Short- and Mid-Term Improvement of Postural Balance after a Neurorehabilitation Program via Hippotherapy in Patients with Sensorimotor Impairment after Cerebral Palsy: A Preliminary Kinetic Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Inclusion Criteria
- Diagnosis: CP consisting of spastic tetraparesis, diparesis, monoparesis or hemiparesis
- Degree of impairment according to GMFCS: levels II to IV (Walking with Limitations to Self-Mobility with Limitations/May Use Powered Mobility)
- Good comprehension ability (no cognitive or behavioral impairment)
- Normal hip joint function and sufficient abduction to allow riding
- No concomitant pathology that may impair sensorimotor and/or cognitive function
- No history of treatment with botulinum toxin in the 6 months preceding the start of the protocol
- No therapeutic intervention planned during the duration of the study (injection of botulinum toxin, tenotomy, tendon transfer, etc.)
- No history of uncontrolled pain
- Certificate of non-contraindication issued by the responsible physician
2.2. Study Design
- (1)
- during the first two minutes: 60 s walk—20 s trot—20 s walk—20 s trot
- (2)
- during the last two minutes: 60 s walk—30 s trot—30 s walk
- Short term effect comparing postural balance at the beginning and at the end of the 10-minute simulator’s exercise (n = 5)
- Mid term effect comparing the remaining effect after 5 sessions, separated by one week each, on (i) postural balance evolution (at the beginning of the session) and (ii) efficacy (adjustment speed during the session) (n = 5)
2.3. Outcome Measures
2.4. Measurement Accuracy
2.5. Data Analysis
- Comparing the postural balance behavior at different times within each session: first two minutes of session 1 versus last two minutes of session 1 and first two minutes of session 5 versus last two minutes of session 5;
- Comparing the evolution of the compensatory postural adjustments (CPA) or feedback mechanisms (activated by sensory events following loss of stable posture) during 3 seconds after each change of pace (T0, T60, T80, T100, T480, T540 and T570) within each session;
- Comparing the evolution of the anticipatory postural adjustments (APA) or feedforward mechanisms (predicting disturbances and producing preprogrammed responses that maintain stability) during 3 seconds before each change of pace (T57, T77, T97, T117, T537, T567 and T597) within each session;
- Comparing the compensatory versus the anticipatory postural adjustment (CPA versus APA) around each pace change, i.e., 3 seconds before and after the change (T57 vs. T60, T77 vs. T80, T97 vs. T100, T537 vs. T540, T567 vs. T570) within each session.
- Comparing the general shape of the displacement of session 1 versus session 5 (the first and last two minutes) to have a general picture of the overall mid-term effect of the therapy on dynamic postural balance;
- Comparing the postural balance behavior at corresponding times: first two minutes of session 1 versus last two minutes of session 1 versus first two minutes of session 5 and versus last two minutes of session 5;
- Comparing the evolution of the compensatory postural adjustments (CPA) during 3 seconds after each change of pace (T0, T60, T80, T100, T480, T540 and T570) at corresponding times of session 1 and 5;
- Comparing the evolution of the anticipatory postural adjustments (APA) during 3 seconds before each change of pace (T57, T77, T97, T117, T537, T567 and T597) at corresponding times of session 1 and 5.
2.6. Statistical Analysis
3. Results
3.1. Study Population
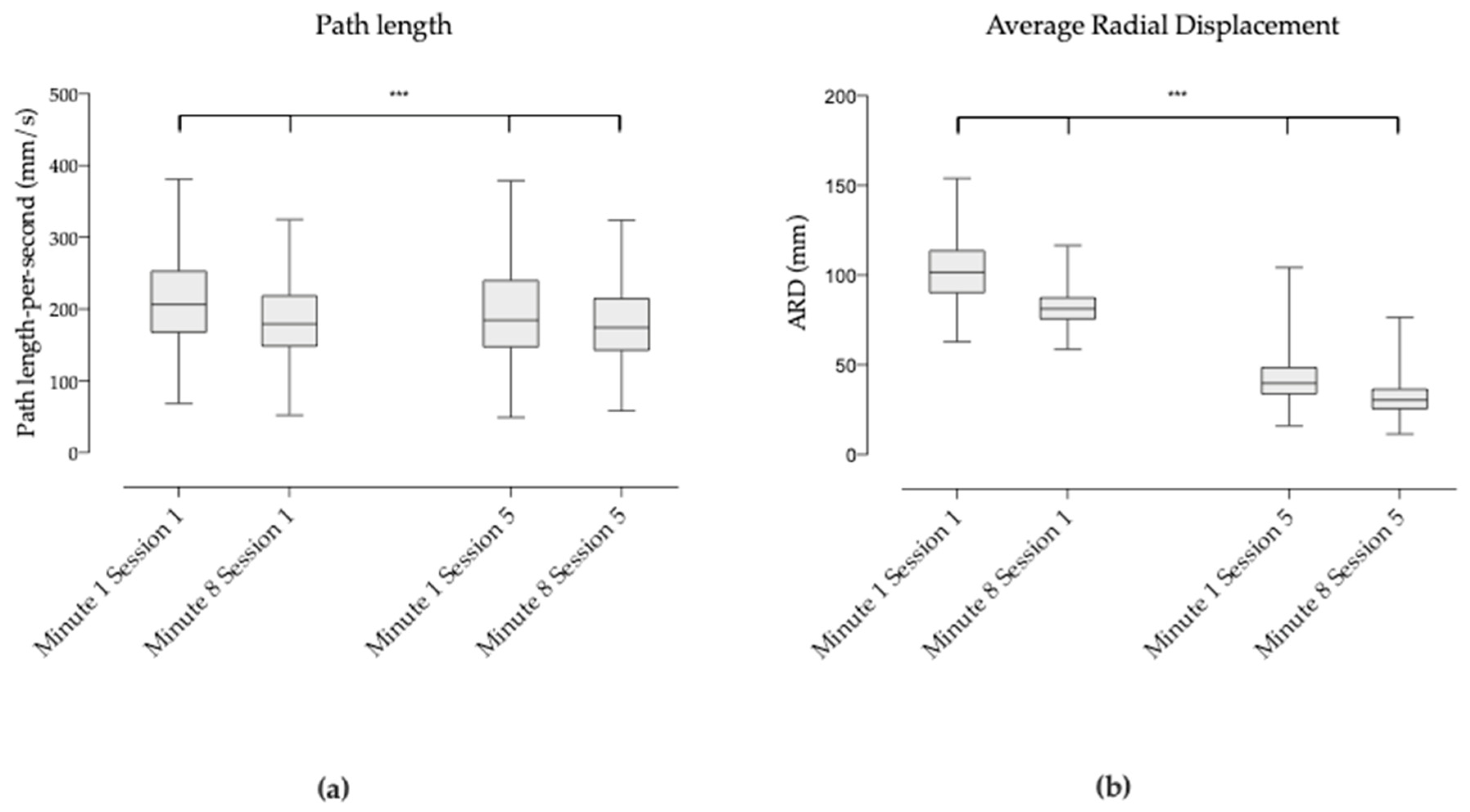
3.2. General Shape of the Displacement of COP between Session 1 and 5
3.3. COP Behavior at the Different Times
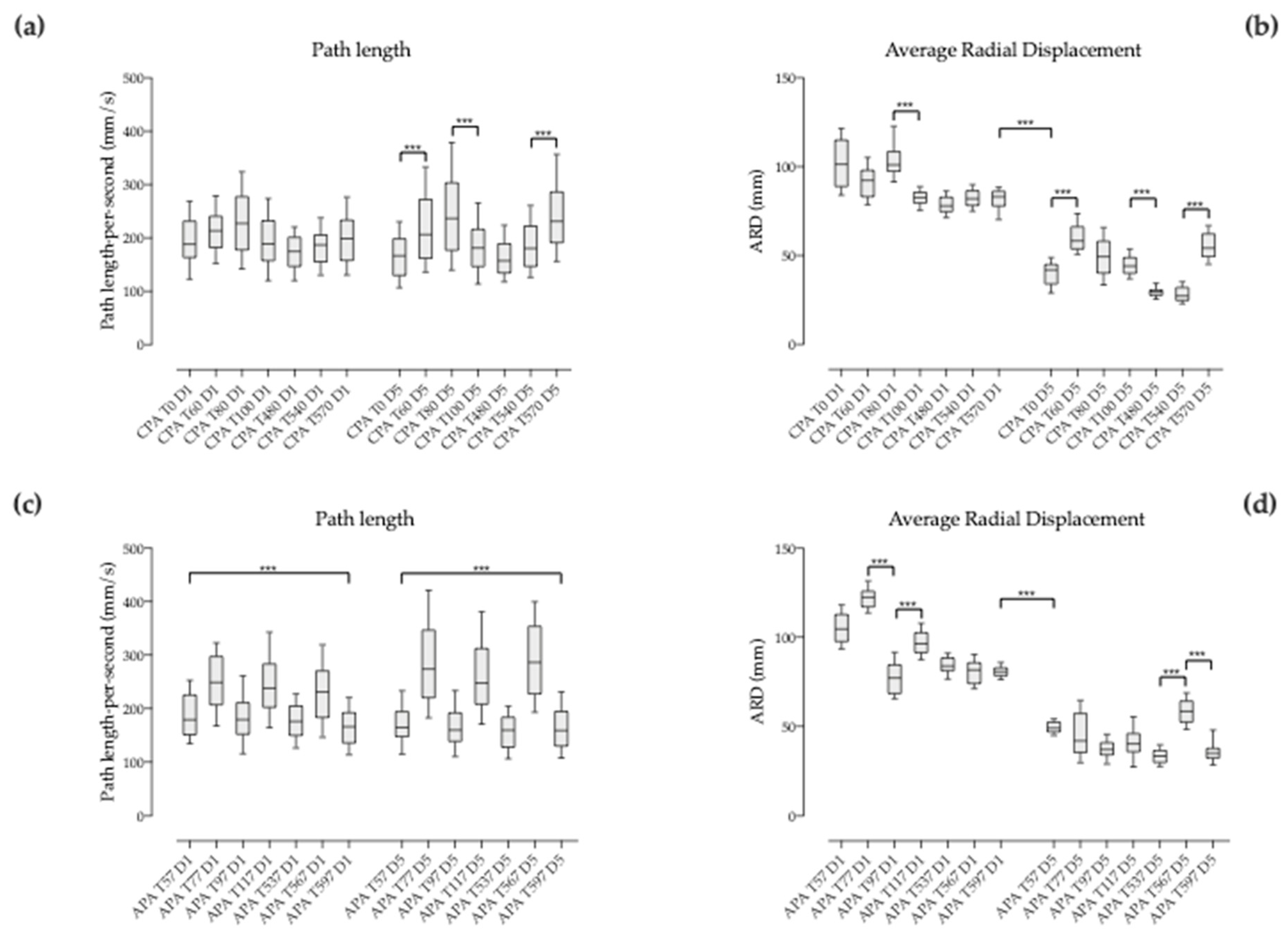
3.4. Behavior of the Compensatory Postural Adjustments (CPA) with Pace Changes
3.4.1. Between Successive Pace Changes within the Same Sequence
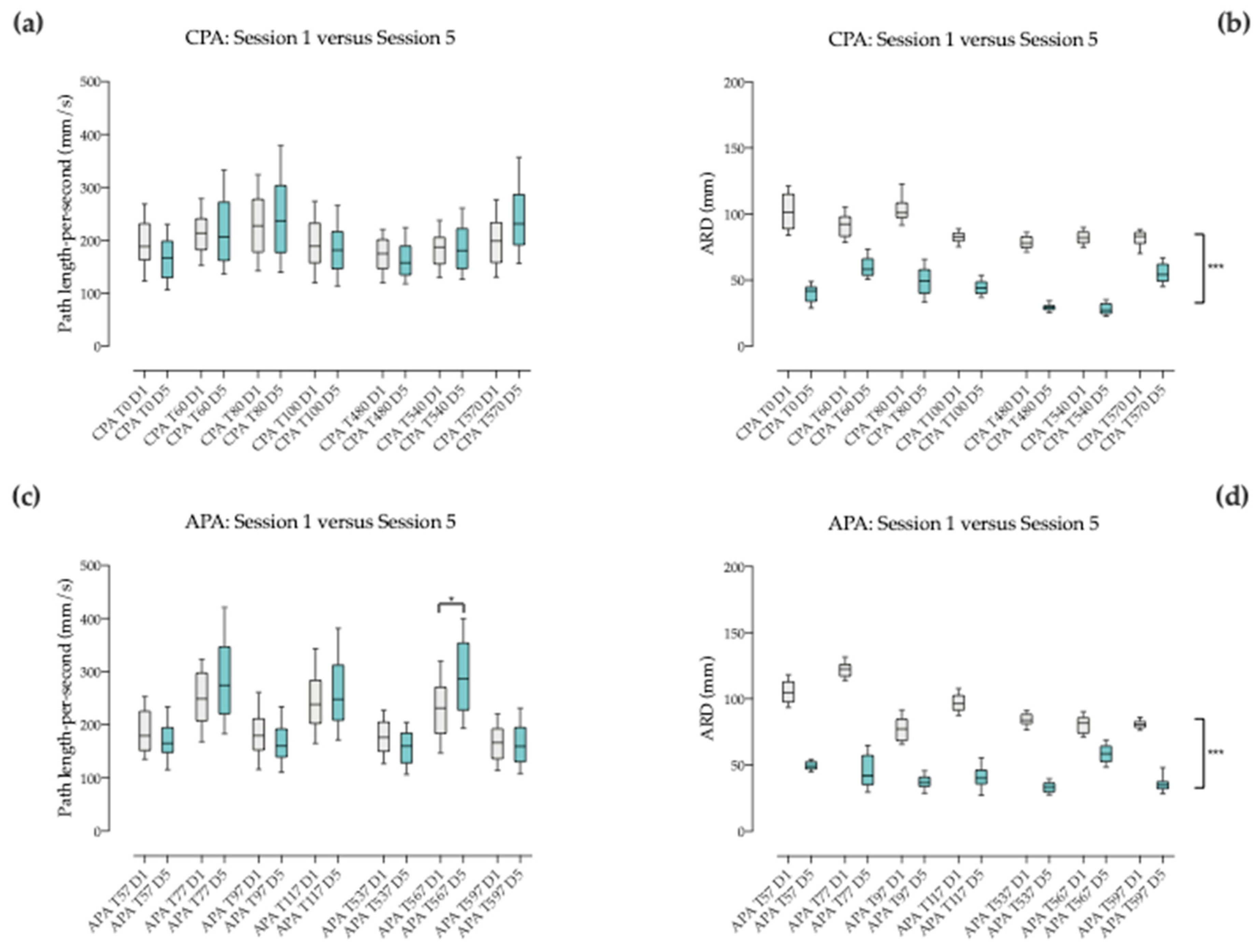
3.4.2. Between Corresponding Times of Session 1 and 5 when Pace Changes
3.5. Behavior of the Anticipatory Postural Adjustments (APA) before Pace Changes
3.5.1. Between Successive Pace Changes within the Same Sequence
3.5.2. Between Corresponding Times of Session 1 and 5 when Pace Changes
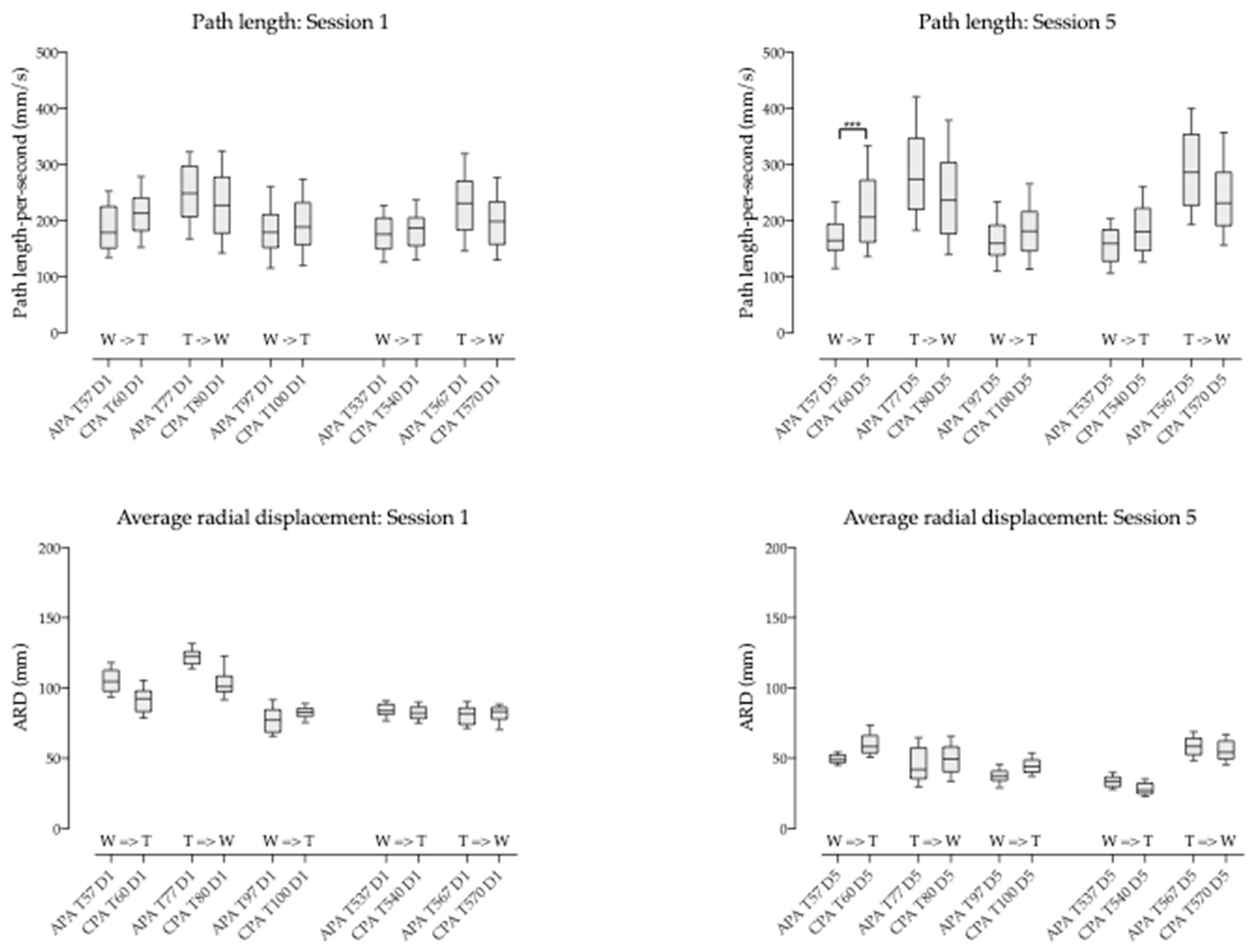
3.6. Compensatory Postural Adjustment (CPA) versus Anticipatory Postural Adjustment (APA)
4. Discussion
5. Conclusions
6. Study Limitations and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winter, D.A.; Patla, A.E.; Prince, F.; Ishac, M.; Gielo-Perczak, K. Stiffness Control of Balance in Quiet Standing. J. Neurophysiol. 1998, 80, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Rinalduzzi, S.; Trompetto, C.; Marinelli, L.; Alibardi, A.; Missori, P.; Fattapposta, F.; Pierelli, F.; Currà, A. Balance Dysfunction in Parkinson’s Disease. BioMed Res. Int. 2015, 2015, 434683. [Google Scholar] [CrossRef] [PubMed]
- Kawato, M. Internal models for motor control and trajectory planning. Curr. Opin. Neurobiol. 1999, 9, 718–727. [Google Scholar] [CrossRef]
- Chiba, R.; Takakusaki, K.; Ota, J.; Yozu, A.; Haga, N. Human upright posture control models based on multisensory inputs: In fast and slow dynamics. Neurosci. Res. 2016, 104, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Westcott, S.L.; Burtner, P. Postural control in children. Phys. Occup. Ther. Pediatr. 2004, 24, 5–55. [Google Scholar] [CrossRef]
- Harris, S.R.; Roxborough, L. Efficacy and Effectiveness of Physical Therapy in Enhancing Postural Control in Children with Cerebral Palsy. Neural Plast. 2005, 12, 229–243. [Google Scholar] [CrossRef]
- Dewar, R.; Love, S.; Johnston, L.M. Exercise interventions improve postural control in children with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2015, 57, 504–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Markopoulos, P.; Yu, B.; Chen, W.; Timmermans, A. Interactive wearable systems for upper body rehabilitation: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 20. [Google Scholar] [CrossRef]
- Vera-Garcia, F.J.; Grenier, S.G.; McGill, S.M. Abdominal Muscle Response during Curl-ups on Both Stable and Labile Surfaces. Phys. Ther. 2000, 80, 564–569. [Google Scholar] [PubMed]
- Lehman, G.J.; Hoda, W.; Oliver, S. Trunk muscle activity during bridging exercises on and off a Swissball. Chiropr. Osteopat. 2005, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, Y.; Chung, Y. The Influence of an Unstable Surface on Trunk and Lower Extremity Muscle Activities during Variable Bridging Exercises. J. Phys. Ther. Sci. 2014, 26, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Sekendiz, B.; Cug, M.; Korkusuz, F. Effects of Swiss-Ball Core Strength Training on Strength, Endurance, Flexibility, and Balance in Sedentary Women. J. Strength Cond. Res. 2010, 24, 3032–3040. [Google Scholar] [CrossRef]
- Sterba, J.A. Does horseback riding therapy or therapist-directed hippotherapy rehabilitate children with cerebral palsy? Dev. Med. Child Neurol. 2007, 49, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Bronson, C.; Brewerton, K.; Ong, J.; Palanca, C.; Sullivan, S.J. Does hippotherapy improve balance in persons with multiple sclerosis: A systematic review. Eur. J. Phys. Rehabil. Med. 2010, 46, 347–353. [Google Scholar]
- Gabriels, R.L.; Agnew, J.A.; Holt, K.D.; Shoffner, A.; Pan, Z.; Ruzzano, S.; Clayton, G.H.; Mesibov, G. Pilot study measuring the effects of therapeutic horseback riding on school-age children and adolescents with autism spectrum disorders. Res. Autism Spectr. Disord. 2012, 6, 578–588. [Google Scholar] [CrossRef]
- Han, J.Y.; Kim, J.M.; Kim, S.K.; Chung, J.S.; Lee, H.-C.; Lim, J.K.; Lee, J.; Park, K.Y. Therapeutic Effects of Mechanical Horseback Riding on Gait and Balance Ability in Stroke Patients. Ann. Rehabil. Med. 2012, 36, 762–769. [Google Scholar]
- McGibbon, N.H.; Benda, W.; Duncan, B.R.; Silkwood-Sherer, D. Immediate and Long-Term Effects of Hippotherapy on Symmetry of Adductor Muscle Activity and Functional Ability in Children with Spastic Cerebral Palsy. Arch. Phys. Med. Rehabil. 2009, 90, 966–974. [Google Scholar] [CrossRef]
- Shurtleff, T.L.; Standeven, J.W.; Engsberg, J.R. Changes in Dynamic Trunk/Head Stability and Functional Reach after Hippotherapy. Arch. Phys. Med. Rehabil. 2009, 90, 1185–1195. [Google Scholar] [CrossRef]
- Kwon, J.-Y.; Chang, H.J.; Lee, J.Y.; Ha, Y.; Lee, P.K.; Kim, Y.-H. Effects of Hippotherapy on Gait Parameters in Children with Bilateral Spastic Cerebral Palsy. Arch. Phys. Med. Rehabil. 2011, 92, 774–779. [Google Scholar] [CrossRef]
- Lee, C.-W.; Gil Kim, S.; Na, S.S. The Effects of Hippotherapy and a Horse Riding Simulator on the Balance of Children with Cerebral Palsy. J. Phys. Ther. Sci. 2014, 26, 423–425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silkwood-Sherer, D.J.; Killian, C.B.; Long, T.M.; Martin, K.S. Hippotherapy—An Intervention to Habilitate Balance Deficits in Children With Movement Disorders: A Clinical Trial. Phys. Ther. 2012, 92, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.B.S.; Werneck, M.J.D.S.; Silva, M.D.L.D.; Gandolfi, L.; Pratesi, R. Therapeutic effects of a horse riding simulator in children with cerebral palsy. Arq. Neuro-Psiquiatr. 2011, 69, 799–804. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, S.-G.; Hwangbo, G. The effects of horse-riding simulator exercise and Kendall exercise on the forward head posture. J. Phys. Ther. Sci. 2015, 27, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- Temcharoensuk, P.; Lekskulchai, R.; Akamanon, C.; Ritruechai, P.; Sutcharitpongsa, S. Effect of horseback riding versus a dynamic and static horse riding simulator on sitting ability of children with cerebral palsy: A randomized controlled trial. J. Phys. Ther. Sci. 2015, 27, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.G.; Lee, B.J.; Lee, W.H. The effects of horse riding simulation exercise with blindfolding on healthy subjects’ balance and gait. J. Phys. Ther. Sci. 2016, 28, 3165–3167. [Google Scholar] [CrossRef][Green Version]
- Kim, S.-K.; Kim, S.-G.; Hwangbo, G. The effect of horse-riding simulator exercise on the gait, muscle strength and muscle activation in elderly people with knee osteoarthritis. J. Phys. Ther. Sci. 2017, 29, 693–696. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baillet, H.; Thouvarecq, R.; Vérin, E.; Tourny, C.; Benguigui, N.; Komar, J.; Leroy, D. Human Energy Expenditure and Postural Coordination on the Mechanical Horse. J. Mot. Behav. 2017, 49, 441–457. [Google Scholar] [CrossRef]
- Silkwood-Sherer, D.; Warmbier, H. Effects of Hippotherapy on Postural Stability, in Persons with Multiple Sclerosis: A Pilot Study. J. Neurol. Phys. Ther. 2007, 31, 77–84. [Google Scholar] [CrossRef]
- Sunwoo, H.; Chang, W.H.; Kwon, J.-Y.; Kim, T.-W.; Lee, J.-Y.; Kim, Y.-H. Hippotherapy in Adult Patients with Chronic Brain Disorders: A Pilot Study. Ann. Rehabil. Med. 2012, 36, 756–761. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Lee, J.; Lee, D. The Effects of Horseback Riding Simulator Exercise on Postural Balance of Chronic Stroke Patients. J. Phys. Ther. Sci. 2013, 25, 1169–1172. [Google Scholar] [CrossRef]
- Herrero, P.; Asensio, A.; García, E.; Marco, Á.; Oliván, B.; Ibarz, A.; Gómez-Trullén, E.M.; Casas, R. Study of the therapeutic effects of an advanced hippotherapy simulator in children with cerebral palsy: A randomised controlled trial. BMC Musculoskelet. Disord. 2010, 11, 71. [Google Scholar] [CrossRef]
- Araujo, T.B.; Silva, N.A.; Costa, J.N.; Pereira, M.M.; Safons, M.P. Effect of equine-assisted therapy on the postural balance of the elderly. Braz. J. Phys. Ther. 2011, 15, 414–419. [Google Scholar] [CrossRef]
- Clayton, H.M.; Kaiser, L.J.; De Pue, B. Center-of-Pressure Movements during Equine-Assisted Activities. Am. J. Occup. Ther. 2011, 65, 211–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fradkin, A.J.; Zazryn, T.R.; Smoliga, J. Effects of Warming-up on Physical Performance: A Systematic Review with Meta-analysis. J. Strength Cond. Res. 2010, 24, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.J.; Leach, J.; Owen-Lynch, P.J.; Sünram-Lea, S.I. Stress reactivity and cognitive performance in a simulated firefighting emergency. Aviat. Space Environ. Med. 2013, 84, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-H.; Kim, S.-E.; Lee, M.-G.; Jin, J.-J.; Hong, J.; Choi, Y.-T.; Kim, M.-H.; Jee, Y.-S. The effect of horse simulator riding on visual analogue scale, body composition and trunk strength in the patients with chronic low back pain. Int. J. Clin. Pract. 2014, 68, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.; Wolff, D.R.; Jones, V.K.; Bloch, D.A.; Oehlert, J.W.; Gamble, J.G. Postural balance in children with cerebral palsy. Dev. Med. Child Neurol. 2002, 44, 58–63. [Google Scholar] [CrossRef]
- Campbell, S.K. Efficacy of Physical Therapy in Improving Postural Control in Cerebral Palsy. Pediatr. Phys. Ther. 1990, 2, 135–140. [Google Scholar] [CrossRef]
- Baram, Y.; Miller, A. Auditory feedback control for improvement of gait in patients with Multiple Sclerosis. J. Neurol. Sci. 2007, 254, 90–94. [Google Scholar] [CrossRef]
- Sterba, J.A.; Rogers, B.T.; France, A.P.; Vokes, D.A. Horseback riding in children with cerebral palsy: Effect on gross motor function. Dev. Med. Child Neurol. 2002, 44, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, E.; Jahnsen, R.; Stanghelle, J.K.; Strand, L.I. Body weight supported treadmill training versus traditional training in patients dependent on walking assistance after stroke: A randomized controlled trial. Disabil. Rehabil. 2012, 34, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.J.; Zimbelman, J.; Roenigk, K.L.; McCabe, J.P.; Rogers, J.M.; Butler, K.; Burdsall, R.; Holcomb, J.P.; Marsolais, E.B.; Ruff, R.L. Recovery of coordinated gait: Randomized controlled stroke trial of functional electrical stimulation (FES) versus no FES, with weight-supported treadmill and over-ground training. Neurorehabil. Neural Repair 2011, 25, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hwang, W.-H.; Tsai, Y.-C.; Liu, F.-K.; Hsieh, L.-F.; Chern, J.-S. Improving Balance Skills in Patients Who Had Stroke Through Virtual Reality Treadmill Training. Am. J. Phys. Med. Rehabil. 2011, 90, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Brogårdh, C.; Flansbjer, U.-B.; Lexell, J. No Specific Effect of Whole-Body Vibration Training in Chronic Stroke: A Double-Blind Randomized Controlled Study. Arch. Phys. Med. Rehabil. 2012, 93, 253–258. [Google Scholar] [CrossRef]
- Fisher, S.; Lucas, L.; Thrasher, T.A. Robot-Assisted Gait Training for Patients with Hemiparesis Due to Stroke. Top. Stroke Rehabil. 2011, 18, 269–276. [Google Scholar] [CrossRef]
- Bütefisch, C.M. Plasticity in the Human Cerebral Cortex: Lessons from the Normal Brain and from Stroke. Neuroscientist 2004, 10, 163–173. [Google Scholar] [CrossRef]
- Weerdesteyn, V.; Laing, A.C.; Robinovitch, S.N. Automated postural responses are modified in a functional manner by instruction. Exp. Brain Res. 2008, 186, 571–580. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Cortical control of postural responses. J. Neural Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef]
- Deliagina, T.G.; Beloozerova, I.N.; Zelenin, P.V.; Orlovsky, G.N. Spinal and supraspinal postural networks. Brain Res. Rev. 2008, 57, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Butler, J.E.; Taylor, J.L.; Gandevia, S.C. Motor cortex may be involved in feedforward postural responses of the deep trunk muscles. In International Society for Posture and Gait Research; Menz, L.H.B., Ed.; International Society for Posture and Gait Research: Sydney, Australia, 2003; pp. 53–54. [Google Scholar]
- Tsao, H.; Galea, M.P.; Hodges, P.W.; Galea, M. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 2008, 131, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Karl, A.; Birbaumer, N.; Lutzenberger, W.; Cohen, L.G.; Flor, H. Reorganization of Motor and Somatosensory Cortex in Upper Extremity Amputees with Phantom Limb Pain. J. Neurosci. 2001, 21, 3609–3618. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, K.; Donoghue, J. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 1991, 251, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Florence, S.L.; Taub, H.B.; Kaas, J.H. Large-Scale Sprouting of Cortical Connections after Peripheral Injury in Adult Macaque Monkeys. Science 1998, 282, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Hogg, T.M.; Vandenberg, P.M.; Cooper, N.R.; Bruneau, R.; Remple, M. Cortical Synaptogenesis and Motor Map Reorganization Occur during Late, But Not Early, Phase of Motor Skill Learning. J. Neurosci. 2004, 24, 628–633. [Google Scholar] [CrossRef]
- Ziemann, U.; Hallett, M.; Cohen, L.G. Mechanisms of Deafferentation-Induced Plasticity in Human Motor Cortex. J. Neurosci. 1998, 18, 7000–7007. [Google Scholar] [CrossRef]
- Kolb, B.; Muhammad, A. Harnessing the power of neuroplasticity for intervention. Front. Hum. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef]
- Heijtz, R.D.; Kolb, B.; Forssberg, H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? Eur. J. Neurosci. 2003, 18, 3394–3399. [Google Scholar] [CrossRef] [PubMed]
- Horn, G. Pathways of the past: The imprint of memory. Nat. Rev. Neurosci. 2004, 5, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Ham, T.E.; Bonnelle, V.; Hellyer, P.; Jilka, S.; Robertson, I.H.; Leech, R.; Sharp, D.J. The neural basis of impaired self-awareness after traumatic brain injury. Brain 2014, 137, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Van Criekinge, T.; Saeys, W.; Vereeck, L.; De Hertogh, W.; Truijen, S. Are unstable support surfaces superior to stable support surfaces during trunk rehabilitation after stroke? A systematic review. Disabil. Rehabil. 2017, 40, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Gjelsvik, B.E.B.; Hofstad, H.; Smedal, T.; Eide, G.E.; Næss, H.; Skouen, J.S.; Frisk, B.; Daltveit, S.; Strand, L.I. Balance and walking after three different models of stroke rehabilitation: Early supported discharge in a day unit or at home, and traditional treatment (control). BMJ Open 2014, 4, e004358. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.W.; Murphy, B.A. Core stability exercises on and off a Swiss ball. Arch. Phys. Med. Rehabil. 2005, 86, 242–249. [Google Scholar] [CrossRef] [PubMed]


| Age | Sex | Motor deficit | GMFCS | Axial Hypotonia * | Sitting Postural Deficit ** | Adductor’s Hypertonia *** |
|---|---|---|---|---|---|---|
| 12 | M | spastic tetraparesis | IV | + | ++ | 2 |
| 24 | F | spastic tetraparesis | III | ++ | + | 2 |
| 19 | M | spastic tetraparesis | IV | ++ | ++ | 2 |
| 8 | M | spastic tetraparesis | III | ++ | ++ | 1+ |
| 14 | F | tetraparesis | II | +++ | ++ | 0 |
| COP Path Length during CPA | Value 1 | Value 2 | p Value |
|---|---|---|---|
| Session 1 | |||
| T0 (Walk 1) vs. T60 (Trot 1) | 196.1 ± 56.1 | 215.9 ± 56.1 | ns |
| T60 (Trot 1) vs. T80 (Walk 2) | 215.9 ± 56.1 | 229.3 ± 71.2 | ns |
| T80 (Walk 2) vs. T100 (Trot 2) | 229.3 ± 71.2 | 196.9 ± 54.9 | ns |
| T100 (Trot 2) vs. T480 (Walk 3) | 196.9 ± 54.9 | 172.4 ± 39.6 | ns |
| T480 (Walk 3) vs. T540 (Trot 3) | 172.4 ± 39.6 | 183.9 ± 44.2 | ns |
| T540 (Trot 3) vs. T570 (Walk 4) | 183.9 ± 44.2 | 200.6 ± 58.5 | ns |
| Session 5 | |||
| T0 (Walk 1) vs. T60 (Trot 1) | 167.9 ± 48.4 | 219.3 ± 75.2 | *** |
| T60 (Trot 1) vs. T80 (Walk 2) | 219.3 ± 75.2 | 254.5 ± 98.1 | ns |
| T80 (Walk 2) vs. T100 (Trot 2) | 254.5 ± 98.1 | 185.8 ± 58.4 | *** |
| T100 (Trot 2) vs. T480 (Walk 3) | 185.8 ± 58.4 | 163.5 ± 42.1 | ns |
| T480 (Walk 3) vs. T540 (Trot 3) | 163.5 ± 42.1 | 187.4 ± 49.2 | ns |
| T540 (Trot 3) vs. T570 (Walk 4) | 187.4 ± 49.2 | 241.4 ± 73.6 | *** |
| COP average radial displacement during CPA | |||
| Session 1 | |||
| T0 (Walk 1) vs. T60 (Trot 1) | 102.2 ± 14.4 | 91.3 ± 9.5 | ns |
| T60 (Trot 1) vs. T80 (Walk 2) | 91.3 ± 9.5 | 103.9 ± 11.6 | ns |
| T80 (Walk 2) vs. T100 (Trot 2) | 103.9 ± 11.6 | 82.4 ± 6.0 | *** |
| T100 (Trot 2) vs. T480 (Walk 3) | 82.4 ± 6.0 | 78.6 ± 5.4 | ns |
| T480 (Walk 3) vs. T540 (Trot 3) | 78.6 ± 5.4 | 82.4 ± 6.0 | ns |
| T540 (Trot 3) vs. T570 (Walk 4) | 82.4 ± 6.0 | 81.5 ± 6.5 | ns |
| Session 5 | |||
| T0 (Walk 1) vs. T60 (Trot 1) | 40.1 ± 7.2 | 60.3 ± 8.7 | *** |
| T60 (Trot 1) vs. T80 (Walk 2) | 60.3 ± 8.7 | 49.1 ± 11.3 | ns |
| T80 (Walk 2) vs. T100 (Trot 2) | 49.1 ± 11.3 | 44.8 ± 6.1 | ns |
| T100 (Trot 2) vs. T480 (Walk 3) | 44.8 ± 6.1 | 29.5 ± 3.0 | *** |
| T480 (Walk 3) vs. T540 (Trot 3) | 29.5 ± 3.0 | 28.2 ± 4.5 | ns |
| T540 (Trot 3) vs. T570 (Walk 4) | 28.2 ± 4.5 | 55.6 ± 8.4 | *** |
| COP Path Length during CPA | Session 1 | Session 5 | p Value |
|---|---|---|---|
| T0 D1 (Walk 1) vs. T0 D5 (Walk 1) | 196.1 ± 56.1 | 167.9 ± 48.4 | ns |
| T60 D1 (Trot 1) vs. T60 D5 (Trot 1) | 215.9 ± 56.1 | 219.3 ± 75.2 | ns |
| T80 D1 (Walk 2) vs. T80 D5 (Walk 2) | 229.3 ± 71.2 | 254.5 ± 98.1 | ns |
| T100 D1 (Trot 2) vs. T100 D5 (Trot 2) | 196.9 ± 54.9 | 185.8 ± 58.4 | ns |
| T480 D1 (Walk 3) vs. T480 D5 (Walk 3) | 172.4 ± 39.6 | 163.5 ± 42.1 | ns |
| T540 D1 (Trot 3) vs. T540 D5 (Trot 3) | 183.9 ± 44.2 | 187.4 ± 49.2 | ns |
| T570 D1 (Walk 4) vs. T570 D5 (Walk 4) | 200.6 ± 58.5 | 241.4 ± 73.6 | ns |
| COP average radial displacement during CPA | |||
| T0 D1 (Walk 1) vs. T0 D5 (Walk 1) | 102.2 ± 14.4 | 40.1 ± 7.2 | *** |
| T60 D1 (Trot 1) vs. T60 D5 (Trot 1) | 91.3 ± 9.5 | 60.3 ± 8.7 | *** |
| T80 D1 (Walk 2) vs. T80 D5 (Walk 2) | 103.9 ± 11.6 | 49.1 ± 11.3 | *** |
| T100 D1 (Trot 2) vs. T100 D5 (Trot 2) | 82.4 ± 6.0 | 44.8 ± 6.1 | *** |
| T480 D1 (Walk 3) vs. T480 D5 (Walk 3) | 78.6 ± 5.4 | 29.5 ± 3.0 | *** |
| T540 D1 (Trot 3) vs. T540 D5 (Trot 3) | 82.4 ± 6.0 | 28.2 ± 4.5 | *** |
| T570 D1 (Walk 4) vs. T570 D5 (Walk 4) | 81.5 ± 6.5 | 55.6 ± 8.4 | *** |
| COP Path Length during APA | Value 1 | Value 2 | p Value |
|---|---|---|---|
| Session 1 | |||
| T57 D1 vs. T77 D1 | 188.6 ± 46.7 | 250.9 ± 62.0 | *** |
| T77 D1 vs. T97 D1 | 250.9 ± 62.0 | 184.5 ± 49.6 | *** |
| T97 D1 vs. T117 D1 | 184.5 ± 49.6 | 247.2 ± 66.4 | *** |
| T117 D1 vs. T537 D1 | 247.2 ± 66.4 | 177.7 ± 40.2 | *** |
| T537 D1 vs. T567 D1 | 177.7 ± 40.2 | 232.3 ± 67.4 | *** |
| T567 D1 vs. T597 D1 | 232.3 ± 67.4 | 167.3 ± 41.8 | *** |
| Session 5 | |||
| T57 D5 vs. T77 D5 | 169.6 ± 40.3 | 290.8 ± 94.2 | *** |
| T77 D5 vs. T97 D5 | 290.8 ± 94.2 | 166.5 ± 44.0 | *** |
| T97 D5 vs. T117 D5 | 166.5 ± 44.0 | 268.2 ± 92.1 | *** |
| T117 D5 vs. T537 D5 | 268.2 ± 92.1 | 157.5 ± 40.3 | *** |
| T537 D5 vs. T567 D5 | 157.5 ± 40.3 | 289.4 ± 82.1 | *** |
| T567 D5 vs. T597 D5 | 289.4 ± 82.1 | 164.5 ± 46.5 | *** |
| COP average radial displacement during APA | |||
| Session 1 | |||
| T57 D1 vs. T77 D1 | 104.9 ± 9.5 | 122.8 ± 8.6 | ns |
| T77 D1 vs. T97 D1 | 122.8 ± 8.6 | 77.3 ± 9.2 | *** |
| T97 D1 vs. T117 D1 | 77.3 ± 9.2 | 96.8 ± 7.4 | *** |
| T117 D1 vs. T537 D1 | 96.8 ± 7.4 | 84.1 ± 5.1 | ns |
| T537 D1 vs. T567 D1 | 84.1 ± 5.1 | 80.5 ± 7.2 | ns |
| T567 D1 vs. T597 D1 | 80.5 ± 7.2 | 80.7 ± 3.6 | ns |
| Session 5 | |||
| T57 D5 vs. T77 D5 | 49.5 ± 3.8 | 45.5 ± 13.6 | ns |
| T77 D5 vs. T97 D5 | 45.5 ± 13.6 | 37.3 ± 5.8 | ns |
| T97 D5 vs. T117 D5 | 37.3 ± 5.8 | 41.0 ± 10.4 | ns |
| T117 D5 vs. T537 D5 | 41.0 ± 10.4 | 33.5 ± 4.6 | ns |
| T537 D5 vs. T567 D5 | 33.5 ± 4.6 | 58.3 ± 7.8 | *** |
| T567 D5 vs. T597 D5 | 58.3 ± 7.8 | 36.1 ± 6.5 | *** |
| COP Path Length during APA | Session 1 | Session 5 | p Value |
|---|---|---|---|
| T57 D1 vs. T57 D5 | 188.6 ± 46.7 | 169.6 ± 40.3 | ns |
| T77 D1 vs. T77 D5 | 250.9 ± 62.0 | 290.8 ± 94.2 | ns |
| T97 D1 vs. T97 D5 | 184.5 ± 49.6 | 166.5 ± 44.0 | ns |
| T117 D1 vs. T117 D5 | 247.2 ± 66.4 | 268.2 ± 92.1 | ns |
| T537 D1 vs. T537 D5 | 177.7 ± 40.2 | 157.5 ± 40.3 | ns |
| T567 D1 vs. T567 D5 | 232.3 ± 67.4 | 289.4 ± 82.1 | * |
| T597 D1 vs. T597 D5 | 167.3 ± 41.8 | 164.5 ± 46.5 | ns |
| COP average radial displacement during APA | |||
| T57 D1 vs. T57 D5 | 104.9 ± 9.5 | 49.5 ± 3.8 | *** |
| T77 D1 vs. T77 D5 | 122.8 ± 8.6 | 45.5 ± 13.6 | *** |
| T97 D1 vs. T97 D5 | 77.3 ± 9.2 | 37.3 ± 5.8 | *** |
| T117 D1 vs. T117 D5 | 96.8 ± 7.4 | 41.0 ± 10.4 | *** |
| T537 D1 vs. T537 D5 | 84.1 ± 5.1 | 33.5 ± 4.6 | *** |
| T567 D1 vs. T567 D5 | 80.5 ± 7.2 | 58.3 ± 7.8 | *** |
| T597 D1 vs. T597 D5 | 80.7 ± 3.6 | 36.1 ± 6.5 | *** |
| COP Path Length | APA | CPA | p value |
|---|---|---|---|
| Session 1 | |||
| T57 (walk) vs. T60 (trot) | 188.6 ± 46.7 | 215.9 ± 56.1 | ns |
| T77 (trot) vs. T80 (walk) | 250.9 ± 62.0 | 229.3 ± 71.2 | ns |
| T97 (walk) vs. T100 (trot) | 184.5 ± 49.6 | 196.9 ± 54.9 | ns |
| T537 (walk) vs. T540 (trot) | 177.7 ± 40.2 | 183.9 ± 44.2 | ns |
| T567 (trot) vs. T570 (walk) | 232.3 ± 67.4 | 200.6 ± 58.5 | ns |
| Session 5 | |||
| T57 (walk) vs. T60 (trot) | 169.6 ± 40.3 | 219.3 ± 75.2 | *** |
| T77 (trot) vs. T80 (walk) | 290.8 ± 94.2 | 254.5 ± 98.1 | ns |
| T97 (walk) vs. T100 (trot) | 166.5 ± 44.0 | 185.8 ± 58.4 | ns |
| T537 (walk) vs. T540 (trot) | 157.5 ± 40.3 | 187.4 ± 49.2 | ns |
| T567 (trot) vs. T570 (walk) | 289.4 ± 82.1 | 241.4 ± 73.6 | ns |
| COP average radial displacement | |||
| Session 1 | |||
| T57 (walk) vs. T60 (trot) | 104.9 ± 9.5 | 91.3 ± 9.5 | ns |
| T77 (trot) vs. T80 (walk) | 122.8 ± 8.6 | 103.9 ± 11.6 | ns |
| T97 (walk) vs. T100 (trot) | 77.3 ± 9.2 | 82.4 ± 6.0 | ns |
| T537 (walk) vs. T540 (trot) | 84.1 ± 5.1 | 82.4 ± 6.0 | ns |
| T567 (trot) vs. T570 (walk) | 80.5 ± 7.2 | 81.5 ± 6.5 | ns |
| Session 5 | |||
| T57 (walk) vs. T60 (trot) | 49.5 ± 3.8 | 60.3 ± 8.7 | ns |
| T77 (trot) vs. T80 (walk) | 45.5 ± 13.6 | 49.1 ± 11.3 | ns |
| T97 (walk) vs. T100 (trot) | 37.3 ± 5.8 | 44.8 ± 6.1 | ns |
| T537 (walk) vs. T540 (trot) | 33.5 ± 4.6 | 28.2 ± 4.5 | ns |
| T567 (trot) vs. T570 (walk) | 58.3 ± 7.8 | 55.6 ± 8.4 | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viruega, H.; Gaillard, I.; Carr, J.; Greenwood, B.; Gaviria, M. Short- and Mid-Term Improvement of Postural Balance after a Neurorehabilitation Program via Hippotherapy in Patients with Sensorimotor Impairment after Cerebral Palsy: A Preliminary Kinetic Approach. Brain Sci. 2019, 9, 261. https://doi.org/10.3390/brainsci9100261
Viruega H, Gaillard I, Carr J, Greenwood B, Gaviria M. Short- and Mid-Term Improvement of Postural Balance after a Neurorehabilitation Program via Hippotherapy in Patients with Sensorimotor Impairment after Cerebral Palsy: A Preliminary Kinetic Approach. Brain Sciences. 2019; 9(10):261. https://doi.org/10.3390/brainsci9100261
Chicago/Turabian StyleViruega, Hélène, Inès Gaillard, John Carr, Bill Greenwood, and Manuel Gaviria. 2019. "Short- and Mid-Term Improvement of Postural Balance after a Neurorehabilitation Program via Hippotherapy in Patients with Sensorimotor Impairment after Cerebral Palsy: A Preliminary Kinetic Approach" Brain Sciences 9, no. 10: 261. https://doi.org/10.3390/brainsci9100261
APA StyleViruega, H., Gaillard, I., Carr, J., Greenwood, B., & Gaviria, M. (2019). Short- and Mid-Term Improvement of Postural Balance after a Neurorehabilitation Program via Hippotherapy in Patients with Sensorimotor Impairment after Cerebral Palsy: A Preliminary Kinetic Approach. Brain Sciences, 9(10), 261. https://doi.org/10.3390/brainsci9100261






