Altering Temporal Dynamics of Sleepiness and Mood During Sleep Deprivation: Evidence from Resting-State EEG Microstates
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
2.3. Questionnaires and Behavior Measurement
- Sleep diary: A sleep diary is a tool for self-evaluation of sleep [35]. Sleep diaries are simple to use and only require a few minutes a day to complete. It contains items such as “Time I went to bed last night” and “How alert did I feel when I got up this morning?”
- Subjective sleepiness: Subjective sleepiness was assessed using the KSS [36]. Participants chose one of nine descriptions to describe their current level of subjective sleepiness, ranging from s the first statement, “Extremely alert,” to the ninth statement, “Extremely sleepy, cannot keep awake.”
- Mood: Mood was evaluated using the PANAS, which includes 20 items describing emotional states, such as hostility. Each item is rated from 1 (nearly no) to 5 (extremely a lot) and grouped into two dimensions: negative affect (PANAS-NA) and positive affect (PANAS-PA).
- Objective sleepiness: The PVT was used to measure objective sleepiness [8]. The screen was centered on a square frame. Participants had to click on a mouse when the red number appeared in the box. The numbers reappeared after a random time. The PVT lasted for 5 min, with median RT and lapses (SC, RT > 500 ms; SD, RT > 700 ms) used to objectively measure vigilance.
2.4. EEG Data Acquisition and Data Preprocessing
2.5. Microstate Analysis
2.6. Statistical Analysis
3. Results
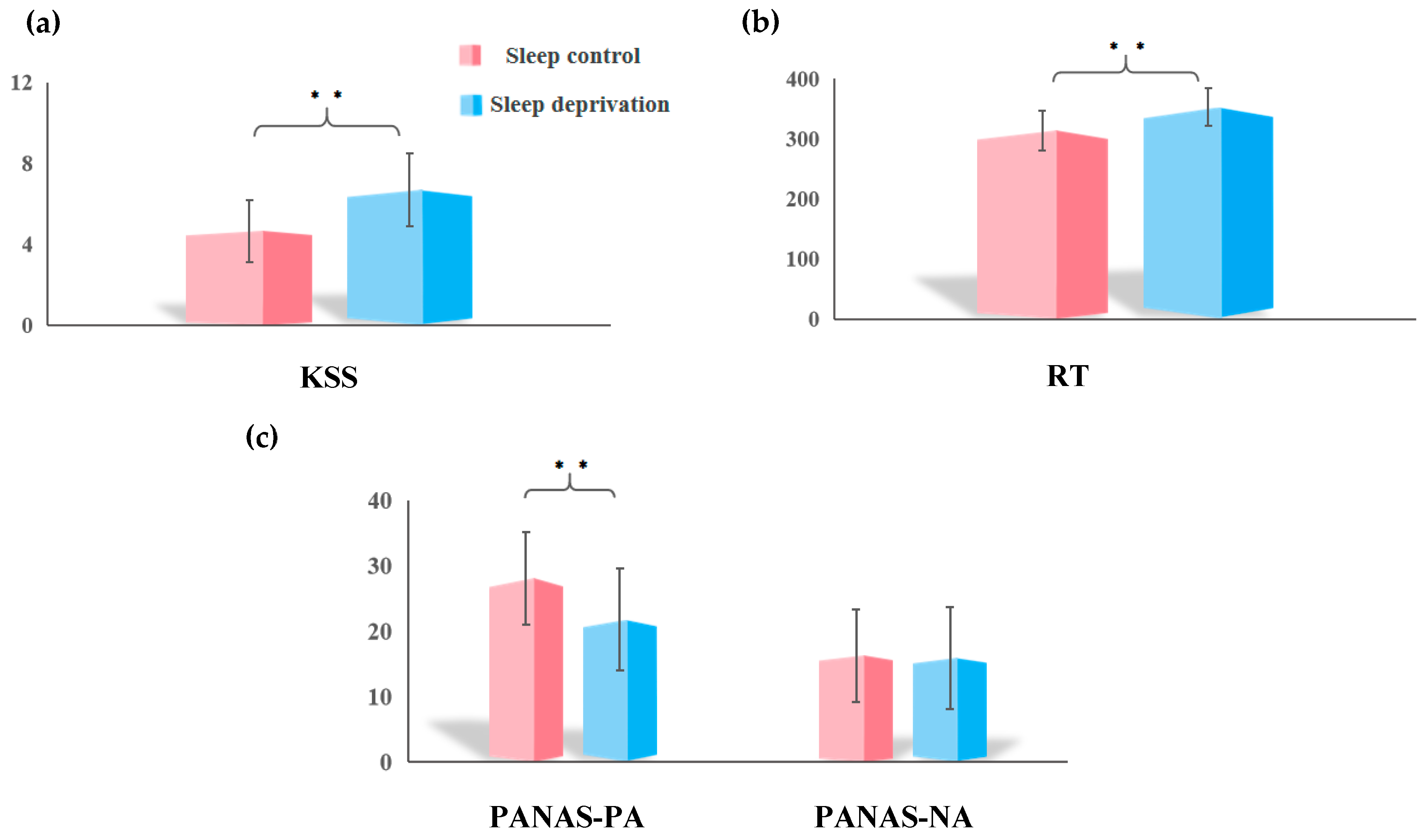
3.1. Sleep Deprivation on Subjective Sleepiness, Objective Vigilance, and Mood
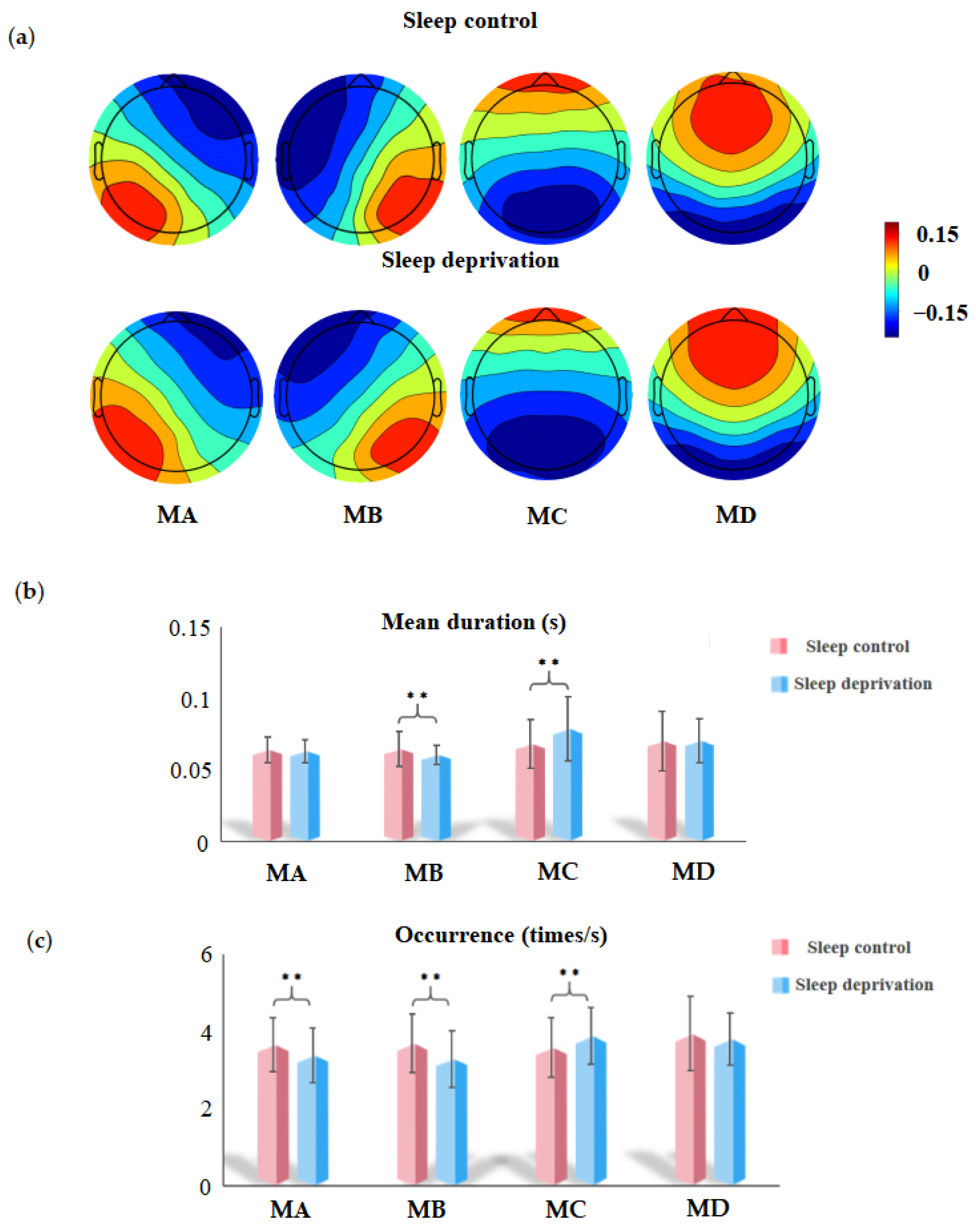
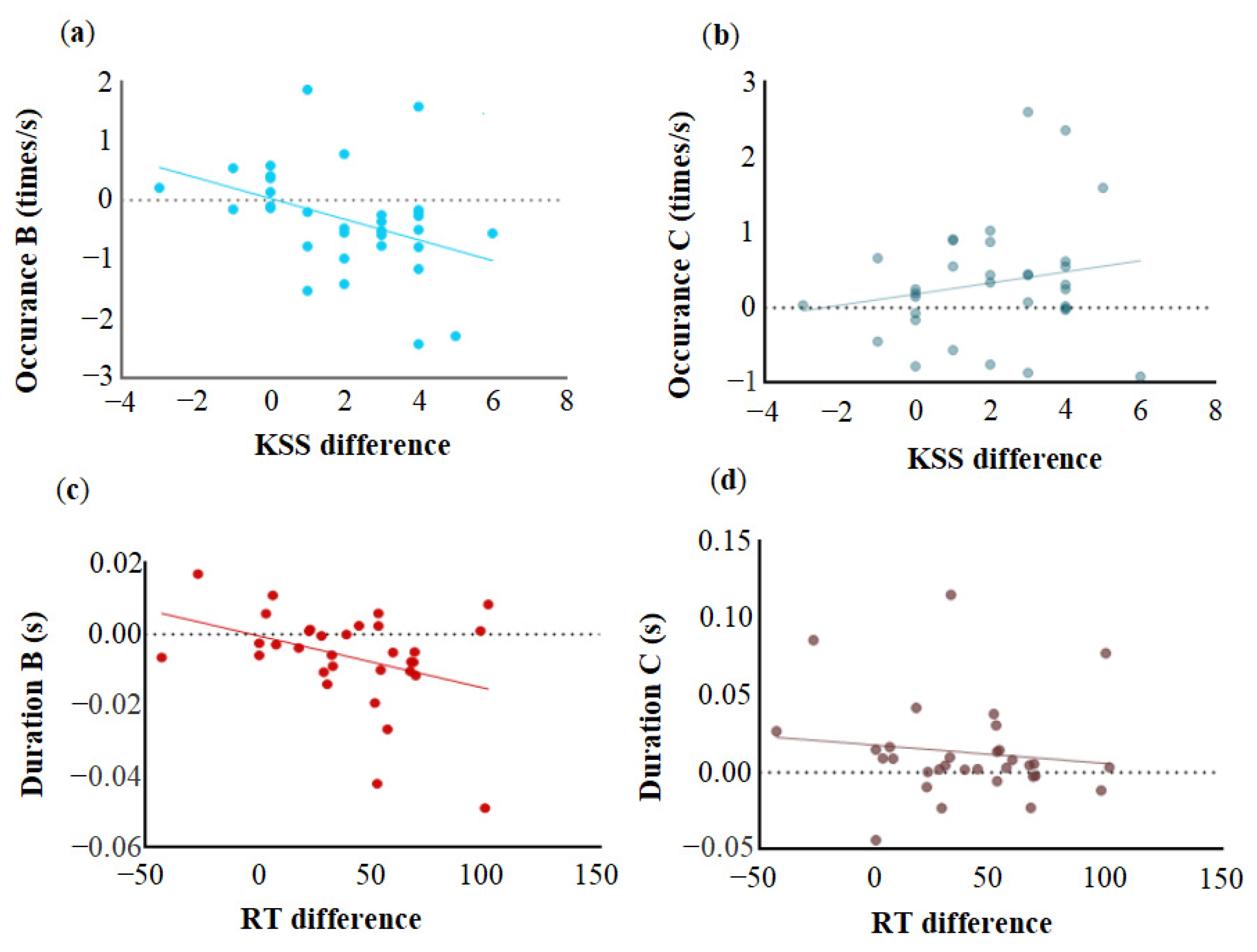
3.2. EEG Microstate Analysis
4. Discussion
4.1. Sleep Deprivation Altered Microstate B
4.2. Sleep Deprivation Decreases Positive Mood
4.3. Sleep Deprivation Altered Microstate C
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irwin, M.R. Why sleep is important for health: A psychoneuroimmunology perspective. Annu. Rev. Psychol. 2015, 66, 143–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abrams, R.M. Sleep Deprivation. Obstet. Gynecol. Clin. 2015, 42, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, J.J.; Huffcutt, A.I. Effects of sleep deprivation on performance: A meta-analysis. Sleep 1996, 19, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Durmer, J.S.; Dinges, D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005, 25, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Herscovitch, J.; Broughton, R. Sensitivity of the stanford sleepiness scale to the effects of cumulative partial sleep deprivation and recovery oversleeping. Sleep 1981, 4, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kaida, K.; Takahashi, M.; Akerstedt, T.; Nakata, A.; Otsuka, Y.; Haratani, T.; Fukasawa, K. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin. Neurophysiol. 2006, 117, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.J.; Safati, A.; Hall, P.A. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neurosci. Biobehav. Rev. 2017, 80, 586–604. [Google Scholar] [CrossRef] [PubMed]
- Balkin, T.J. Behavioral biomarkers of sleepiness. J. Clin. Sleep Med. 2011, 7, S12–S15. [Google Scholar] [CrossRef]
- Killgore, W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Roach, G.D.; Dawson, D.; Lamond, N. Can a shorter psychomotor vigilance task be used as a reasonable substitute for the ten-minute psychomotor vigilance task? Chronobiol. Int. 2006, 23, 1379–1387. [Google Scholar] [CrossRef]
- Manousakis, J.E.; Mann, N.; Jeppe, K.J.; Anderson, C. Awareness of sleepiness: Temporal dynamics of subjective and objective sleepiness. Psychophysiology 2021, 58, e13839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Duan, W.; Lei, X. Impaired Coupling of the Brain’s Default Network During Sleep Deprivation: A Resting-State EEG Study. Nat. Sci. Sleep 2020, 12, 937–947. [Google Scholar] [CrossRef]
- Gorgoni, M.; Ferlazzo, F.; Ferrara, M.; Moroni, F.; D’Atri, A.; Fanelli, S.; Gizzi Torriglia, I.; Lauri, G.; Marzano, C.; Rossini, P.M.; et al. Topographic electroencephalogram changes associated with psychomotor vigilance task performance after sleep deprivation. Sleep Med. 2014, 15, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xie, C.; Lei, X. Isolation of subjectively reported sleepiness and objectively measured vigilance during sleep deprivation: A resting-state fMRI study. Cogn. Neurodyn. 2022, 16, 1151–1162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomaso, C.C.; Johnson, A.B.; Nelson, T.D. The effect of sleep deprivation and restriction on mood, emotion, and emotion regulation: Three meta-analyses in one. Sleep 2021, 44, zsaa289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ben Simon, E.; Maron-Katz, A.; Lahav, N.; Shamir, R.; Hendler, T. Tired and misconnected: A breakdown of brain modularity following sleep deprivation. Hum. Brain Mapp. 2017, 38, 3300–3314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khanna, A.; Pascual-Leone, A.; Michel, C.M.; Farzan, F. Microstates in resting-state EEG: Current status and future directions. Neurosci. Biobehav. Rev. 2015, 49, 105–113. [Google Scholar] [CrossRef]
- Chivu, A.; Pascal, S.A.; Damborská, A.; Tomescu, M.I. EEG Microstates in Mood and Anxiety Disorders: A Meta-analysis. Brain Topogr. 2024, 37, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Tarailis, P.; Koenig, T.; Michel, C.M.; Griškova-Bulanova, I. The Functional Aspects of Resting EEG Microstates: A Systematic Review. Brain Topogr. 2024, 37, 181–217. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Koenig, T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. NeuroImage 2018, 180, 577–593. [Google Scholar] [CrossRef]
- Britz, J.; Van De Ville, D.; Michel, C.M. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. NeuroImage 2010, 52, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Ma, M.; Meng, F.; Liang, H.; Liang, C.; Liu, X.; Zhang, B.; Ju, Y.; Liu, S.; Ming, D. Diminished attention network activity and heightened salience-default mode transitions in generalized anxiety disorder: Evidence from resting-state EEG microstate analysis. J. Affect. Disord. 2025, 373, 227–236. [Google Scholar] [CrossRef]
- Clawson, B.C.; Durkin, J.; Suresh, A.K.; Pickup, E.J.; Broussard, C.G.; Aton, S.J. Sleep Promotes, and Sleep Loss Inhibits, Selective Changes in Firing Rate, Response Properties and Functional Connectivity of Primary Visual Cortex Neurons. Front. Syst. Neurosci. 2018, 12, 40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Guo, S.; Wang, C.; Wang, B.; Sun, H.; Zhang, X. Increased interhemispheric resting-state functional connectivity in healthy participants with insomnia symptoms: A randomized clinical consort study. Medicine 2017, 96, e7037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, R.; He, Z.; Chang, M.; Wang, F.; Wei, W.; Zhang, X.; Zhu, Y.; Xi, Y.; Yang, X.; et al. Abnormal dynamic functional connectivity after sleep deprivation from temporal variability perspective. Hum. Brain Mapp. 2022, 43, 3824–3839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marques, D.R.; Gomes, A.A.; Caetano, G.; Castelo-Branco, M. Insomnia Disorder and Brain’s Default-Mode Network. Curr. Neurol. Neurosci. Rep. 2018, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Zanesco, A.P.; King, B.G.; Skwara, A.C.; Saron, C.D. Within and between-person correlates of the temporal dynamics of resting EEG microstates. Neuroimage 2020, 211, 116631. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Li, J.; Wang, L. Alteration in Resting-State EEG Microstates Following 24 Hours of Total Sleep Deprivation in Healthy Young Male Subjects. Front. Hum. Neurosci. 2021, 15, 636252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- An, X.; Lian, J.; Xu, L.; Peng, Z.; Chen, S.; Cheng, M.Y.; Shao, Y. Changes in electroencephalography microstates are associated with reduced levels of vigilance after sleep deprivation. Brain Res. 2024, 1825, 148729. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; Shen, X.; Lv, Z.; Song, S.; Zhang, D. The EEG microstate representation of discrete emotions. Int. J. Psychophysiol. 2023, 186, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Fan, X.; Bai, D.; Lv, K.; Lei, X. A Resting-state EEG Dataset for Sleep Deprivation. OpenNeuro 2025. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Fan, X.; Bai, D.; Lv, K.; Lei, X. A resting-state EEG dataset for sleep deprivation. Sci. Data 2024, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- van Hees, V.T.; Sabia, S.; Anderson, K.N.; Denton, S.J.; Oliver, J.; Catt, M.; Abell, J.G.; Kivimäki, M.; Trenell, M.I.; Singh-Manoux, A. A Novel, Open Access Method to Assess Sleep Duration Using a Wrist-Worn Accelerometer. PLoS ONE 2015, 10, e0142533. [Google Scholar] [CrossRef]
- Akerstedt, T.; Gillberg, M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef]
- Zhang, S.; Lyu, H. EEG Microstate Associated with Trait Nostalgia. Brain Topogr. 2024, 37, 826–833. [Google Scholar] [CrossRef]
- Seitzman, B.A.; Abell, M.; Bartley, S.C.; Erickson, M.A.; Bolbecker, A.R.; Hetrick, W.P. Cognitive manipulation of brain electric microstates. NeuroImage 2017, 146, 533–543. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Nagabhushan Kalburgi, S.; Kleinert, T.; Aryan, D.; Nash, K.; Schiller, B.; Koenig, T. MICROSTATELAB: The EEGLAB Toolbox for Resting-State Microstate Analysis. Brain Topogr. 2024, 37, 621–645. [Google Scholar] [CrossRef] [PubMed]
- Custo, A.; Van De Ville, D.; Wells, W.M.; Tomescu, M.I.; Brunet, D.; Michel, C.M. Electroencephalographic resting-state networks: Source localization of microstates. Brain Connect. 2017, 7, 671–682. [Google Scholar] [CrossRef]
- Koenig, T.; Prichep, L.; Lehmann, D.; Sosa, P.V.; Braeker, E.; Kleinlogel, H.; Isenhart, R.; John, E.R. Millisecond by millisecond, year by year: Normative EEG microstates and developmental stages. NeuroImage 2002, 16, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, T.; Ma, N. Resting-State EEG Microstates Dynamics Associated with Interindividual Vulnerability to Sleep Deprivation. Nat. Sci. Sleep 2024, 16, 1937–1948. [Google Scholar] [CrossRef]
- Yeo, B.T.; Tandi, J.; Chee, M.W. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. NeuroImage 2015, 111, 147–158. [Google Scholar] [CrossRef]
- Kaufmann, T.; Elvsåshagen, T.; Alnæs, D.; Zak, N.; Pedersen, P.Ø.; Norbom, L.B.; Quraishi, S.H.; Tagliazucchi, E.; Laufs, H.; Bjørnerud, A.; et al. The brain functional connectome is robustly altered by lack of sleep. NeuroImage 2016, 127, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Mai, Z.; Li, M.; Zhou, X.; Ma, N. Altered frontal connectivity after sleep deprivation predicts sustained attentional impairment: A resting-state functional magnetic resonance imaging study. J. Sleep Res. 2021, 30, e13329. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, J.; Liu, B.X.; Dai, X.J. Altered connection properties of important network hubs may be neural risk factors for individuals with primary insomnia. Sci. Rep. 2018, 8, 5891. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Monti, J.M.; Burman, D.; Karthikeyan, R.; BaHammam, A.S.; Spence, D.W.; Brown, G.M.; Narashimhan, M. Clarifying the role of sleep in depression: A narrative review. Psychiatry Res. 2020, 291, 113239. [Google Scholar] [CrossRef] [PubMed]
- Bosch, O.G.; Rihm, J.S.; Scheidegger, M.; Landolt, H.P.; Stämpfli, P.; Brakowski, J.; Esposito, F.; Rasch, B.; Seifritz, E. Sleep deprivation increases dorsal nexus connectivity to the dorsolateral prefrontal cortex in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 19597–19602. [Google Scholar] [CrossRef]
- Lei, L.; Liu, Z.; Zhang, Y.; Guo, M.; Liu, P.; Hu, X.; Yang, C.; Zhang, A.; Sun, N.; Wang, Y.; et al. EEG microstates as markers of major depressive disorder and predictors of response to SSRIs therapy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 116, 110514. [Google Scholar] [CrossRef]
- Yan, D.; Liu, J.; Liao, M.; Liu, B.; Wu, S.; Li, X.; Li, H.; Ou, W.; Zhang, L.; Li, Z.; et al. Prediction of clinical outcomes with EEG microstate in patients with Major Depressive Disorder. Front. Psychiatry 2021, 12, 695272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ng, S.-C.; Khoo, S.; Chi, A. Temporal and spatial Dynamics of EEG features in female College students with subclinical depression. Int. J. Environ. Res. Public Health 2022, 19, 1778. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, T.; Nash, K.; Koenig, T.; Wascher, E. Normative Intercorrelations Between EEG Microstate Characteristics. Brain Topogr. 2024, 37, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Tramonti Fantozzi, M.P.; Banfi, T.; Di Galante, M.; Ciuti, G.; Faraguna, U. Sleep Deprivation-Induced Changes in Baseline Brain Activity and Vigilant Attention Performance. Brain Sci. 2022, 12, 1690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DiFrancesco, M.W.; Van Dyk, T.; Altaye, M.; Drummond, S.P.A.; Beebe, D.W. Network-based Responses to the Psychomotor Vigilance Task during Lapses in Adolescents after Short and Extended Sleep. Sci. Rep. 2019, 9, 13913. [Google Scholar] [CrossRef]
- Fu, W.; Dai, C.; Chen, J.; Wang, L.; Song, T.; Peng, Z.; Xu, M.; Xu, L.; Tang, Y.; Shao, Y. Altered insular functional connectivity correlates to impaired vigilant attention after sleep deprivation: A resting-state functional magnetic resonance imaging study. Front. Neurosci. 2022, 16, 889009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Havas, J.A.; Parimal, S.; Soon, C.S.; Chee, M.W. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage 2012, 59, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.L. Levels of analysis and the organization of affect. Rev. Gen. Psychol. 1998, 2, 247–270. [Google Scholar] [CrossRef]
- Ma, N.; Dinges, D.F.; Basner, M.; Rao, H. How acute total sleep loss affects the attending brain: A meta-analysis of neuroimaging studies. Sleep 2015, 38, 233–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, X.J.; Gong, H.H.; Wang, Y.X.; Zhou, F.Q.; Min, Y.J.; Zhao, F.; Wang, S.Y.; Liu, B.X.; Xiao, X.Z. Gender differences in brain regional homogeneity of healthy subjects after normal sleep and after sleep deprivation: A resting-state fMRI study. Sleep Med. 2012, 13, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, B.Z.; Zhang, Y.; Pan, B.; Gao, Y.H.; Zhan, H.; Liu, Y.; Shao, Y.C.; Zhang, X. Altered insula-prefrontal functional connectivity correlates to decreased vigilant attention after total sleep deprivation. Sleep Med. 2021, 84, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zheng, Y.; Zhan, Q.; Dong, J.; Peng, H.; Zhai, J.; Zhao, J.; She, S.; Wu, C. Covariation between spontaneous neural activity in the insula and affective temperaments is related to sleep disturbance in individuals with major depressive disorder. Psychol. Med. 2021, 51, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Berntson, G.G.; Norman, G.J.; Bechara, A.; Bruss, J.; Tranel, D.; Cacioppo, J.T. The insula and evaluative processes. Psychol. Sci. 2011, 22, 80–86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alfini, A.J.; Won, J.; Weiss, L.R.; Nyhuis, C.C.; Shackman, A.J.; Spira, A.P.; Smith, J.C. Impact of exercise on older adults’ mood is moderated by sleep and mediated by altered brain connectivity. Soc. Cogn. Affect. Neurosci. 2020, 15, 1238–1251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ben Simon, E.; Rossi, A.; Harvey, A.G.; Walker, M.P. Overanxious and underslept. Nat. Hum. Behav. 2020, 4, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Lü, W.; Hughes, B.M.; Howard, S.; James, J.E. Sleep restriction undermines cardiovascular adaptation during stress, contingent on emotional stability. Biol. Psychol. 2018, 132, 125–132. [Google Scholar] [CrossRef]
- Zhao, Z.; Ran, X.; Wang, J.; Lv, S.; Qiu, M.; Niu, Y.; Wang, C.; Xu, Y.; Gao, Z.; Ren, W.; et al. Common and differential EEG microstate of major depressive disorder patients with and without response to rTMS treatment. J. Affect. Disord. 2024, 367, 777–787. [Google Scholar] [CrossRef]
- Remmers, C.; Topolinski, S.; Dietrich, D.E.; Michalak, J. Impaired intuition in patients with major depressive disorder. Br. J. Clin. Psychol. 2015, 54, 200–213. [Google Scholar] [CrossRef]
- Bagley, S.L.; Weaver, T.L.; Buchanan, T.W. Sex differences in physiological and affective responses to stress in remitted depression. Physiol. Behav. 2011, 104, 180–186. [Google Scholar] [CrossRef]
- Watkins-Martin, K.; Bolanis, D.; Richard-Devantoy, S.; Pennestri, M.H.; Malboeuf-Hurtubise, C.; Philippe, F.; Guindon, J.; Gouin, J.P.; Ouellet-Morin, I.; Geoffroy, M.C. The effects of walking in nature on negative and positive affect in adult psychiatric outpatients with major depressive disorder: A randomized-controlled study. J. Affect. Disord. 2022, 318, 291–298. [Google Scholar] [CrossRef]
- He, C.; Xiao, L.; Xu, J.; Cui, Y.; Huang, Y.; Li, Y.; Tang, Y.; Xu, S.; Wang, H.; Cai, Y.; et al. Effect of sleep deprivation plus existing therapies on depression: A systematic review and meta-analysis of randomized controlled trials. Int. J. Psychophysiol. 2023, 184, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.Y.; Lai, W.H.; On, S.H.; On, Y.Y.; Adam, B.M.; Law, W.C.; Ng, B.H.S.; Fong, A.Y.Y.; Anselm, S.T. Resting-state electroencephalography (EEG) microstates of healthy individuals following mild sleep deprivation. Sci. Rep. 2024, 14, 16820. [Google Scholar] [CrossRef] [PubMed]
- Croce, P.; Zappasodi, F.; Capotosto, P. Offline stimulation of human parietal cortex differently affects resting EEG microstates. Sci. Rep. 2018, 8, 1287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| NSC (NSD) | SC | SD | t | p | |
|---|---|---|---|---|---|
| Age (year) | 71 | 20.00 ± 1.44 | - | - | |
| Sex (female%) | 71 | 47.89% | - | - | |
| PSQI | 66 | 5.05 ± 2.45 | - | - | |
| Diary | 53 (51) | 7.51 ± 1.42 | - | - | - |
| KSS | 33 | 4.67 ± 1.53 | 6.70 ± 1.81 | −5.72 | <0.001 |
| PANAS-PA | 71 (68) | 27.96 ± 6.83 | 21.72 ± 7.96 | 5.22 | <0.001 |
| PANAS-NA | 71 (68) | 16.73 ± 5.99 | 16.63 ± 6.23 | 0.20 | 0.840 |
| Median RT (RT, ms) | 38 | 315.54 ± 32.04 | 355.26 ± 32.73 | −7.42 | <0.001 |
| Number of lapses (times) | 38 | 2.00 ± 2.37 | 2.24 ± 2.75 | −0.25 | 0.808 |
| Standard deviation of RT | 38 | 58.27 ± 16.54 | 78.55 ± 18.61 | −4.27 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, D.; Fan, X.; Xiang, C.; Lei, X. Altering Temporal Dynamics of Sleepiness and Mood During Sleep Deprivation: Evidence from Resting-State EEG Microstates. Brain Sci. 2025, 15, 423. https://doi.org/10.3390/brainsci15040423
Bai D, Fan X, Xiang C, Lei X. Altering Temporal Dynamics of Sleepiness and Mood During Sleep Deprivation: Evidence from Resting-State EEG Microstates. Brain Sciences. 2025; 15(4):423. https://doi.org/10.3390/brainsci15040423
Chicago/Turabian StyleBai, Duo, Xinrui Fan, Chuqin Xiang, and Xu Lei. 2025. "Altering Temporal Dynamics of Sleepiness and Mood During Sleep Deprivation: Evidence from Resting-State EEG Microstates" Brain Sciences 15, no. 4: 423. https://doi.org/10.3390/brainsci15040423
APA StyleBai, D., Fan, X., Xiang, C., & Lei, X. (2025). Altering Temporal Dynamics of Sleepiness and Mood During Sleep Deprivation: Evidence from Resting-State EEG Microstates. Brain Sciences, 15(4), 423. https://doi.org/10.3390/brainsci15040423







