Can Musical Training Influence Brain Connectivity? Evidence from Diffusion Tensor MRI
Abstract
:1. Introduction
Investigation of White Matter Using Structural MRI
2. Diffusion Tensor MRI
2.1. Overview
2.2. Region of Interest Analysis
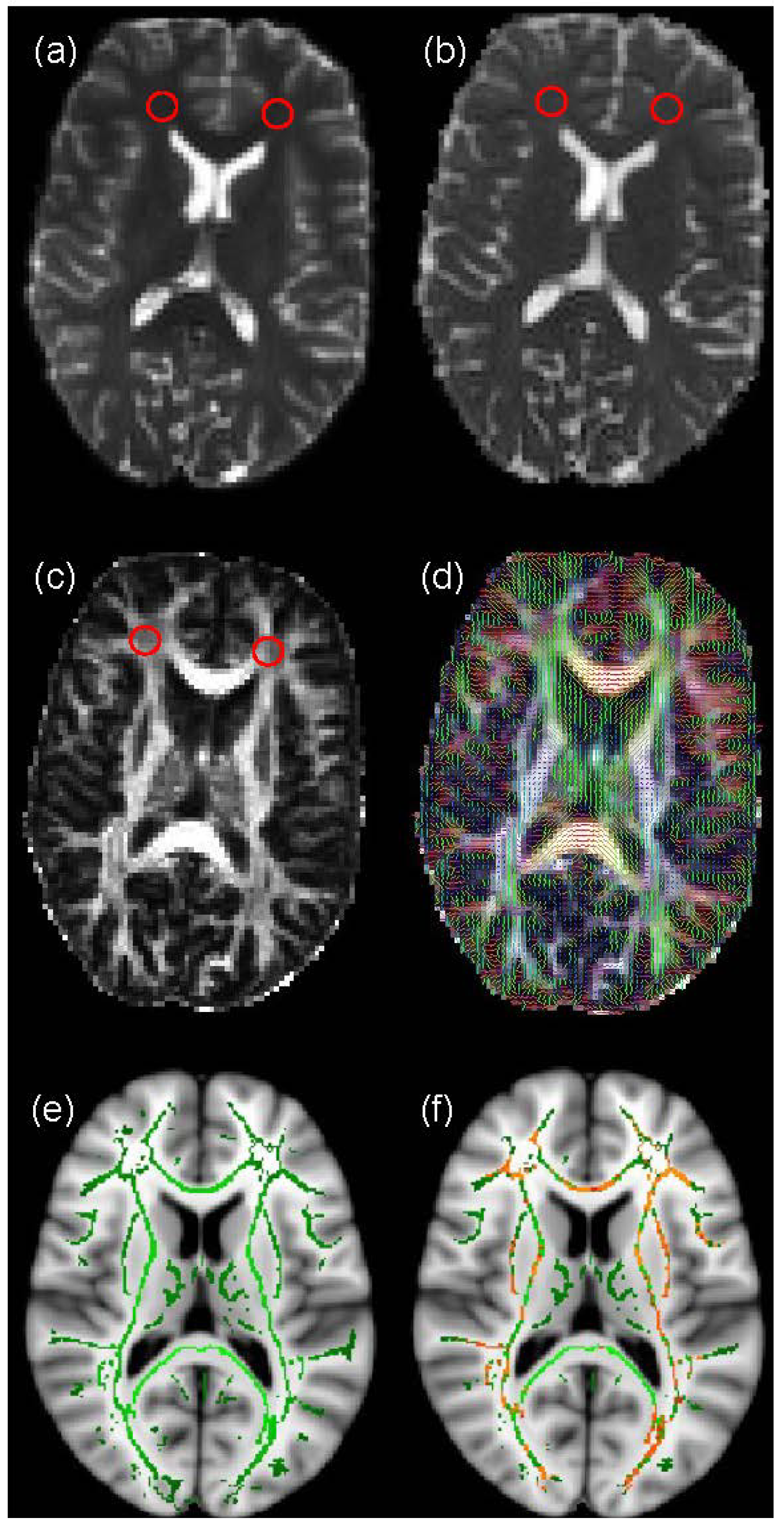
2.3. Voxel-Based Analysis
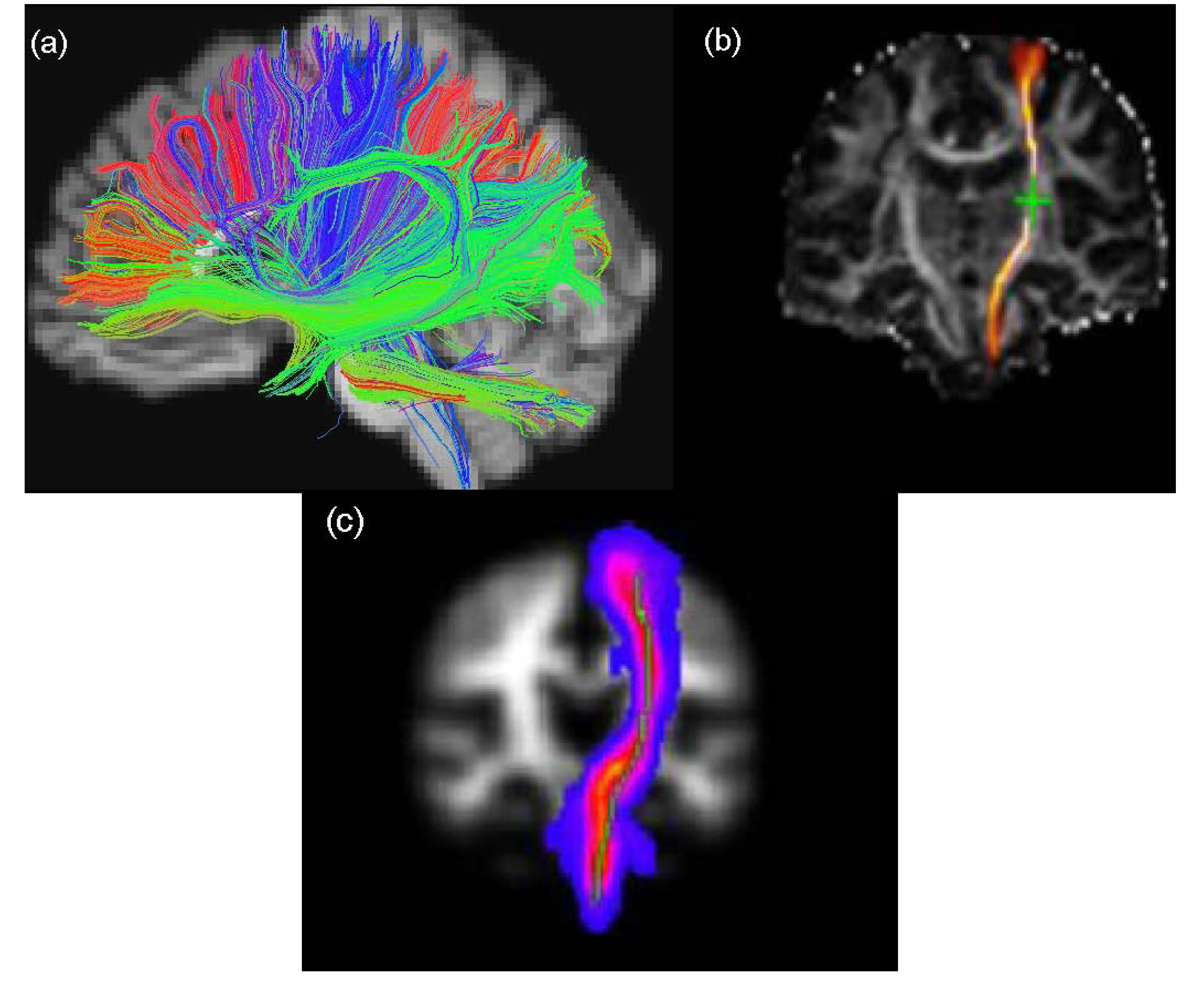
2.4. Tractography Techniques
2.4.1. Deterministic Tractography
2.4.2. Probabilistic Tractography
2.5. Applications to Musicians
3. Using DT-MRI to Investigate the Effects of Musical Training on White Matter Architecture
3.1. Cross-Sectional Studies
| Reference | No. of Participants | Analysis Method | Key Findings |
|---|---|---|---|
| Schmithorst & Wilke 2002 [34] | 5 Musicians 6 Non-Musicians | Voxel-Based | Significantly greater FA in the genu of the corpus callosum, but significantly lower FA in the corona radiata and internal capsule in musicians compared with non-musicians |
| Bengtsson et al., 2005 [36] | 8 Pianists 8 Non-Pianists | Voxel-Based | Significantly greater FA in the right posterior limb of the internal capsule in musicians compared with non-musicians. FA in several brain regions was positively correlated with mean total number of hours practice time in childhood, adolescence and adulthood. |
| Han et al., 2009 [37] | 18 Pianists 18 Non-Musicians | Voxel-Based | Significantly greater FA in the right posterior limb of the internal capsule in musicians compared with non-musicians. No significant correlation between either the age of training onset or total number of years training and FA. |
| Halwani et al., 2009 [38] | 11 Instrumentalists 11 Singers 11 non-musicians | ROI & Probabilistic Tractography | Tract volume of the arcuate fasciculus was greatest in singers, then instrumentalists and then non-musicians. FA in singers was significantly lower at the midpoint of the longitudinal portion of the left dorsal arcuate fasciculus compared with instrumentalists and non-musicians. |
| Imfeld et al., 2009 [39] | 13 Early Trained (ET) musicians 13 Late Trained (LT) Musicians 13 Non-Musicians | Deterministic Tractography, ROI & Voxel-Based | Significantly lower FA values in the CST of musicians compared with non-musicians. Significantly higher MD in both the left and right CST in ET musicians compared with LT musicians. No significant differences between absolute pitch (AP) musicians and non-AP musicians. No correlation between FA in the bilateral CST and age of training onset. MD in the CST was negatively correlated with age of training onset. |
| Oechslin et al., 2010 [40] | 13 AP Musicians 13 Non-AP Musicians 13 Non-Musicians | Deterministic Tractography & ROI | Correlation between AP ability and FA in the superior longitudinal fasciculus. AP demonstrated a greater-left-than-right asymmetry of FA in the superior longitudinal fasciculus. |
| Loui et al., 2011 [41] | 12 AP Musicians 12 non-AP Musicians | Deterministic Tractography & ROI | Higher volume and fibre number in tracts connecting the posterior superior temporal gyrus to the middle temporal gyrus in AP compared with non-AP musicians. Correlations between performance accuracy on a pitch-naming test, designed to test perfect pitch skills, and fibre volume connecting the left superior temporal gyrus and left middle temporal gyrus. |
| Abdul-Kareem et al., 2011 [42] | 10 Musicians 10 Non-Musicians | ROI & Deterministic Tractography | Significantly greater right middle cerebellar peduncle volume, right superior cerebellar peduncle volume and number of streamlines in right superior cerebellar peduncles in musicians compared with non-musicians. No correlation between age of training onset and WM volume differences or number of streamlines. |
| Dohn et al., 2013 [43] | 17 AP Musicians 18 Non-AP Musicians | TBSS | Significantly greater FA in a single WM cluster within the path of the inferior fronto-occipital fasciculus, uncinate fasciculus and the inferior longitudinal fasciculus in AP compared with non-AP musicians. AP ability associated with a rightward FA asymmetry. |
| Steele et al., 2013 [35] | 18 ET Musicians 18 LT Musicians 17 Non-Musicians | TBSS, ROI & Probabilistic Tractography | Significantly greater FA in the posterior midbody of the corpus callosum, and in the anterior portion of the isthmus in ET musicians compared with both LT musicians and non-musicians. Age of training onset correlated with FA in the posterior midbody of the corpus callosum. |
| Rüber et al., 2013 [44] | 10 Keyboard Players 10 String Players (Violin and Cello) 10 Non-musicians | Probabilistic Tractography Voxel-wise analysis within the tracts | Significantly greater FA in PT in right hemisphere of string players and keyboard players compared with non-musicians. Significantly greater FA in the PT in the left hemisphere of pianists. FA values in left and right PT and aMF significantly correlated with maximal tapping speed of the contralateral index finger. |
| Engel et al., 2014 [45] | 18 Non-Musicians | TBSS | FA values in the bilateral CST and right superior longitudinal fasciculus were correlated with learning speeds of piano melodies with the right hand. |
3.2. Experimental Musical Training Paradigms
3.3. DT-MRI and Motor Skills
4. Discussion
4.1. Effects of Participant Recruitment
4.2. Effects of Analysis Methods
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Schlaug, G. The brain of musicians. Ann. N. Y. Acad. Sci. 2001, 930, 281–299. [Google Scholar] [CrossRef]
- Münte, T.F.; Altenmüller, E.; Jäncke, L. The musician’s brain as a model of neuroplasticity. Nat. Rev. Neurosci. 2002, 3, 473–478. [Google Scholar]
- Bangert, M.; Schlaug, G. Specialization of the specialized in features of external human brain morphology. Eur. J. Neurosci. 2006, 24, 1832–1834. [Google Scholar] [CrossRef]
- Gaser, C.; Schlaug, G. Brain structures differ between musicians and non-musicians. J. Neurosci. 2003, 23, 9240–9245. [Google Scholar]
- Schneider, P.; Scherg, M.; Dosch, H.G.; Specht, H.J.; Gutschalk, A.; Rupp, A. Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nat. Neurosci. 2002, 5, 688–694. [Google Scholar] [CrossRef]
- Hutchinson, S.; Lee, L.H.L.; Gaab, N.; Schlaug, G. Cerebellar volume of musicians. Cereb. Cortex 2003, 13, 943–949. [Google Scholar] [CrossRef]
- Tervaniemi, M. Musicians—Same or different? Ann. N. Y. Acad. Sci. 2009, 1169, 151–156. [Google Scholar] [CrossRef]
- Jäncke, L. Music drives brain plasticity. F1000 Biol. Rep. 2009, 1, 78–84. [Google Scholar]
- Wan, C.Y.; Schlaug, G. Music making as a tool for promoting brain plasticity across the life span. Neuroscientist 2010, 16, 566–577. [Google Scholar] [CrossRef]
- Herholz, S.C.; Zatorre, R.J. Musical training as a framework for brain plasticity: Behavior, function, and structure. Neuron 2012, 76, 486–502. [Google Scholar] [CrossRef]
- Schlaug, G.; Jäncke, L.; Huang, Y.; Staiger, J.F.; Steinmetz, H. Increased corpus callosum size in musicians. Neuropsychologia 1995, 33, 1047–1055. [Google Scholar] [CrossRef]
- Penhune, V.B. Sensitive periods in human development: Evidence from musical training. Cortex 2011, 47, 1126–1137. [Google Scholar] [CrossRef]
- Schlaug, G.; Norton, A.; Overy, K.; Winner, E. Effects of music training on the child’s brain and cognitive development. Ann. N. Y. Acad. Sci. 2005, 1060, 219–230. [Google Scholar] [CrossRef]
- Schlaug, G.; Forgeard, M.; Zhu, L.; Norton, A.; Norton, A.; Winner, E. Training induced Neuroplasticity in Young Children. Ann. N. Y. Acad. Sci. 2009, 1169, 205–208. [Google Scholar] [CrossRef]
- Hyde, K.L.; Lerch, J.; Norton, A.; Forgeard, M.; Winner, E.; Evans, A.C.; Schlaug, G. Musical training shapes structural brain development. J. Neurosci. 2009, 29, 3019–3025. [Google Scholar] [CrossRef]
- Beaulieu, C.; Allen, P.S. Determinants of anisotropic water diffusion in nerves. Magn. Reson. Med. 1994, 31, 394–400. [Google Scholar] [CrossRef]
- Basser, P.J.; Mattiello, J.; Le Bihan, D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. B 1994, 103, 247–254. [Google Scholar] [CrossRef]
- Pierpaoli, C.; Jezzard, P.; Basser, P.J.; Barnett, A.; di Chiro, G. Diffusion tensor MR imaging of the human brain. Radiology 1996, 201, 637–648. [Google Scholar]
- Basser, P.J.; Pajevic, S.; Pierpaoli, C.; Duda, J.; Aldroubi, A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 2000, 44, 625–632. [Google Scholar] [CrossRef]
- Le Bihan, D.; Mangin, J.F.; Poupon, C.; Clark, C.A.; Pappata, S.; Molko, N.; Chabriat, H. Diffusion tensor imaging: Concepts and applications. J. Magn. Reson. Imaging 2001, 13, 534–546. [Google Scholar]
- Jones, D.K. Studying connections in the living human brain with diffusion MRI. Cortex 2008, 44, 936–952. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; Behrens, T.E. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Snook, L.; Plewes, C.; Beaulieu, C. Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. NeuroImage 2007, 34, 243–252. [Google Scholar] [CrossRef]
- FMRIB Software Library. Available online: http://www.fmrib.ox.ac.uk/fsl (accessed on 24 February 2014).
- Sprooten, E.; McIntosh, A.M.; Lawrie, S.M.; Hall, J.; Sussmann, J.E.; Dahmen, N.; Konrad, A.; Bastin, M.E.; Winterer, G. An investigation of a genomewide supported psychosis variant in ZNF804A and white matter integrity in the human brain. Magn. Reson. Imaging 2012, 30, 1373–1380. [Google Scholar] [CrossRef]
- Conturo, T.E.; Lori, N.F.; Cull, T.S.; Akbudak, E.; Snyder, A.Z.; Shimony, J.S.; McKinstry, R.C.; Burton, H.; Raichle, M.E. Tracking neuronal fiber pathways in the living human brain. Proc. Natl. Acad. Sci. USA 1999, 96, 10422–10427. [Google Scholar] [CrossRef]
- Clayden, J.D.; Storkey, A.J.; Bastin, M.E. A probabilistic model-based approach to consistent white matter tract segmentation. IEEE Trans. Med. Imaging 2007, 26, 1555–1561. [Google Scholar] [CrossRef]
- Behrens, T.E.; Berg, H.J.; Jbabdi, S.; Rushworth, M.F.; Woolrich, M.W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage 2007, 34, 144–155. [Google Scholar] [CrossRef]
- Lilja, Y.; Ljungberg, M.; Starck, G.; Malmgren, K.; Rydenhag, B.; Nilsson, D.T. Visualizing Meyer’s loop: A comparison of deterministic and probabilistic tractography. Epilepsy Res. 2014, 108, 481–490. [Google Scholar] [CrossRef]
- Buchanan, C.R.; Pernet, C.R.; Gorgolewski, K.J.; Storkey, A.J.; Bastin, M.E. Test-retest reliability of structural brain networks from diffusion MRI. NeuroImage 2014, 86, 231–243. [Google Scholar]
- TrackVis. Available online: http://trackvis.org (accessed on 20 February 2014).
- TractoR. Available online: http://www.tractor-mri.org.uk/ (access on 21 February 2014).
- Bastin, M.E.; Maniega, S.M.; Ferguson, K.J.; Brown, L.J.; Wardlaw, J.M.; MacLullich, A.M.; Clayden, J.D. Quantifying the effects of normal ageing on white matter structure using unsupervised tract shape modelling. NeuroImage 2010, 51, 1–10. [Google Scholar]
- Schmithorst, V.J.; Wilke, M. Differences in white matter architecture between musicians and non-musicians: A diffusion tensor imaging study. Neurosci. Lett. 2002, 321, 57–60. [Google Scholar] [CrossRef]
- Steele, C.J.; Bailey, J.A.; Zatorre, R.J.; Penhune, V.B. Early musical training and white-matter plasticity in the corpus callosum: Evidence for a sensitive period. J. Neurosci. 2013, 33, 1282–1290. [Google Scholar] [CrossRef]
- Bengtsson, S.L.; Nagy, Z.; Skare, S.; Forsman, L.; Forssberg, H.; Ullén, F. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 2005, 8, 1148–1150. [Google Scholar] [CrossRef]
- Han, Y.; Yang, H.; Lv, Y.T.; Zhu, C.Z.; He, Y.; Tang, H.H.; Gong, Q-Y.; Luo, Y.J.; Zang, Y-F.; Dong, Q. Gray matter density and white matter integrity in pianists’ brain: A combined structural and diffusion tensor MRI study. Neurosci. Lett. 2009, 459, 3–6. [Google Scholar] [CrossRef]
- Halwani, G.F.; Loui, P.; Rüber, T.; Schlaug, G. Effects of practice and experience on the arcuate fasciculus: Comparing singers, instrumentalists, and non-musicians. Front. Psychol. 2011, 2. [Google Scholar] [CrossRef]
- Imfeld, A.; Oechslin, M.S.; Meyer, M.; Loenneker, T.; Jancke, L. White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. NeuroImage 2009, 46, 600–607. [Google Scholar] [CrossRef]
- Oechslin, M.S.; Imfeld, A.; Loenneker, T.; Meyer, M.; Jäncke, L. The plasticity of the superior longitudinal fasciculus as a function of musical expertise: A diffusion tensor imaging study. Front. Hum. Neurosci. 2010, 3. [Google Scholar] [CrossRef]
- Loui, P.; Li, H.C.; Hohmann, A.; Schlaug, G. Enhanced cortical connectivity in absolute pitch musicians: A model for local hyperconnectivity. J. Cogn. Neurosci. 2011, 23, 1015–1026. [Google Scholar] [CrossRef]
- Abdul-Kareem, I.A.; Stancak, A.; Parkes, L.M.; Al-Ameen, M.; AlGhamdi, J.; Aldhafeeri, F.M.; Embleton, K.; Morris, D.; Sluming, V. Plasticity of the superior and middle cerebellar peduncles in musicians revealed by quantitative analysis of volume and number of streamlines based on diffusion tensor tractography. Cerebellum 2011, 10, 611–623. [Google Scholar] [CrossRef]
- Dohn, A.; Garza-Villarreal, E.A.; Chakravarty, M.M.; Hansen, M.; Lerch, J.P.; Vuust, P. Gray-and White-Matter Anatomy of Absolute Pitch Possessors. Cereb. Cortex 2013. [Google Scholar] [CrossRef]
- Rüber, T.; Lindenberg, R.; Schlaug, G. Differential adaptation of descending motor tracts in musicians. Cereb. Cortex 2013. [Google Scholar] [CrossRef]
- Engel, A.; Hijmans, B.S.; Cerliani, L.; Bangert, M.; Nanetti, L.; Keller, P.E.; Keysers, C. Inter-individual differences in audio-motor learning of piano melodies and white matter fiber tract architecture. Hum. Brain Mapp. 2014, 35, 2483–2497. [Google Scholar] [CrossRef]
- Engvig, A.; Fjell, A.M.; Westlye, L.T.; Moberget, T.; Sundseth, Ø.; Larsen, V.A.; Walhovd, K.B. Memory training impacts short-term changes in aging white matter: A longitudinal diffusion tensor imaging study. Hum. Brain Mapp. 2012, 33, 2390–2406. [Google Scholar] [CrossRef]
- Mackey, A.P.; Whitaker, K.J.; Bunge, S.A. Experience-dependent plasticity in white matter microstructure: Reasoning training alters structural connectivity. Front. Neuroanat. 2012, 6. [Google Scholar] [CrossRef]
- Thomas, C.; Baker, C.I. Teaching an adult brain new tricks: A critical review of evidence for training-dependent structural plasticity in humans. NeuroImage 2013, 73, 225–236. [Google Scholar] [CrossRef]
- Chang, Y. Reorganization and plastic changes of the human brain associated with skill learning and expertise. Front. Hum. Neurosci. 2014, 8. [Google Scholar]
- Hänggi, J.; Koeneke, S.; Bezzola, L.; Jäncke, L. Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Hum. Brain Mapp. 2010, 31, 1196–1206. [Google Scholar]
- Wang, B.; Fan, Y.; Lu, M.; Li, S.; Song, Z.; Peng, X.; Zhang, R.; Lin, Q.; He, Y.; Wang, J.; Huang, R. Brain anatomical networks in world class gymnasts: A DTI tractography study. NeuroImage 2013, 65, 476–487. [Google Scholar] [CrossRef]
- Scholz, J.; Klein, M.C.; Behrens, T.E.; Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009, 12, 1370–1371. [Google Scholar] [CrossRef]
- Wang, X.; Casadio, M.; Weber, K.A., II.; Mussa-Ivaldi, F.A.; Parrish, T.B. White matter microstructure changes induced by motor skill learning utilizing a body machine interface. NeuroImage 2014, 88, 32–40. [Google Scholar]
- Taubert, M.; Draganski, B.; Anwander, A.; Müller, K.; Horstmann, A.; Villringer, A.; Ragert, P. Dynamic properties of human brain structure: Learning-related changes in cortical areas and associated fiber connections. J. Neurosci. 2010, 30, 11670–11677. [Google Scholar] [CrossRef]
- Sagi, Y.; Tavor, I.; Hofstetter, S.; Tzur-Moryosef, S.; Blumenfeld-Katzir, T.; Assaf, Y. Learning in the fast lane: New insights into neuroplasticity. Neuron 2012, 73, 1195–1203. [Google Scholar] [CrossRef]
- Landi, S.M.; Baguear, F.; Della-Maggiore, V. One Week of Motor Adaptation Induces Structural Changes in Primary Motor Cortex That Predict Long-Term Memory One Year Later. J. Neurosci. 2011, 31, 11808–11813. [Google Scholar] [CrossRef]
- Palmer, H.S.; Håberg, A.K.; Fimland, M.S.; Solstad, G.M.; Iversen, V.M.; Hoff, J.; Helgerud, J.; Eikenes, L. Structural brain changes after 4 wk of unilateral strength training of the lower limb. J. Appl. Physiol. 2013, 115, 167–175. [Google Scholar] [CrossRef]
- Bailey, J.A.; Penhune, V.B. The relationship between the age of onset of musical training and rhythm synchronization performance: Validation of sensitive period effects. Front. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Watanabe, D.; Savion-Lemieux, T.; Penhune, V.B. The effect of early musical training on adult motor performance: Evidence for a sensitive period in motor learning. Exp. Brain Res. 2007, 176, 332–340. [Google Scholar] [CrossRef]
- Bailey, J.; Penhune, V.B. A sensitive period for musical training: Contributions of age of onset and cognitive abilities. Ann. N. Y. Acad. Sci. 2012, 1252, 163–170. [Google Scholar] [CrossRef]
- Bailey, J.; Zatorre, R.J.; Penhune, V.B. Early musical training is linked to gray matter structure in the ventral premotor cortex and auditory-motor rhythmic synchronisation performance. J. Cogn. Neurosci. 2014, 26, 755–767. [Google Scholar] [CrossRef]
- Draganski, B.; Gaser, C.; Busch, V.; Schuierer, G.; Bogdahn, U.; May, A. Changes in grey matter induced by training. Nature 2004, 427, 311–312. [Google Scholar] [CrossRef]
- James, C.E.; Oechslin, M.S.; van de Ville, D.; Hauert, C.A.; Descloux, C.; Lazeyras, F. Musical training intensity yields opposite effects on grey matter density in cognitive versus sensorimotor networks. Brain Struct. Funct. 2014, 219, 353–366. [Google Scholar] [CrossRef]
- Kinney, D.W. Selected nonmusic predictors of urban students’ decisions to enroll and persist in middle school band programs. J. Res. Music Educ. 2010, 57, 334–350. [Google Scholar] [CrossRef]
- Jones, D.K.; Cercignani, M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010, 23, 803–820. [Google Scholar] [CrossRef]
- Jones, D.K.; Williams, S.C.; Gasston, D.; Horsfield, M.A.; Simmons, A.; Howard, R. Isotropic resolution diffusion tensor imaging with whole brain acquisition in a clinically acceptable time. Hum. Brain Mapp. 2002, 15, 216–230. [Google Scholar] [CrossRef]
- Wedeen, V.J.; Hagmann, P.; Tseng, W.Y.; Reese, T.G.; Weisskoff, R.M. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn. Reson. Med. 2005, 54, 1377–1386. [Google Scholar] [CrossRef]
- Wedeen, V.J.; Wang, R.P.; Schmahmann, J.D.; Benner, T.; Tseng, W.Y.I.; Dai, G.; Pandya, D.N.; Hagmann, P.; D’Arceuil, H.; de Crespigny, A.J. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. NeuroImage 2008, 41, 1267–1277. [Google Scholar] [CrossRef]
- Kuo, L.W.; Chen, J.H.; Wedeen, V.J.; Tseng, W.Y. Optimization of diffusion spectrum imaging and q-ball imaging on clinical MRI system. NeuroImage 2008, 41, 7–18. [Google Scholar] [CrossRef]
- De Santis, S.; Drakesmith, M.; Bells, S.; Assaf, Y.; Jones, D.K. Why diffusion tensor MRI does well only some of the time: Variance and covariance of white matter tissue microstructure attributes in the living human brain. NeuroImage 2014, 89, 35–44. [Google Scholar] [CrossRef]
- Sporns, O. The human connectome: A complex network. Ann. N. Y. Acad. Sci. 2011, 1224, 109–125. [Google Scholar] [CrossRef]
- Jäncke, L.; Langer, N.; Hänggi, J. Diminished whole-brain but enhanced peri-sylvian connectivity in absolute pitch musicians. J. Cogn. Neurosci. 2012, 24, 1447–1461. [Google Scholar] [CrossRef]
- Dauguet, J.; Peled, S.; Berezovskii, V.; Delzescaux, T.; Warfield, S.K.; Born, R.; Westin, C.F. Comparison of fiber tracts derived from in vivo DTI tractography with 3D histological neural tract tracer reconstruction on a macaque brain. NeuroImage 2007, 37, 530–538. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Moore, E.; Schaefer, R.S.; Bastin, M.E.; Roberts, N.; Overy, K. Can Musical Training Influence Brain Connectivity? Evidence from Diffusion Tensor MRI. Brain Sci. 2014, 4, 405-427. https://doi.org/10.3390/brainsci4020405
Moore E, Schaefer RS, Bastin ME, Roberts N, Overy K. Can Musical Training Influence Brain Connectivity? Evidence from Diffusion Tensor MRI. Brain Sciences. 2014; 4(2):405-427. https://doi.org/10.3390/brainsci4020405
Chicago/Turabian StyleMoore, Emma, Rebecca S. Schaefer, Mark E. Bastin, Neil Roberts, and Katie Overy. 2014. "Can Musical Training Influence Brain Connectivity? Evidence from Diffusion Tensor MRI" Brain Sciences 4, no. 2: 405-427. https://doi.org/10.3390/brainsci4020405
APA StyleMoore, E., Schaefer, R. S., Bastin, M. E., Roberts, N., & Overy, K. (2014). Can Musical Training Influence Brain Connectivity? Evidence from Diffusion Tensor MRI. Brain Sciences, 4(2), 405-427. https://doi.org/10.3390/brainsci4020405




