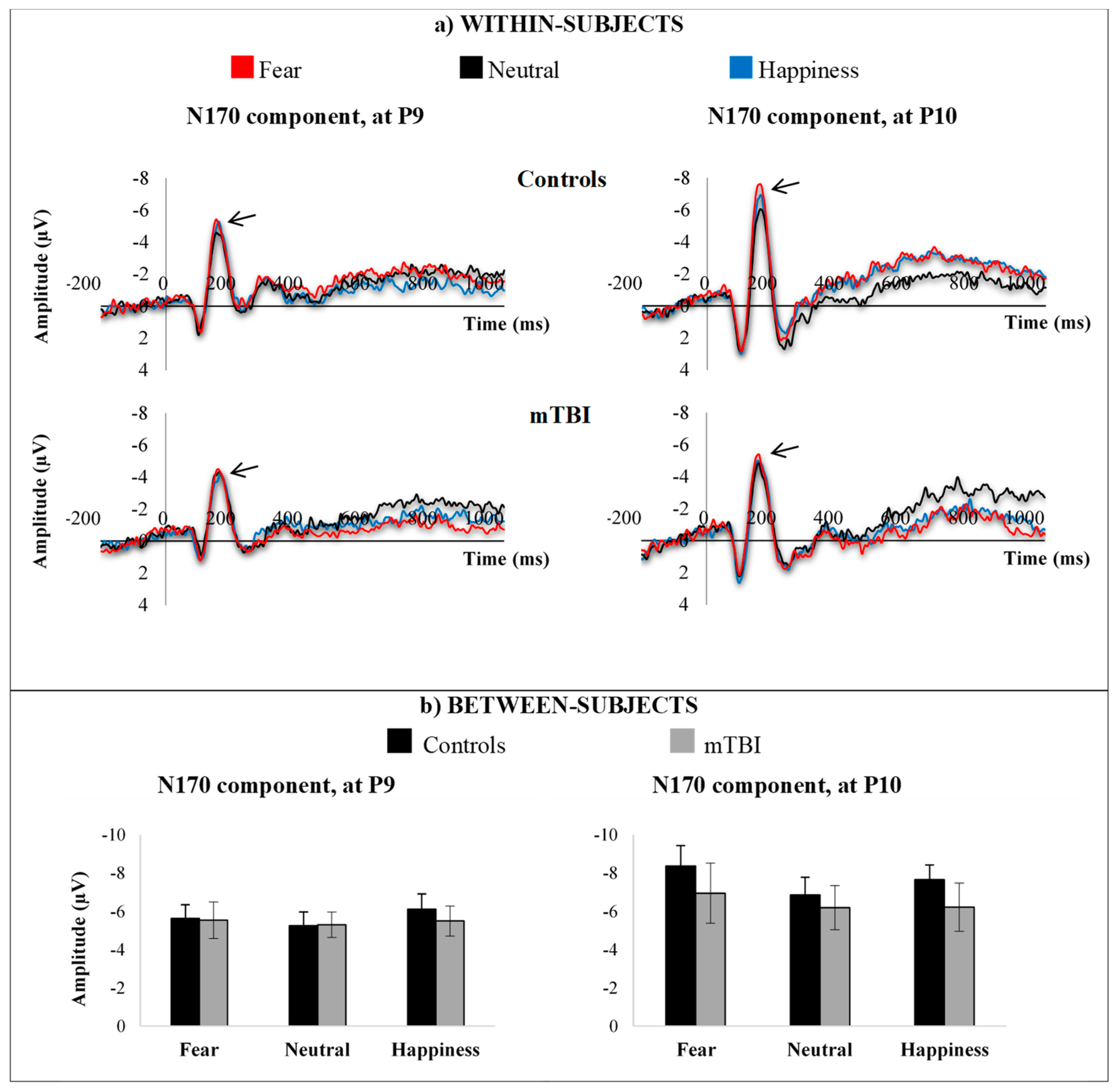
3.3.2. N170
3.3.3. N2
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Guise, E.; leBlanc, J.; Feyz, M.; Meyer, K.; Duplantie, J.; Thomas, H.; Abouassaly, M.; Champoux, M.C.; Couturier, C.; Lin, H.; et al. Long-term outcome after severe traumatic brain injury: The McGill interdisciplinary prospective study. J. Head Trauma. Rehabil. 2008, 23, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Temkin, N.R.; Corrigan, J.D.; Dikmen, S.S.; Machamer, J. Social Functioning After Traumatic Brain Injury. J. Head Trauma. Rehabil. 2009, 24, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Knox, L.; Douglas, J. Long-term ability to interpret facial expression after traumatic brain injury and its relation to social integration. Brain Cogn. 2009, 69, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Milders, M.; Ietswaart, M.; Crawford, J.R.; Currie, D. Social behavior following traumatic brain injury and its association with emotion recognition, understanding of intentions, and cognitive flexibility. J. Int. Neuropsychol. Soc. 2008, 14, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Spikman, J.M.; Milders, M.V.; Visser-Keizer, A.C.; Westerhof-Evers, H.J.; Herben-Dekker, M.; van der Naalt, J. Deficits in Facial Emotion Recognition Indicate Behavioral Changes and Impaired Self-Awareness after Moderate to Severe Traumatic Brain Injury. PLOS ONE 2013, 8, e65581. [Google Scholar] [CrossRef]
- Babbage, D.R.; Yim, J.; Zupan, B.; Neumann, D.; Tomita, M.R.; Willer, B. Meta-analysis of facial affect recognition difficulties after traumatic brain injury. Neuropsychology 2011, 25, 277–285. [Google Scholar] [CrossRef]
- Bornhofen, C.; McDonald, S. Emotion perception deficits following traumatic brain injury: A review of the evidence and rationale for intervention. J. Int. Neuropsychol. Soc. 2008, 14, 511–525. [Google Scholar] [CrossRef]
- Callahan, B.L.; Ueda, K.; Sakata, D.; Plamondon, A.; Murai, T. Liberal bias mediates emotion recognition deficits in frontal traumatic brain injury. Brain Cogn. 2011, 77, 412–418. [Google Scholar] [CrossRef]
- Ietswaart, M.; Milders, M.; Crawford, J.R.; Currie, D.; Scott, C.L. Longitudinal aspects of emotion recognition in patients with traumatic brain injury. Neuropsychology 2008, 46, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, J.; Gosselin, N.; Peretz, I.; McKerral, M. Emotional recognition from dynamic facial, vocal and musical expressions following traumatic brain injury. Brain Inj. 2017, 31, 221–229. [Google Scholar] [CrossRef]
- Zupan, B.; Babbage, D.; Neumann, D.; Willer, B. Recognition of facial and vocal affect following traumatic brain injury. Brain Inj. 2014, 28, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Adolphs, R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002, 12, 169–177. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Placentino, A.; Carletti, F.; Landi, P.; Allen, P.; Surguladze, S.; Benedetti, F.; Abbamonte, M.; Gasparotti, R.; Barale, F.; et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci. 2009, 34, 418–432. [Google Scholar] [PubMed]
- Phillips, M.L.; Drevets, W.C.; Rauch, S.L.; Lane, R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Boil. Psychiatry 2003, 54, 504–514. [Google Scholar] [CrossRef]
- Bigler, E.D. Structural imaging. In Textbook of Traumatic Brain Injury, 2nd ed; Silver, J.M., MacAllister, T.W., Yudofsky, S.C., Eds.; American Psychiatric Publishing: Arlington, VA, USA, 2011; pp. 73–91. [Google Scholar]
- Eierud, C.; Craddock, R.C.; Fletcher, S.; Aulakh, M.; King-Casas, B.; Kuehl, D.; LaConte, S.M. Neuroimaging after mild traumatic brain injury: Review and meta-analysis. NeuroImage Clin. 2014, 4, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Adolphs, R.; Tranel, D.; Damasio, H.; Damasio, A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 1994, 372, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, N.; Peretz, I.; Hasboun, D.; Baulac, M.; Samson, S. Impaired recognition of musical emotions and facial expressions following anteromedial temporal lobe excision. Cortex 2011, 47, 1116–1125. [Google Scholar] [CrossRef]
- Vandekerckhove, M.; Plessers, M.; Van Mieghem, A.; Beeckmans, K.; Van Acker, F.; Maex, R.; Markowitsch, H.; Mariën, P.; Van Overwalle, F. Impaired facial emotion recognition in patients with ventromedial prefrontal hypoperfusion. Neuropsychology 2014, 28, 605–612. [Google Scholar] [CrossRef]
- Dockree, P.M.; Robertson, I.H. Electrophysiological markers of cognitive deficits in traumatic brain injury: A review. Int. J. Psychophysiol. 2011, 82, 53–60. [Google Scholar] [CrossRef]
- Elting, J.W.; Maurits, N.; Van Weerden, T.; Spikman, J.; De Keyser, J.; Van Der Naalt, J. P300 analysis techniques in cognitive impairment after brain injury: Comparison with neuropsychological and imaging data. Brain Inj. 2008, 22, 870–881. [Google Scholar] [CrossRef]
- Beauchamp, M.H.; Ditchfield, M.; Maller, J.J.; Catroppa, C.; Godfrey, C.; Rosenfeld, J.V.; Kean, M.J.; Anderson, V.A. Hippocampus, amygdala and global brain changes 10 Years after childhood traumatic brain injury. Int. J. Dev. Neurosci. 2001, 29, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.R.; Mannell, M.V.; Ling, J.; Gasparovic, C.; Yeo, R.A. Functional Connectivity in Mild Traumatic Brain Injury. Hum. Brain Mapp. 2011, 32, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Yeo, R.A.; Gasparovic, C.; Merideth, F.; Ruhl, D.; Doezema, D.; Mayer, A.R. A Longitudinal Proton Magnetic Resonance Spectroscopy Study of Mild Traumatic Brain Injury. J. Neurotrauma 2011, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, N.; Bottari, C.; Chen, J.-K.; Huntgeburth, S.C.; De Beaumont, L.; Petrides, M.; Cheung, B.; Ptito, A. Evaluating the cognitive consequences of mild traumatic brain injury and concussion by using electrophysiology. Neurosurg. Focus 2012, 33, E7. [Google Scholar] [CrossRef] [PubMed]
- Lachapelle, J.; Bolduc-Teasdale, J.; Ptito, A.; McKerral, M. Deficits in complex visual information processing after mild TBI: Electrophysiological markers and vocational outcome prognosis. Brain Inj. 2008, 22, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Hajcak, G.; Weinberg, A.; MacNamara, A.; Foti, D. ERPs and the study of emotion. In The Oxford Handbook of Event-Related Potential Components; Kappenman, E.S., Luck, S.L., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 441–472. [Google Scholar]
- Olofsson, J.K.; Nordin, S.; Sequeira, H.; Polich, J. Affective picture processing: An integrative review of ERP findings. Boil. Psychol. 2008, 77, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.K.; Luck, S.J. The visual N1 component as an index of a discrimination process. Psychophysiol. 2000, 37, 190–203. [Google Scholar] [CrossRef]
- Hinojosa, J.; Mercado, F.; Carretié, L. N170 sensitivity to facial expression: A meta-analysis. Neurosci. Biobehav. Rev. 2015, 55, 498–509. [Google Scholar] [CrossRef]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef]
- Luck, S.J. A closer look at ERPs and ERP components. In An Introduction to the Event-Related Potential Technique, 2nd Ed.; Luck, S.J., Ed.; The MIT Press: Cambridge, UK, 2014; pp. 35–70. [Google Scholar]
- Luo, W.; Feng, W.; He, W.; Wang, N.-Y.; Luo, Y.-J. Three stages of facial expression processing: ERP study with rapid serial visual presentation. NeuroImage 2010, 49, 1857–1867. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, W.; Luo, Y. Single-trial ERP analysis reveals facial expression category in a three-stage scheme. Brain Res. 2013, 1512, 78–88. [Google Scholar] [CrossRef]
- Blau, V.C.; Maurer, U.; Tottenham, N.; McCandliss, B.D. The face-specific N170 component is modulated by emotional facial expression. Behav. Brain Funct. 2007, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Foti, D.; Hajcak, G.; Dien, J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology 2009, 46, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, J.M.; Moulson, M.C.; Vogel-Farley, V.K.; Nelson, C.A. An ERP Study of Emotional Face Processing in the Adult and Infant Brain. Child Dev. 2007, 78, 232–245. [Google Scholar] [CrossRef]
- LeDoux, J.E. The Emotional Brain: The Mysterious Underpinnings of Emotional Life; Simon & Schuster Paperbacks: New York, NY, USA, 1996. [Google Scholar]
- Schupp, H.T.; Schmälzle, R.; Flaisch, T. Explicit semantic stimulus categorization interferes with implicit emotion processing. Soc. Cogn. Affect. Neurosci. 2014, 9, 1738–1745. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zuj, D.V.; Felmingham, K.L.; Palmer, M.A.; Lawrence-Wood, E.; van Hooff, M.; Lawrence, A.J.; Bryant, R.A.; McFarlane, A.C. Neural activity and emotional processing following military deployment: Effects of mild traumatic brain injury and posttraumatic stress disorder. Brain Cogn. 2017, 118, 19–26. [Google Scholar] [CrossRef]
- D’Hont, F.; Lassonde, M.; Thebault-Dagher, F.; Bernier, A.; Gravel, J.; Vannasing, P.; Beauchamp, M.H. Electrophysiological correlates of emotional face processing after mild traumatic brain injury in preschool children. Cogn. Affec. Behav. Neurosci. 2017, 17, 124–142. [Google Scholar] [CrossRef]
- Arlinghaus, K.A.; Pastorek, N.J.; Graham, D.P. Neuropsychiatric assessment. In Textbook of traumatic brain injury, 2nd Edition; Silver, J.M., MacAllister, T.W., Yudofsky, S.C., Eds.; American Psychiatric Publishing: Arlington, VA, USA, 2011; pp. 55–73. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III); Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Lovell, M.R.; Iverson, G.L.; Collins, M.W.; Podell, K.; Johnston, K.M.; Pardini, D.; Pardini, J.; Norwig, J.; Maroon, J.C. Measurement of Symptoms Following Sports-Related Concussion: Reliability and Normative Data for the Post-Concussion Scale. Appl. Neuropsychol. 2006, 13, 166–174. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 2013. [Google Scholar]
- Roy, S.; Roy, C.; Éthier-Majcher, C.; Fortin, I.; Belin, P.; Gosselin, F. STOIC: A database of dynamic and static faces expressing highly recognizable emotions. J. Vis. 2007, 7, 944. [Google Scholar] [CrossRef]
- Jasper, H.H. The ten twenty electrode system of the International Federation. Electroencephalography Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Kwok, F.Y.; Lee, T.M.C.; Leung, C.H.S.; Poon, W.S. Changes of cognitive functioning following mild traumatic brain injury over a 3-month period. Brain Inj. 2008, 22, 740–751. [Google Scholar] [CrossRef] [PubMed]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2017, 12, 0174847. [Google Scholar] [CrossRef] [PubMed]
- Carrier-Toutant, F.; Guay, S.; Beaulieu, C.; Léveillé, É.; Turcotte-Giroux, A.; Papineau, S.; Brisson, B.; D’Hondt, F. Effects of Repeated Concussions and Sex on Early Processing of Emotional Facial Expressions as Revealed by Electrophysiology. J. Int. Neuropsychol. Soc. 2018, 24, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Carlozzi, N.E.; Kirsch, N.L.; Kisala, P.A.; Tulsky, D.S. An Examination of the Wechsler Adult Intelligence Scales, Fourth Edition (WAIS-IV) in Individuals with Complicated Mild, Moderate and Severe Traumatic Brain Injury (TBI). Clin. Neuropsychol. 2015, 29, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Lange, R.T.; Iverson, G.L.; Franzen, M.D. Neuropsychological functioning following complicated vs. uncomplicated mild traumatic brain injury. Brain Inj. 2009, 23, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Van Dillen, L.F.; Derks, B. Working memory load reduces facilitated processing of threatening faces: An ERP study. Emotion 2012, 12, 1340–1349. [Google Scholar] [CrossRef]
- Pessoa, L.; McKenna, M.; Gutiérrez, E.; Ungerleider, L.G. Neural processing of emotional faces requires attention. Proc. Natl. Acad. Sci. USA 2002, 99, 11458–11463. [Google Scholar] [CrossRef]
- Mancuso, M.; Magnani, N.; Cantagallo, A.; Rossi, G.; Capitani, D.; Galletti, V.; Robertson, I.H. Emotion recognition impairment in traumatic brain injury compared with schizophrenia spectrum: Similar deficits with different origins. J. Nerv. Ment. Dis. 2015, 203, 87–95. [Google Scholar] [CrossRef]
- Yim, J.; Babbage, D.R.; Zupan, B.; Neumann, D.; Willer, B. The relationship between facial affect recognition and cognitive functioning after traumatic brain injury. Brain Inj. 2013, 27, 1155–1161. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Langenbahn, D.M.; Braden, C.; Malec, J.F.; Kalmar, K.; Fraas, M.; Felicetti, T.; Laatsch, L.; Harley, J.P.; Bergquist, T.; et al. Evidence-Based Cognitive Rehabilitation: Updated Review of the Literature From 2003 Through 2008. Arch. Phys. Med. Rehabilit. 2011, 92, 519–530. [Google Scholar] [CrossRef]
- McKerral, M.; Léveillé, G. Chapter 13: Return to work. When and how should I return to work after a concussion. In Sports Concussions Continuum: A Complete Guide to Recovery and Management; Gagnon, I., Ptito, A., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Cicerone, K.D. Remediation of ’working attention’ in mild traumatic brain injury. Brain Inj. 2002, 16, 185–195. [Google Scholar] [CrossRef] [PubMed]

| TBI Participants | Sex | Age (years) | Education (years) | Cause of TBI | GCSScore | Lesion Site on CT Scan | Months Post-Injury | BDI-II | PCS | Verbal IQ Estimate | Performance IQ Estimate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mTBI16 | F | 26 | 13 | Fall on ice | 15 | Normal | 31 | 11 | 31 | 105 | 120 |
| mTBI27 | M | 36 | 14 | Fall >1-meter, work accident | 15 | Normal | 28 | 6 | 4 | 95 | 95 |
| mTBI30 | F | 33 | 18 | Face-to-face hit, hockey | 15 | Normal | 18 | 0 | 7 | 105 | 120 |
| mTBI34 | F | 20 | 13 | Car accident | 15 | Normal | 4 | 20 | 26 | 100 | 115 |
| mTBI41 | F | 46 | 17 | Car accident | 14 | Normal | 5 | 9 | 16 | 105 | 100 |
| mTBI42 | F | 32 | 18 | Knee-to-head hit, basketball | 15 | Normal | 24 | 15 | 28 | 100 | 100 |
| mTBI44 | F | 29 | 16 | Fall backward sitting on bench | 15 | Normal | 7 | 7 | 18 | 110 | 125 |
| mTBI46 | F | 42 | 18 | Hit on head by basketball | 15 | Normal | 27 | 4 | 20 | 100 | 110 |
| mTBI47 | F | 40 | 17 | Car accident | 15 | Normal | 6 | 27 | 40 | 95 | 120 |
| mTBI48 | F | 42 | 16 | Fall on head, martial arts | 14 | Normal | 26 | 26 | 64 | 95 | 95 |
| CTRL101 | F | 44 | 13 | 2 | 6 | 105 | 115 | ||||
| CTRL103 | F | 29 | 18 | 6 | 14 | 105 | 115 | ||||
| CTRL105 | M | 31 | 14 | 0 | 0 | 95 | 120 | ||||
| CTRL106 | M | 26 | 16 | 3 | 4 | 115 | 115 | ||||
| CTRL107 | M | 26 | 16 | 0 | 23 | 100 | 125 | ||||
| CTRL108 | F | 51 | 14 | 8 | 15 | 105 | 115 | ||||
| CTRL109 | M | 35 | 16 | 6 | 3 | 100 | 125 | ||||
| CTRL112 | M | 29 | 12 | 3 | 4 | 100 | 115 | ||||
| CTRL114 | F | 30 | 18 | 0 | 1 | 115 | 105 | ||||
| CTRL115 | F | 30 | 20 | 0 | 1 | 105 | 120 | ||||
| CTRL116 | M | 28 | 17 | 0 | 3 | 120 | 135 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drapeau, J.; Gosselin, N.; Peretz, I.; McKerral, M. Electrophysiological Responses to Emotional Facial Expressions Following a Mild Traumatic Brain Injury. Brain Sci. 2019, 9, 142. https://doi.org/10.3390/brainsci9060142
Drapeau J, Gosselin N, Peretz I, McKerral M. Electrophysiological Responses to Emotional Facial Expressions Following a Mild Traumatic Brain Injury. Brain Sciences. 2019; 9(6):142. https://doi.org/10.3390/brainsci9060142
Chicago/Turabian StyleDrapeau, Joanie, Nathalie Gosselin, Isabelle Peretz, and Michelle McKerral. 2019. "Electrophysiological Responses to Emotional Facial Expressions Following a Mild Traumatic Brain Injury" Brain Sciences 9, no. 6: 142. https://doi.org/10.3390/brainsci9060142
APA StyleDrapeau, J., Gosselin, N., Peretz, I., & McKerral, M. (2019). Electrophysiological Responses to Emotional Facial Expressions Following a Mild Traumatic Brain Injury. Brain Sciences, 9(6), 142. https://doi.org/10.3390/brainsci9060142





