Abstract
Background: Dorsal raphe serotonin (5-hydroxytryptamine, 5-HT) neurons are spontaneously active and release 5-HT that is critical for normal brain function and regulates mood and emotion. Serotonin reuptake inhibitors (SSRIs) increase the synaptic and extracellular 5-HT level and are effective in treating depression. Treatment of two weeks or longer is often required for SSRIs to produce clinical benefits. The cellular mechanism underlying this delay is not fully understood. Methods and Results: Using whole-cell patch clamp recording in brain slices, here we show that the GABAergic inputs inhibit the spike firing of raphe 5-HT neurons. This GABAergic regulation was reduced by 5-HT; additionally, this 5-HT effect was prevented by the G-protein-activated inwardly rectifying potassium (GirK) channel inhibitor tertiapin-Q, indicating a contribution of 5-HT activation of GirK channels in GABAergic presynaptic axon terminals. Equally important, after 14 days of treatment with fluoxetine, a widely used SSRI type antidepressant, the 5-HT inhibition of GABAergic inputs was downregulated. Furthermore, chronic fluoxetine treatment downregulated the 5-HT activation of the inhibitory GirK current in 5-HT neurons. Conclusions: Taken together, our results suggest that chronic fluoxetine treatment, by blocking 5-HT reuptake and hence increasing the extracellular 5-HT level, can downregulate the function of 5-HT1B receptors on the GABAergic afferent axon terminals synapsing onto 5-HT neurons, allowing extrinsic GABAergic neurons to more effectively influence 5-HT neurons; simultaneously, chronic fluoxetine treatment also downregulated somatic 5-HT autoreceptor-activated GirK channel-mediated hyperpolarization and decrease in input resistance, rendering 5-HT neurons resistant to autoinhibition and leading to increased 5-HT neuron activity. These neuroplastic changes in raphe 5-HT neurons and their GABAergic afferents may contribute to the behavioral effect of SSRIs.
1. Introduction
The serotonin (5-hydroxytryptamine, 5-HT) neurons in the dorsal raphe nucleus (DRN) project to every forebrain area in rodents and primates including humans [1,2,3,4,5,6,7]. 5-HT, the key neurotransmitter of these neurons, contributes critically to the development and synaptic connectivity of these brain areas [8,9,10,11,12]. 5-HT is also important for the normal operation and cognition of the adult brain, as indicated by the fact that 5-HT agonists such as lysergic acid diethylamide (LSD) can alter human cognition and trigger powerful hallucinations [13,14,15]. Further supporting the importance of the 5-HT system, selective serotonin reuptake inhibitors (SSRIs) that increases the extracellular 5-HT level are effective for treating depression symptoms in humans, at least in sub-groups of patients [16,17]. Thus, controlling and regulating 5-HT neuron spiking activity that trigger 5-HT release in brain target areas is important for normal brain function and may contribute to the pathogenesis of depression and other neuropsychiatric-behavioral disorders.
5-HT neurons often fire around 3 Hz in a quiet awake state [18,19,20,21,22]. This tonic firing is altered when the animal’s cognitive and behavioral state and its environment are changed. Synaptic inputs likely contribute to the behavior-related changes in 5-HT neuron firing. Studies have indicated that GABAergic inputs inhibit raphe 5-HT neuron firing [23,24]. For example, inhibition of 5-HT neuron spike firing or decreased 5-HT release affects sleep [21,25,26,27,28,29,30].
This inhibition of 5-HT neuron activity may be mediated, at least partially, by GABAergic input from extrinsic sources such as the hypothalamus, lateral habenula nucleus, mesopontine rostromedial tegmental nucleus (RMTg), lateral preoptic area and the pontine ventral periaqueductal gray including the DRN, ventral tegmental area (VTA), and substantia nigra pars reticulata (SNr) [31,32,33,34,35,36,37,38,39,40,41]. These GABAergic inputs can affect the spike firing of the raphe 5-HT neurons. Further, GABAergic afferents to 5-HT neurons may express 5-HT1B receptors that may inhibit GABA release, providing another mechanism that regulates 5-HT neuron activity [42].
Raphe 5-HT neurons can also inhibit themselves (autoinhibition) by releasing 5-HT in the raphe and expressing inhibitory 5-HT1A autoreceptors [43,44,45,46,47]. 5-HT1A receptor activation triggers the opening of G-protein-activated inwardly rectifying K (GirK) channels, leading to hyperpolarization and inhibition of these neurons [18,22,48,49,50,51,52,53]. Furthermore, chronic (≥2 weeks) fluoxetine treatment, matching the required duration for clinical antidepressant effects to appear, has been shown to downregulate or desensitize 5-HT1A receptors [45,54,55,56] and remove the initial 5-HT neuron firing inhibition after selective 5-HT reuptake inhibitor treatment (detected by in vivo extracellular spike recording) [57,58,59,60,61,62]. This 5-HT1A receptor neuroadaptive downregulation has been suggested to be critical to fluoxetine’s antidepressant effect [16,17,63]. Indeed, molecular genetics-mediated inactivation of or reduction in these 5-HT1A autoreceptors had antidepressant-like effects [64,65]. These previous studies used biochemical methods and extracellular recording methods. Thus, a knowledge gap exists about (1) the potential effects of chronic fluoxetine on 5-HT-activated GirK-mediated hyperpolarization and the associated changes in intrinsic excitability in dorsal raphe 5-HT neurons, and (2) potential changes in 5-HT regulation of GABAergic inputs to these 5-HT neurons. In our study, we sought to bridge this knowledge gap by chronically treating mice with fluoxetine and then performing whole-cell patch-clamp recording in raphe 5-HT neurons.
2. Materials and Methods
2.1. Animals
All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center, Memphis, Tennessee (animal protocol #17-033.0). Normal wild-type C57Black/6J breeder mice were purchased from The Jackson Laboratory and offspring mice were used for experiments. No transgenic were used in this study. Mice had free access to food and water. The room light was on from 7:00 a.m. to 7:00 p.m. and off for the night. Male and female mice (starting on PN 13 days) were administered intraperitoneally the antidepressant fluoxetine (10 mg/kg) or saline for two weeks (once daily). The 2-week duration was mainly based on the published results of Blier & de Montigny (1983) [57] and Czachura & Rasmussen (2000) [61]. Fluoxetine was chosen because it is a commonly prescribed and hence important antidepressant. The fluoxetine dose (10 mg/kg) was based on the mouse and rat fluoxetine dose-plasma concentration data and in vivo raphe 5-HT neuron spiking data of Czachura & Rasmussen (2000) [61], Ansorge et al. (2008) [66] and Sawyer (2011) [67]. The required numbers of mice for sufficient statistical power for our quantitative experiments were estimated by power analysis performed using a power analysis calculator provided by Boston University.
2.2. Brain Slice Preparation
Male and female mice (PN27-30) were rapidly decapitated and their brains were quickly dissected. The brainstem was rapidly removed and placed in ice-cold, high-sucrose, artificial cerebral spinal fluid (ACSF) that contained the following (in mM): 220 sucrose, 2.5 KCl, 1.25 NaH2PO4, 2 ascorbic acid, 25 NaH2CO3, 0.5 CaCl2, 7 MgCl2, and 20 D-glucose, pH 7.4 (continuously bubbled with 95% O2–5% CO2). Three to four coronal midbrain slices containing the dorsal raphe nucleus were cut (300 μm thick) using a Leica vibratome (VT1200S, Leica Microsystems, Wetzlar, Germany) and immediately transferred to a holding chamber filled with the normal extracellular solution (in mM): 2 ascorbic acid, 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaH2CO3, 2.5 CaCl2, 1.3 MgCl2, and 10 D-glucose, pH 7.4 (when continuously bubbled with 95% O2–5% CO2). This extracellular solution was also the normal perfusing solution. After incubation at 34 °C for 30 min, the slices were maintained at room temperature (22–24 °C) until being transferred to the recording chamber on the stage of the microscope. Drugs were applied via the perfusing solution. The perfusion rate was 2 mL/min.
2.3. Electrophysiological Recording
Conventional whole-cell patch-clamp recordings were performed in the recording chamber maintained at 30 °C by a solution heater. As illustrated in Figure 1A,B, the dorsal raphe neurons were visualized in the midline region ventral to the aqueduct, using an Olympus upright microscope (BX51WI) fitted with a 60x water immersion lens and DIC optics and a Zeiss Axiocam MRm digital camera. The slice was submerged in and perfused with a normal extracellular solution equilibrated with 95% O2–5% CO2. Whole-cell recording pipettes were pulled from borosilicate glass capillary tubing (KG-33, 1.65 mm outer diameter, 1.10 mm inner diameter, King Precision Glass, Claremont, CA, USA) on a two-stage PC-10 micropipette puller (Narishige, Tokyo, Japan).
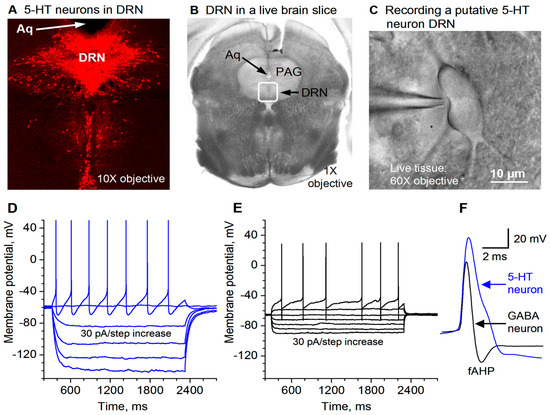
Figure 1.
Recording and identification of 5-HT neurons in the dorsal raphe nucleus (DRN) in mouse brain slices. (A) Tryptophan hydoxylase (TPH) immunostaining identifies 5-HT neurons in mouse DRN. Aq: aqueduct. (B) A live coronal brain slice containing the DRN viewed under a low-power objective. The boxed area is roughly the DRN. Aq: aqueduct. PAG: periaqueductal gray. (C) A putative 5-HT neuron in the DRN being patch-clamped. (D) Membrane potential responses to current injection into a putative 5-HT neuron in the DRN. The current strength started at −120 pA and the step size was 30 pA. For display purposes, the 5-HT neuron spike amplitudes were truncated by ~5 mV (the peak reached ~ +55 mV in this neuron). (E) Membrane potential responses to current injection into a putative GABA neuron in the DRN. The current strength started at −120 pA and the step size was 30 pA. (F) A high temporal resolution display showing that the action potential duration and amplitude for DRN 5-HT neurons are clearly longer and larger than those for DRN GABA neurons.
Cells were recorded in whole cell configuration in voltage- and/or current-clamp mode. Recordings were made with an Axopatch 200B patch-clamp amplifier (Axon Instruments, USA) and pCLAMP 9.2 software (Clampex for data acquisition and Clampfit for data analysis). Signals were filtered at 5 kHz, digitized at 10 kHz with the Digidiata 1322A interface (Axon Instruments), and stored in a computer hard disk for off-line analysis using Clampfit.
A potassium methanesulfonate (KSO3CH3)-based low Cl- intracellular solution (in mM: 135 KSO3CH3, 5 KCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 4 Na2-phosphocreatine, pH 7.25, 280–290 mOsm) was used for recording the natural hyperpolarizing GABAA inhibitory postsynaptic potentials (IPSPs) and their effects on spiking activity (Figure 2). A high Cl- intracellular solution (in mM: 135 KCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 4 Na2-phosphocreatine, pH 7.25, 285 mOsm) was used for recording inward GABAA inhibitory postsynaptic currents (IPSCs) at –70 mV. The patch pipette resistance was about 2 MΩ. Access resistance change was <15% during recording. Liquid junction potentials were not corrected.
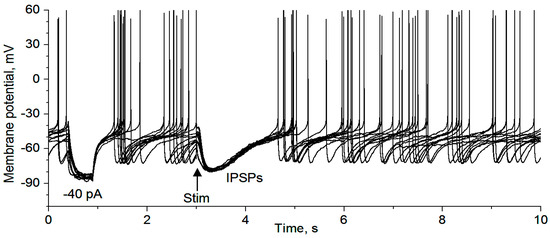
Figure 2.
Qualitative demonstration that IPSPs inhibit raphe 5-HT neuron action potential firing. The recording was made with a KSO3CH4-based intracellular solution (for producing hyperpolarizing IPSPs) in a saline-treated mouse. The first hyperpolarization pulse was induced by a −40 pA pulse to monitor input resistance and to provide an estimate of the strength of the synaptic input underlying the IPSPs: for this the 5-HT neurons, the synaptic current was less than 40 pA. Ten sweeps are displayed here. Stim, local electrical stimulation (20 μA 0.1 ms, delivered every 30 s).
Evoked IPSCs (eIPSC) were electrically stimulated via a bipolar stimulating electrode; stimulus timing and intensity were controlled by using a Master-8 pulse generator (A.M.P.I.) and an A360 stimulus isolator (World Precision Instruments, Sarasota, FL, USA) in the presence of ionotropic glutamate receptor blockers 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM, to block non-NMDA glutamate receptors) and D-2-amino-5-phosphonopetanoic acid (D-APV, 20 μM, to block NMDA receptors). The dorsal raphe neuron under recording was usually 100 μm away from the two tips of the stimulating electrode. Stimulus intervals were 30 s unless otherwise stated. Input resistance was measured in the whole cell configuration in current-clamp mode by injecting a current pulse of –20 pA with a duration of 1000 ms.
2.4. Statistics
Each mouse served as one replicate or data point: we either recorded one 5-HT neuron from one mouse, or averaged the data from one mouse when Two or Three neurons were recorded in the mouse (counted the average as one neuron). The data are presented as the mean ± SEM. The Kolmogorov–Smirnov test (K-S test), one-way ANOVA with post-hoc Tukey’s test and an unpaired t-test were used for statistical comparison. p < 0.05 was used as one indicator of significant difference; we are aware that the overall data provide important indications if there are significant differences between experimental groups [68,69]. Calculations were performed using StatMost software (version 3.6) (Dataxiom Inc., Los Angeles, CA, USA).
2.5. Drugs
Picrotoxin, DL-2-amino-5-phosphonopentanoic acid (AP-5), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 5-HT, tetrodotoxin (TTX), and tertiapin-Q were purchased from Tocris or Sigma. Fluoxetine was either purchased from Tocris or supplied by the NIMH Drug Supply program. 5-HT was purchased from Sigma, prepared as a 10 mM stock solution in pure water, and stored at −20 °C and was used at the final concentration of 10 μM.
2.6. DRN 5-HT Neuron Mapping with Tryptophan Hydroxylase (TPH) Immunostaining
To guide our electrophysiological recording, we also immunostained the tissue sections for TPH, a key 5-HT synthesis enzyme, to visualize DRN 5-HT neurons (Figure 1A). We used conventional immunohistochemical procedures that we have published [70,71]. Briefly, free-floating sections (50 μm in thickness) were incubated with 2% fat-free milk, 1% bovine serum albumin (BSA), and 0.8% Triton X-100 in 0.01 M PBS for 1 h at room temperature to block nonspecific staining. Then, these free-floating tissue sections were incubated with a primary mouse anti-TPH antibody (diluted 1:500, purchased from Sigma, Cat. # MAB5278) for 48 h at 4 °C. After 3 × 10 min PBS rinses to wash out excess primary antibody, the tissue sections were incubated with Alexa Fluor 568 (red) donkey anti-mouse secondary antibody (diluted 1:400, purchased from Invitrogen) for 2 h at room temperature, followed by rinsing 3 × 10 min, and being cover-slipped and digitally photographed on a fluorescence microscope.
3. Results
3.1. Electrophysiological Identification of Dorsal Raphe 5-HT Neurons
Under visual guidance of a video-microscope equipped with a 60x water immersion lens and DIC optics, we made whole-cell patch clamp-recordings in neurons in the dorsal raphe of mice. To record putative 5-HT neurons, we targeted relatively large neurons in the DRN (~20–25 μm in the longest dimension, Figure 1B,C) because literature data indicate that large neurons in the dorsal raphe are 5-HT neurons. Our presumed 5-HT neurons (5-HT neurons hereafter) had the following basic electrophysiological properties: a resting membrane potential of –61.3 ± 1.2 mV, a high spike peak amplitude of 93.3 ± 1.3 mV, a wide action potential duration of 2.94 ± 0.11 m at the spike base and a whole-cell input resistance of 362.2 ± 5.4 MΩ (n = 18). Although not our focus in this study, for the purpose of comparison, we also recorded presumed GABAergic neurons by targeting smaller neurons (≤15 μm in the longest dimension). As shown in Figure 1D–F, these presumed GABAergic neurons had the following electrophysiological properties that are clearly different from those in 5-HT neurons: the action potential duration was 1.19 ± 0.11 ms at the base, the action potential amplitude was 76.4 ± 2.3 mV, and the input resistance was 185.4 ± 8.5 MΩ (n = 10 cells). These putative GABAergic neurons also had deep fast afterhyperpolarization (fAHP) (Figure 1D–F). It is equally important to note that our 5-HT neurons were hyperpolarized by 5-HT. These characteristics are consistent with electrophysiological parameters for dorsal raphe 5-HT and GABAergic neurons in the literature [72,73,74,75,76], indicating that under our recording conditions, 5-HT neurons can be identified based on the whole-cell electrophysiological properties.
3.2. GABAergic Inhibition of DR 5-HT Cell Firing
We first determined if IPSPs inhibit the spiking activity of 5-HT neurons in our mouse brain slices, under the condition where fast ionotropic glutamate receptors were blocked by 10 μM CNQX and 20 μM D-APV. As shown in Figure 2, local electrical stimulation evoked typical hyperpolarizing IPSPs and these IPSPs clearly inhibited the generation of spontaneous action potentials in DR 5-HT neurons (n = 6). These data indicate that regulation of the GABAergic inputs may exert an important influence on 5-HT neuron activity, providing functional relevance for the experiments described below.
3.3. 5-HT Inhibition of GABAergic Inputs to DR 5-HT Neurons
In the presence of 10 μM CNQX and 20 μM D-APV for blocking fast ionotropic glutamate receptors, we recorded local extracellular stimulation-evoked inhibitory postsynaptic currents (evoked IPSC or eIPSC) in 5-HT neurons voltage-clamped at −70 mV. To observe the repetitive GABA release properties of the afferent axon terminals, we used a train stimulation with an intra-train stimulation frequency of 10 Hz, a common spiking frequency of the neurons projecting to the raphe. At this frequency, the axon terminal can sustain the IPSC amplitude or GABA release--after a moderate depression during the first several IPSCs (Figure 3A). To examine the potential effects of 5-HT on GABAergic inputs to raphe 5-HT neurons in normal mice, we first electrically evoked this train of 20 repetitive eIPSCs. After a stable baseline recording, bath application of 10 μM 5-HT reduced the peak amplitude of each eIPSC in the train by about 70% (n = 6 neurons; Figure 3B,C).
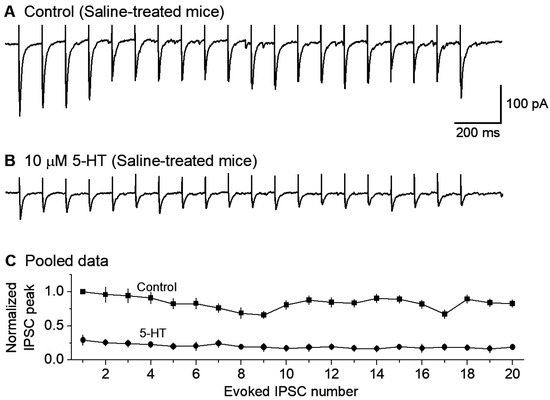
Figure 3.
Bath-applied 5-HT substantially reduced the eIPSC amplitude in DRN 5-HT neurons in saline-treated control mice. (A,B) an example recording of a train of 20 10-Hz stimuli evoking 20 IPSCs under a basal condition (A) and during the application of 10 μM 5-HT (B). The interval between each stimulation train was 30 s. The sharp upward signals in (A,B) are stimulus artifacts. The cells were voltage-clamped at −70 mV and a 135 KCl-based intracellular solution was used, leading to inward GABAA receptor-mediated eIPSCs. (C) Pooled data to show the 5-HT-induced reduction in eIPSC amplitude; each IPSC peak was normalized to the first IPSC peak under the control condition for each neuron. The reduction is obvious; p < 0.001, according to the K-S test, using data from 6 mice.
3.4. GirK Channel Blocker Tertiapin-Q Prevents 5-HT Inhibition of GABAergic Inputs to 5-HT Neurons
Our observation that 5-HT reduced the amplitude of repetitive eIPSCs globally suggested that 5-HT, probably via 5-HT1B receptor activation, may reduce the general excitability and hence transmitter release of the GABAergic axon terminals synapsing onto the 5-HT neurons. Specifically, 5-HT may activate GirK channels expressed in the GABAergic afferent axon terminals; recent anatomical and functional studies support this possibility in both the raphe and other brain areas [77,78,79,80]. In raphe 5-HT neurons, somatodendritic 5-HT1A couples to GirK channels that cause hyperpolarization and hence inhibition of spike firing, accompanied by decreased whole-cell input resistance and excitability. Thus, we reasoned that GirK channels expressed on raphe afferent axon terminals may reduce axonal excitability and hence cause a general decrease in GABA release. To test this possibility, we used tertiapin-Q, which is a known selective inhibitor of GirK channels [52,80,81]. As shown in Figure 4, in the presence of 1 μM tertiapin-Q, bath application of 10 μM 5-HT did not reduce eIPSCs (n = 6). These results indicate that blockade of GirK channels is a key mechanism for 5-HT to inhibit the GABAergic inputs to and GABAAR IPSCs in raphe 5-HT neurons.
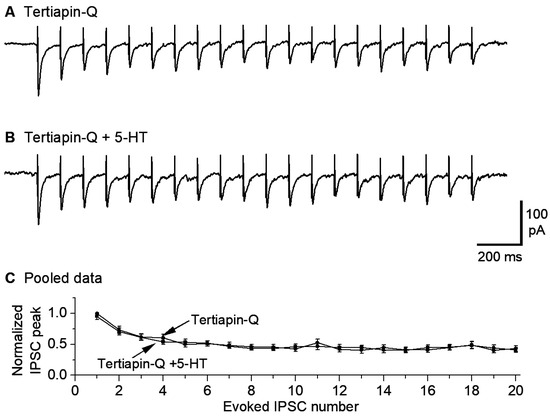
Figure 4.
GirK channel blocker tertiapin-Q prevents 5-HT inhibition of eIPSCs in saline treated mice. A train of 20 10-Hz stimuli evoked 20 IPSCs in the presence of 1 μM tertiapin-Q only (A) and 1 μM tertiapin-Q and 10 μM 5-HT (B). The sharp upward signals in (A,B) are stimulus artifacts. The cells were voltage-clamped at −70 mV and a 135 KCl-based intracellular solution was used. For quantitative comparison (C), the amplitude of all evoked IPSCs in (A,B) was normalized to the first IPSC under control (i.e., 1 μM tertiapin-Q only) for each neuron; there was obviously no difference. p = 0.82, according to the K-S test, using data from 6 mice.
3.5. Chronic Fluoxetine Treatment Downregulates 5-HT Inhibition of GABAergic Inputs to 5-HT Neurons
Chronic fluoxetine treatment is known to downregulate or desensitize 5-HT1A autoreceptors on the somatodendritic areas and axon terminals of raphe 5-HT neurons (via decreased receptor expression, receptor-Gi/o coupling, and/or downregulation of downstream signaling pathway). A similar downregulation of 5-HT1B receptors on non-5-HT neuron axon terminals is entirely possible but has not been reported. We hypothesized that chronic fluoxetine may induce desensitization or downregulation of presynaptic 5-HT1B inhibition of IPSCs in raphe 5-HT neurons.
To test this hypothesis, we treated the mice for ≥14 days with fluoxetine (10 mg/kg per day), with a saline injection as the control. Then, we examined the effects of bath-applied 5-HT (10 μM) on the GABAergic IPSCs in raphe 5-HT neurons in fluoxetine-treated mice. We found that 10 μM 5-HT decreased the 10 Hz stimulus-train-evoked eIPSC amplitude only minimally (n = 9) (Figure 5A–C). These results indicate that chronic treatment with fluoxetine downregulates or reduces the 5-HT inhibition of GABAergic inputs to 5-HT neurons.
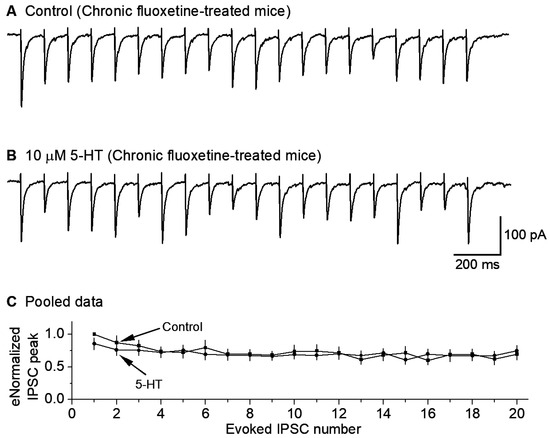
Figure 5.
Chronic fluoxetine treatment downregulates 5-HT inhibition of GABA inputs to 5-HT neurons. A train of 20 10-Hz stimuli evoked 20 IPSCs under the basal condition (A) and during 10 μM 5-HT application (B). The cells were voltage-clamped at −70 mV and a 135 KCl-based intracellular solution was used. For quantitative comparison (C), each eIPSC peak was normalized to the first IPSC peak under the control condition for each neuron. p = 0.56, according to the K-S test, using data from 9 mice.
3.6. Chronic Fluoxetine Treatment Downregulates 5-HT Neuron Autoinhibition
Chronic fluoxetine treatment has been shown to downregulate 5-HT1A receptors and remove the initial inhibition of 5-HT neuron firing (recorded extracellularly) [61]. Here, we used whole-cell recording to directly determine if chronic fluoxetine treatment reduces 5-HT-activated GirK current-induced hyperpolarization and the associated low excitability in raphe 5-HT neurons. We found that in saline-treated mice, bath application of 10 μM 5-HT induced robust hyperpolarization in raphe 5-HT neurons (−26.03 ± 1.82 mV, n = 8; Figure 6(A1,A2,C1)); this hyperpolarization was accompanied by a substantial decrease in input resistance (584.05 ± 23.14 MΩ under control, 128.17 ± 5.65 MΩ under 5-HT, n = 8; Figure 6(A1,A2,C2)). Further, current injection-evoked spike firing was severely inhibited (10.7 ± 0.6 spikes under control, 0 spike under 5-HT, n = 8; Figure 6(A1,A2)). These results are fully consistent with 5-HT activating 5-HT1ARs and opening GirK channels.
In mice treated with fluoxetine for 14 days, the responses to 5-HT were much smaller. Under an identical recording condition, bath application of 10 μM 5-HT induced much smaller hyperpolarization in raphe 5-HT neurons (−8.7 ± 0.9 mV, n = 8; Figure 6(B1,B2,C1)); this smaller hyperpolarization was accompanied by a smaller decrease in input resistance (542.04 ± 20.20 MΩ under control, 343.91 ± 14.26 MΩ under 5-HT, n = 8; Figure 6(B1,B2,C2)); further, current injection-evoked spike firing was only moderately reduced (10.5 ± 0.5 spikes under control, 7.5±0.5 spike under 5-HT, n = 8; Figure 6(B1,B2)). These results indicate that in chronically fluoxetine-treated mice, 5-HT/5-HT1ARs-induced GirK current in raphe 5-HT neurons is reduced. We also need to note here that although 10 μM 5-HT still inhibited DRN 5-HT neurons in brain slices from chronic fluoxetine-treated mice, the endogenous extracellular 5-HT level that probably can substantially inhibit 5-HT neurons in untreated or saline-treated animals is more likely around 1 μM, but it can no longer significantly inhibit these 5-HT neurons in SSRI-treated animals. This level needs to be determined in future studies.
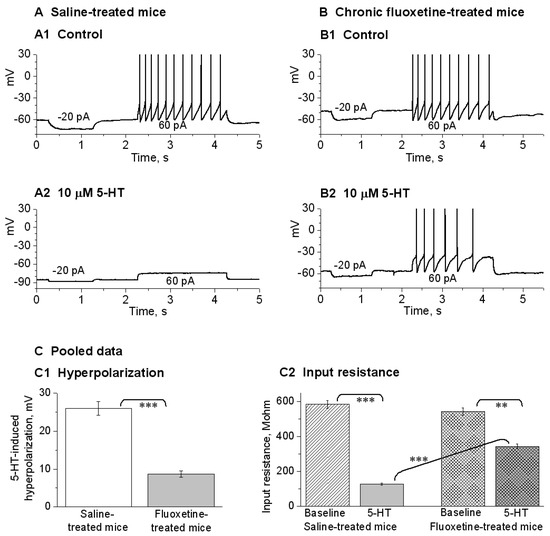
Figure 6.
Chronic fluoxetine treatment renders DRN 5-HT neurons resistant to 5-HT autoinhibition by substantially down-regulating 5-HT autoinhibition of DR 5-HT neurons. A(A1,A2) In brain slices from saline-treated control mice, bath-application of 10 μM 5-HT caused large (26 mV) hyperpolarization, a large decrease in input resistance, and cessation of evoked spike firing. B(B1,B2) In brain slices from fluoxetine-treated mice, bath-application of 10 μM 5-HT caused much smaller (8.7 mV) hyperpolarization, a smaller decrease in input resistance, and a modest decrease in evoked spike firing. In (A,B), action potential peaks are truncated for display. C(C1,C2) Pooled data. The effects are obvious: ***, p < 0.001; **, p < 0.01; unpaired t-test for (C1) and one-way ANOVA for C2. The data are from 8 saline-treated mice and 8 fluoxetine-treated mice.
4. Discussion
The main findings of the present study are that (1) 5-HT, probably via 5-HT1B activating presynaptic GirK channels, reduces GABAergic regulatory inputs to raphe 5-HT neurons, and that (2) chronic treatment with the antidepressant fluoxetine downregulates this 5-HT inhibition of GABAergic inputs and also 5-HT-activated somatodendritic GirK current-mediated autoinhibition (see the summary diagram in Figure 7). Below, we will discuss these results.
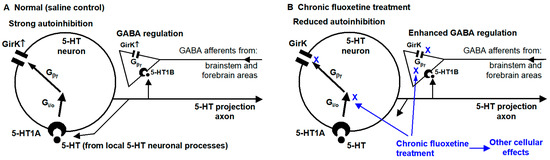
Figure 7.
Summary diagram. In normal and saline-treated animals (A), 5-HT causes autoinhibition by activating somatic 5-HT1A receptors, leading to the release of the Gβγ subunit from the Gi/o protein and opening GirK channels. 5-HT may activate 5-HT1B receptors in GABA afferents and decreases GABA release, which may be mediated by activating GirK channels on axon terminals. These two effects were diminished in animals receiving chronic fluoxetine treatment (B).
4.1. Chronic Fluoxetine Treatment Enhances GABAergic Inhibitory Influence on Dorsal Raphe 5-HT Neurons by Downregulating Presynaptic 5-HT Inhibition
In this study, first, we demonstrated that IPSPs effectively inhibit the spike firing of DRN 5-HT neurons, partly due to their high input resistance such that even a small inhibitory synaptic input can cause significant hyperpolarization and hence inhibition of spike activity. These results are useful and expand prior studies reporting that DRN 5-HT neurons receive inhibitory synaptic inputs but do not show explicitly that these IPSPs inhibit spike firing in DRN 5-HT neurons [42,82,83,84].
Second, our data indicate that 5-HT reduced the GABAergic inputs to DRN 5-HT neurons, probably by activating presynaptic 5-HT1B receptors on GABAergic afferent terminals synapsing on 5-HT neurons based on the established observation that 5-HT1B receptors are commonly on axon terminals [42,71,82,85,86,87,88], although in the present study we did not use 5-HT1B receptor-selective ligands to test this possibility. This mechanism serves to reduce the inhibitory influence of local GABAergic neurons and external GABAergic centers on DRN 5-HT neurons; the sources of these GABAergic afferents include the hypothalamus, lateral habenula nucleus, RMTg, lateral preoptic area, pontine ventral periaqueductal gray, VTA and SNr [32,33,34,35,36,38,39,41,89]. This mechanism may contribute to the overall regulation of 5-HT neuron spiking activity that matches the animal’s mental and behavioral status and needs.
Third, our data indicate that pretreatment with the GirK channel inhibitor tertiapin-Q prevented the inhibitory effect of 5-HT on the IPSCs. Our interpretation is as follows. 5-HT activates presynaptic 5-HT1 receptors (5-HT1B and/or 5-HT1A) that in turn activate GirK channels expressed at the axon terminals, thus reducing axon terminal excitability, leading to fewer spikes, less Ca influx, and less GABA release. GirK channels have been reported to be expressed at axon terminals in multiple brain areas and also at afferent axon terminals in the DRN [77,78,79,80,90]. This is also consistent with the report that presynaptic DA receptors reduce GABA release onto midbrain DA neurons by activating presynaptic GirK channels [91].
Finally, we found that this presynaptic 5-HT inhibition was downregulated after 2-week chronic fluoxetine treatment. Previous studies have established that chronic antidepressant treatment desensitizes 5-HT1 autoreceptors on 5-HT axon terminals inhibiting 5-HT release, and this downregulation temporally coincides with the onset of antidepressant effects in animal models [54,92,93,94,95]. Our present study suggests that chronic SSRI treatment and hence extracellular 5-HT increase may affect 5-HT1B heteroreceptors. Thus, it appears that chronic exposure to high levels of extracellular 5-HT can desensitize the function and/or decrease the cell surface or de novo expression of both 5-HT1B autoreceptors and heteroreceptors. The reduced 5-HT inhibition indicates that after chronic fluoxetine treatment, 5-HT neurons can be more effectively influenced by outside GABAergic neurons in the hypothalamus, SNr, VTA, and other brain areas including the cerebral cortex and brainstem [38].
We also need to note here that literature data indicate that 5-HT1B receptors are probably expressed in GABAergic neurons in the raphe and on the axon collaterals of raphe 5-HT neurons; these 5-HT1B receptors can inhibit GABAergic and 5-HT release, and their functional roles in normal animal physiology and depression pathogenesis are being investigated but have not been established [42,93,95,96,97].
4.2. Chronic Antidepressant Treatment Renders DRN 5-HT Neurons Resistant to 5-HT Autoinhibition by Downregulating 5-HT Inhibition of the Intrinsic Excitability
We found that 2-week daily fluoxetine treatment substantially reduced 5-HT-induced autoinhibition of DRN 5-HT neurons monitored by whole-cell patch clamp recording. Our present results are consistent with and expand the literature data in the field. In their pioneering study using in vivo extracellular spike recording, Blier & De Montigny (1983) [57] found that ip injection of the SSRI zimelidine initially autoinhibited the extracellularly recorded spontaneous spike firing in DRN 5-HT in rats; however, following repeated daily treatment with this SSRI, the autoinhibition gradually declined and eventually disappeared after 2 weeks of treatment, demonstrating that chronic SSRI treatment that induced a chronic increase in the extracellular 5-HT level can desensitize or downregulate 5-HT autoinhibition. In a more detailed study with three doses of fluoxetine (5, 10 and 20 mg/kg per day) combining extracellular spike recording, Czachura & Rasmussen (2000) [61] confirmed that fluoxetine-induced DRN 5-HT neuron autoinhibition, although strong within the first 3 days of fluoxetine treatment, disappeared after 14 days of daily fluoxetine treatment.
The present study provides complementary whole-cell recording data that add new and useful information about the neuroplastic changes in input resistance, intrinsic excitability, and GirK current after chronic fluoxetine treatment. These important details could not be recorded in prior in vivo studies recording extracellular spikes; specifically, our data show that after 2 weeks of daily IP 10 mg/kg fluoxetine treatment, DRN 5-HT neurons became more resistant to 5-HT autoinhibition by producing a much smaller Kir current, much smaller hyperpolarization, and a much smaller decrease in input resistance. These new whole-cell data are consistent with and solidify prior extracellular spike data. Hence, in the presence of fluoxetine or another SSRI type antidepressant, 5-HT neurons can maintain their spiking activity (~1 Hz) and release 5-HT in the projection areas while the SSRI blocks reuptake and increases the extracellular 5-HT level, the intended pharmacological effect that is believed to underlie antidepressant therapeutic efficacy.
Literature evidence indicates that the following chain of events may underlie the chronic fluoxetine-induced downregulation of 5-HT neuron autoinhibition: fluoxetine blocks SERT-mediated 5-HT reuptake and thus increases the extracellular 5-HT level. A chronic increase in the extracellular 5-HT level can desensitize the 5-HT1A and 5-HT1B inhibitory autoreceptors in 5-HT neuron cell bodies and their axon terminals via diminished receptor-G-protein coupling [55,94,98,99,100,101], receptor internalization [45], or a reduction in de novo receptor gene expression [17,92,93]. These molecular events ultimately downregulate 5-HT autoinhibition and lead to sustained increases in extracellular 5-HT and thus contribute to the antidepressant effect [16,54]. The situation with presynaptic 5-HT1B receptors on non-5-HT neurons after SSRI treatment is understudied and less clear. Our study suggests that the downregulating mechanisms may be similar to those of 5-HT1A and 5-HT1B autoreceptors.
4.3. Limitations and Alternative Interpretation
Besides the presynaptic 5-HT1B mechanism described above, we recognize that the postsynaptic 5-HT1A receptor-activated GirK channels may contribute to 5-HT-induced reduction in evoked IPSCs in 5-HT neurons, although it has been reported that the 5-HT1A agonism did not alter the miniature IPSC amplitude in DRN 5-HT neurons recorded with a K-based intracellular solution [42], arguing against the possibility of a lower input resistance contributing to lower eIPSC amplitude. To fully resolve this issue, future studies need to use CsCl-based intracellular solutions to block postsynaptic GirK channels. We also recognize that because our study did not definitively identify the 5-HT receptors involved, future studies will need to use selective 5-HT1A ligands and 5-HT1B ligands together with transgenic 5-HT1A knockout mice and 5-HT1B knockout mice to identify the 5-HT1 receptor subtype mediating the 5-HT effects observed here. Thus, typical for scientific progress, our present interpretation may be revised when additional data are obtained, thereby refining and advancing our understanding of the raphe 5-HT neurons.
5. Conclusions
As depicted in Figure 7, our first conclusion is that chronic fluoxetine downregulates 5-HT inhibition (likely mediated presynaptic 5-HT1B receptors) of GABAergic synaptic inputs to DRN 5-HT neurons; consequently, chronic SSRI-type antidepressant treatment can enable extrinsic, behaviorally important GABAergic neuron activity to more effectively and closely influence DRN 5-HT neurons such that 5-HT neuron activity and hence 5-HT release better match behavioral needs. Our second conclusion is that chronic fluoxetine treatment reduces somatic 5-HT autoreceptor (likely 5-HT1A receptor)-activated GirK channel-mediated hyperpolarization and decreases the input resistance and intrinsic excitability; consequently, chronic antidepressant treatment can render DRN 5-HT neurons resistant to 5-HT autoinhibition and lead to increased 5-HT neuron activity and 5-HT release. These cellular neuroplastic events are potentially key pharmacological mechanisms by which SSRIs exert their antidepressant and other behavioral and cognitive effects.
Author Contributions
Conceptualization, F.-M.Z. and W.Z.; methodology, W.Z., Y.J. and F.-M.Z.; formal analysis, W.Z., Y.J. and F.-M.Z.; data curation, W.Z., Y.J. and F.-M.Z.; writing—original draft preparation, W.Z., Y.J. and F.-M.Z.; writing—review and editing, W.Z., Y.J. and F.-M.Z.; visualization, W.Z., Y.J. and F.-M.Z.; funding acquisition, W.Z. and F.-M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by NIH/NINDS grant R01NS097671. Wei Zhang is a recipient of an award from the China Scholarship Council.
Institutional Review Board Statement
The animal study protocol (#20-0146, approval date 14 May 2020) was approved by the Institutional Animal Care & Use Committee of the University of Tennessee Health Science Center.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to being a part of an ongoing study.
Acknowledgments
This work was supported by NIH/NINDS grant R01NS097671. Wei Zhang is a recipient of an award from the China Scholarship Council.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no influence on any aspect of this study.
References
- Baker, K.G.; Halliday, G.M.; Törk, I. Cytoarchitecture of the human dorsal raphe nucleus. J. Comp. Neurol. 1990, 301, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Charara, A.; Parent, A. Chemoarchitecture of the primate dorsal raphe nucleus. J. Chem. Neuroanat. 1998, 15, 111–127. [Google Scholar] [PubMed]
- Commons, K.G. Ascending serotonin neuron diversity under two umbrellas. Brain Struct. Funct. 2016, 221, 3347–3360. [Google Scholar] [CrossRef] [PubMed]
- Hornung, J.P. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 2003, 26, 331–343. [Google Scholar] [PubMed]
- Parent, M.; Wallman, M.J.; Gagnon, D.; Parent, A. Serotonin innervation of basal ganglia in monkeys and humans. J. Chem. Neuroanat. 2011, 41, 256–265. [Google Scholar] [PubMed]
- Steinbusch, H.W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat—Cell bodies and terminals. Neuroscience 1981, 6, 557–618. [Google Scholar] [PubMed]
- Smiley, J.F.; Goldman-Rakic, P.S. Serotonergic axons in monkey prefrontal cerebral cortex synapse predominantly on interneurons as demonstrated by serial section electron microscopy. J. Comp. Neurol. 1996, 367, 431–443. [Google Scholar] [CrossRef] [PubMed]
- De Stasi, A.M.; Zorrilla de San Martin, J.; Soto, N.; Aguirre, A.; Olusakin, J.; Lourenço, J.; Gaspar, P.; Bacci, A. Alterations of Adult Prefrontal Circuits Induced by Early Postnatal Fluoxetine Treatment Mediated by 5-HT7 Receptors. J. Neurosci. 2025, 45, e2393232024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mosienko, V.; Beis, D.; Pasqualetti, M.; Waider, J.; Matthes, S.; Qadri, F.; Bader, M.; Alenina, N. Life without brain serotonin: Reevaluation of serotonin function with mice deficient in brain serotonin synthesis. Behav. Brain Res. 2015, 277, 78–88. [Google Scholar] [PubMed]
- Soiza-Reilly, M.; Meye, F.J.; Olusakin, J.; Telley, L.; Petit, E.; Chen, X.; Mameli, M.; Jabaudon, D.; Sze, J.Y.; Gaspar, P. SSRIs target pre-frontal to raphe circuits during development modulating synaptic connectivity and emotional behavior. Mol. Psychiatry 2019, 24, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Teissier, A.; Soiza-Reilly, M.; Gaspar, P. Refining the Role of 5-HT in Postnatal Development of Brain Circuits. Front. Cell Neurosci. 2017, 11, 139. [Google Scholar] [PubMed]
- Van Kleef, E.S.; Gaspar, P.; Bonnin, A. Insights into the complex influence of 5-HT signaling on thalamocortical axonal system development. Eur. J. Neurosci. 2012, 35, 1563–1572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holze, F.; Vizeli, P.; Ley, L.; Müller, F.; Dolder, P.; Stocker, M.; Duthaler, U.; Varghese, N.; Eckert, A.; Borgwardt, S.; et al. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology 2021, 46, 537–544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kraehenmann, R.; Pokorny, D.; Vollenweider, L.; Preller, K.H.; Pokorny, T.; Seifritz, E.; Vollenweider, F.X. Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology 2017, 234, 2031–2046. [Google Scholar] [PubMed]
- Preller, K.H.; Vollenweider, F.X. Phenomenology, Structure, and Dynamic of Psychedelic States. Curr. Top. Behav. Neurosci. 2018, 36, 221–256. [Google Scholar] [PubMed]
- Wong, D.T.; Perry, K.W.; Bymaster, F.P. Case history: The discovery of fluoxetine hydrochloride (Prozac). Nat. Rev. Drug Discov. 2005, 4, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Collins, H. Mechanisms of SSRI Therapy and Discontinuation. Curr. Top. Behav. Neurosci. 2024, 66, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Dougalis, A.G.; Matthews, G.A.C.; Liss, B.; Ungless, M.A. Ionic currents influencing spontaneous firing and pacemaker frequency in dopamine neurons of the ventrolateral periaqueductal gray and dorsal raphe nucleus (vlPAG/DRN): A voltage-clamp and computational modelling study. J. Comput. Neurosci. 2017, 42, 275–305. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar]
- Jacobs, B.L.; Martín-Cora, F.J.; Fornal, C.A. Activity of medullary serotonergic neurons in freely moving animals. Brain Res. Brain Res. Rev. 2002, 40, 45–52. [Google Scholar] [CrossRef]
- Sakai, K. Sleep-waking discharge profiles of dorsal raphe nucleus neurons in mice. Neuroscience 2011, 197, 200–224. [Google Scholar] [PubMed]
- Tuckwell, H.C.; Penington, N.J. Computational modeling of spike generation in serotonergic neurons of the dorsal raphe nucleus. Prog. Neurobiol. 2014, 118, 59–101. [Google Scholar] [PubMed]
- Levine, E.S.; Jacobs, B.L. Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus: Microiontophoretic studies in the awake cat. J. Neurosci. 1992, 12, 4037–4044. [Google Scholar] [PubMed]
- Wang, R.Y.; Gallager, D.W.; Aghajanian, G.K. Stimulation of pontine reticular formation suppresses firing of serotonergic neuronses in the dorsal raphe. Nature 1976, 264, 365–368. [Google Scholar] [PubMed]
- Jouvet, M. Sleep and serotonin: An unfinished story. Neuropsychopharmacology 1999, 21 (Suppl. S2), 24S–27S. [Google Scholar] [PubMed]
- Iwasaki, K.; Komiya, H.; Kakizaki, M.; Miyoshi, C.; Abe, M.; Sakimura, K.; Funato, H.; Yanagisawa, M. Ablation of Central Serotonergic Neurons Decreased REM Sleep and Attenuated Arousal Response. Front. Neurosci. 2018, 12, 535. [Google Scholar] [PubMed]
- Nitz, D.; Siegel, J. GABA release in the dorsal raphe nucleus: Role in the control of REM sleep. Am. J. Physiol. 1997, 273, R451–R455. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Crochet, S. Differentiation of presumed serotonergic dorsal raphe neurons in relation to behavior and wake-sleep states. Neuroscience 2001, 104, 1141–1155. [Google Scholar] [PubMed]
- Siegel, J.M. The neurotransmitters of sleep. J. Clin. Psychiatry 2004, 65 (Suppl. S16), 4–7. [Google Scholar] [PubMed]
- Saper, C.B.; Fuller, P.M.; Pedersen, N.P.; Lu, J.; Scammell, T.E. Sleep state switching. Neuron 2010, 68, 1023–1042. [Google Scholar] [CrossRef] [PubMed]
- Bernard, R.; Veh, R.W. Individual neurons in the rat lateral habenular complex project mostly to the dopaminergic ventral tegmental area or to the serotonergic raphe nuclei. J. Comp. Neurol. 2012, 520, 2545–2558. [Google Scholar] [PubMed]
- Gervasoni, D.; Peyron, C.; Rampon, C.; Barbagli, B.; Chouvet, G.; Urbain, N.; Fort, P.; Luppi, P.H. Role and origin of the GABAergic innervation of dorsal raphe serotonergic neurons. J. Neurosci. 2000, 20, 4217–4225. [Google Scholar] [PubMed]
- Kirouac, G.J.; Li, S.; Mabrouk, G. GABAergic projection from the ventral tegmental area and substantia nigra to the periaqueductal gray region and the dorsal raphe nucleus. J. Comp. Neurol. 2004, 469, 170–184. [Google Scholar]
- Lavezzi, H.N.; Parsley, K.P.; Zahm, D.S. Mesopontine rostromedial tegmental nucleus neurons projecting to the dorsal raphe and pedunculopontine tegmental nucleus: Psychostimulant-elicited Fos expression and collateralization. Brain Struct. Funct. 2012, 217, 719–734. [Google Scholar]
- Pollak Dorocic, I.; Fürth, D.; Xuan, Y.; Johansson, Y.; Pozzi, L.; Silberberg, G.; Carlén, M.; Meletis, K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 2014, 83, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Reisine, T.D.; Soubrié, P.; Artaud, F.; Glowinski, J. Involvement of lateral habenula-dorsal raphe neurons in the differential regulation of striatal and nigral serotonergic transmission in cats. J. Neurosci. 1982, 2, 1062–1071. [Google Scholar] [PubMed]
- Sego, C.; Gonçalves, L.; Lima, L.; Furigo, I.C.; Donato, J.; Metzger, M. Lateral habenula and the rostromedial tegmental nucleus innervate neurochemically distinct subdivisions of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 2014, 522, 1454–1484. [Google Scholar] [CrossRef] [PubMed]
- Soiza-Reilly, M.; Commons, K.G. Unraveling the architecture of the dorsal raphe synaptic neuropil using high-resolution neuroanatomy. Front. Neural. Circuits 2014, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; Badurek, S.; Dileone, R.J.; Nashmi, R.; Minichiello, L.; Picciotto, M.R. GABAergic and glutamatergic efferents of the mouse ventral tegmental area. J. Comp. Neurol. 2014, 522, 3308–3334. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Aghajanian, G.K. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science 1977, 197, 89–91. [Google Scholar] [PubMed]
- Zhou, L.; Liu, M.Z.; Li, Q.; Deng, J.; Mu, D.; Sun, Y.G. Organization of Functional Long-Range Circuits Controlling the Activity of Serotonergic Neurons in the Dorsal Raphe Nucleus. Cell Rep. 2017, 18, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.C.; Pan, Y.Z.; Ma, X.; Lamy, C.; Akanwa, A.C.; Beck, S.G. Selective 5-HT receptor inhibition of glutamatergic and GABAergic synaptic activity in the rat dorsal and median raphe. Eur. J. Neurosci. 2006, 24, 3415–3430. [Google Scholar] [PubMed]
- Adell, A.; Celada, P.; Artigas, F. The role of 5-HT1B receptors in the regulation of serotonin cell firing and release in the rat brain. J. Neurochem. 2001, 79, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Adell, A.; Celada, P.; Abellán, M.T.; Artigas, F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res. Brain Res. Rev. 2002, 39, 154–180. [Google Scholar] [CrossRef] [PubMed]
- Descarries, L.; Riad, M. Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2416–2425. [Google Scholar] [PubMed]
- Riad, M.; Garcia, S.; Watkins, K.C.; Jodoin, N.; Doucet, E.; Langlois, X.; el Mestikawy, S.; Hamon, M.; Descarries, L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000, 417, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Stamford, J.A.; Davidson, C.; McLaughlin, D.P.; Hopwood, S.E. Control of dorsal raphe 5-HT function by multiple 5-HT(1) autoreceptors: Parallel purposes or pointless plurality? Trends Neurosci. 2000, 23, 459–465. [Google Scholar] [PubMed]
- Bayliss, D.A.; Li, Y.W.; Talley, E.M. Effects of serotonin on caudal raphe neurons: Activation of an inwardly rectifying potassium conductance. J. Neurophysiol. 1997, 77, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Jones, M.B.; Talley, E.M.; Schrier, A.D.; McIntire, W.E.; Garrison, J.C.; Bayliss, D.A. Activation and inhibition of G protein-coupled inwardly rectifying potassium (Kir3) channels by G protein beta gamma subunits. Proc. Natl. Acad. Sci. USA 2000, 97, 9771–9776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Penington, N.J.; Kelly, J.S.; Fox, A.P. Unitary properties of potassium channels activated by 5-HT in acutely isolated rat dorsal raphe neurones. J. Physiol. 1993, 469, 407–426. [Google Scholar] [PubMed]
- Penington, N.J.; Kelly, J.S.; Fox, A.P. Whole-cell recordings of inwardly rectifying K+ currents activated by 5-HT1A receptors on dorsal raphe neurones of the adult rat. J. Physiol. 1993, 469, 387–405. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, A.; Corradetti, R.; Mlinar, B. Pharmacological Characterization of 5-HT1A Autoreceptor-Coupled GIRK Channels in Rat Dorsal Raphe 5-HT Neurons. PLoS ONE 2015, 10, e0140369. [Google Scholar] [CrossRef] [PubMed]
- Saenz del Burgo, L.; Cortes, R.; Mengod, G.; Zarate, J.; Echevarria, E.; Salles, J. Distribution and neurochemical characterization of neurons expressing GIRK channels in the rat brain. J. Comp. Neurol. 2008, 510, 581–606. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; El Mansari, M. Serotonin and beyond: Therapeutics for major depression. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120536. [Google Scholar] [CrossRef] [PubMed]
- Hensler, J.G. Differential regulation of 5-HT1A receptor-G protein interactions in brain following chronic antidepressant administration. Neuropsychopharmacology 2002, 26, 565–573. [Google Scholar] [CrossRef]
- Rainer, Q.; Nguyen, H.T.; Quesseveur, G.; Gardier, A.M.; David, D.J.; Guiard, B.P. Functional status of somatodendritic serotonin 1A autoreceptor after long-term treatment with fluoxetine in a mouse model of anxiety/depression based on repeated corticosterone administration. Mol. Pharmacol. 2012, 81, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; De Montigny, C. Electrophysiological investigations on the effect of repeated zimelidine administration on serotonergic neurotransmission in the rat. J. Neurosci. 1983, 3, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; Chaput, Y.; de Montigny, C. Long-term 5-HT reuptake blockade, but not monoamine oxidase inhibition, decreases the function of terminal 5-HT autoreceptors: An electrophysiological study in the rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 1988, 337, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Chaput, Y.; Blier, P.; de Montigny, C. In vivo electrophysiological evidence for the regulatory role of autoreceptors on serotonergic terminals. J. Neurosci. 1986, 6, 2796–2801. [Google Scholar] [CrossRef] [PubMed]
- Chaput, Y.; de Montigny, C.; Blier, P. Presynaptic and postsynaptic modifications of the serotonin system by long-term administration of antidepressant treatments. An in vivo electrophysiologic study in the rat. Neuropsychopharmacology 1991, 5, 219–229. [Google Scholar] [PubMed]
- Czachura, J.F.; Rasmussen, K. Effects of acute and chronic administration of fluoxetine on the activity of serotonergic neurons in the dorsal raphe nucleus of the rat. Naunyn Schmiedebergs Arch. Pharmacol. 2000, 362, 266–275. [Google Scholar] [PubMed]
- Guiard, B.P.; Mansari, M.E.; Murphy, D.L.; Blier, P. Altered response to the selective serotonin reuptake inhibitor escitalopram in mice heterozygous for the serotonin transporter: An electrophysiological and neurochemical study. Int. J. Neuropsychopharmacol. 2012, 15, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Boothman, L.; Raley, J.; Quérée, P. Important messages in the ’post’: Recent discoveries in 5-HT neurone feedback control. Trends Pharmacol. Sci. 2007, 28, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Richardson-Jones, J.W.; Craige, C.P.; Guiard, B.P.; Stephen, A.; Metzger, K.L.; Kung, H.F.; Gardier, A.M.; Dranovsky, A.; David, D.J.; Beck, S.G.; et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron 2010, 65, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Ferrés-Coy, A.; Santana, N.; Castañé, A.; Cortés, R.; Carmona, M.C.; Toth, M.; Montefeltro, A.; Artigas, F.; Bortolozzi, A. Acute 5-HT1A autoreceptor knockdown increases antidepressant responses and serotonin release in stressful conditions. Psychopharmacology 2013, 225, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, M.S.; Morelli, E.; Gingrich, J.A. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J. Neurosci. 2008, 28, 199–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawyer, E.K.; Howell, L.L. Pharmacokinetics of fluoxetine in rhesus macaques following multiple routes of administration. Pharmacology 2011, 88, 44–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0.05”. Am. Stat. 2019, 73 (Suppl. S1), 1–19. [Google Scholar] [CrossRef]
- Zhou, F.W.; Jin, Y.; Matta, S.G.; Xu, M.; Zhou, F.M. An ultra-short dopamine pathway regulates basal ganglia output. J. Neurosci. 2009, 29, 10424–10435. [Google Scholar] [PubMed]
- Ding, S.; Li, L.; Zhou, F.M. Robust presynaptic serotonin 5-HT(1B) receptor inhibition of the striatonigral output and its sensitization by chronic fluoxetine treatment. J. Neurophysiol. 2015, 113, 3397–3409. [Google Scholar] [PubMed]
- Liu, R.J.; Van den Pol, A.N.; Aghajanian, G.K. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J. Neurosci. 2002, 22, 9453–9464. [Google Scholar] [PubMed]
- Allers, K.A.; Sharp, T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience 2003, 122, 193–204. [Google Scholar] [PubMed]
- Haj-Dahmane, S. D2-like dopamine receptor activation excites rat dorsal raphe 5-HT neurons in vitro. Eur. J. Neurosci. 2001, 14, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.J.; Lambe, E.K.; Aghajanian, G.K. Somatodendritic autoreceptor regulation of serotonergic neurons: Dependence on L-tryptophan and tryptophan hydroxylase-activating kinases. Eur. J. Neurosci. 2005, 21, 945–958. [Google Scholar] [PubMed]
- Mlinar, B.; Montalbano, A.; Piszczek, L.; Gross, C.; Corradetti, R. Firing Properties of Genetically Identified Dorsal Raphe Serotonergic Neurons in Brain Slices. Front. Cell Neurosci. 2016, 10, 195. [Google Scholar] [PubMed]
- Fernández-Alacid, L.; Aguado, C.; Ciruela, F.; Martín, R.; Colón, J.; Cabañero, M.J.; Gassmann, M.; Watanabe, M.; Shigemoto, R.; Wickman, K.; et al. Subcellular compartment-specific molecular diversity of pre- and post-synaptic GABA-activated GIRK channels in Purkinje cells. J. Neurochem. 2009, 110, 1363–1376. [Google Scholar] [PubMed]
- Fernández-Alacid, L.; Watanabe, M.; Molnár, E.; Wickman, K.; Luján, R. Developmental regulation of G protein-gated inwardly-rectifying K+ (GIRK/Kir3) channel subunits in the brain. Eur. J. Neurosci. 2011, 34, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Ladera, C.; del Carmen Godino, M.; José Cabañero, M.; Torres, M.; Watanabe, M.; Luján, R.; Sánchez-Prieto, J. Pre-synaptic GABA receptors inhibit glutamate release through GIRK channels in rat cerebral cortex. J. Neurochem. 2008, 107, 1506–1517. [Google Scholar] [PubMed]
- Llamosas, N.; Ugedo, L.; Torrecilla, M. Inactivation of GIRK channels weakens the pre- and postsynaptic inhibitory activity in dorsal raphe neurons. Physiol. Rep. 2017, 5, e13141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Johnston, D. Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J. Neurosci. 2005, 25, 3787–3792. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, H.; Manzoni, O.J.; Crabbe, J.C.; Williams, J.T. Regulation of central synaptic transmission by 5-HT1B auto- and heteroreceptors. Mol. Pharmacol. 2000, 58, 1271–1278. [Google Scholar] [PubMed]
- Pan, Z.Z.; Williams, J.T. GABA- and glutamate-mediated synaptic potentials in rat dorsal raphe neurons in vitro. J. Neurophysiol. 1989, 61, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.T.; Colmers, W.F.; Pan, Z.Z. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J. Neurosci. 1988, 8, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Boschert, U.; Amara, D.A.; Segu, L.; Hen, R. The mouse 5-hydroxytryptamine1B receptor is localized predominantly on axon terminals. Neuroscience 1994, 58, 167–182. [Google Scholar] [CrossRef]
- Ding, S.; Li, L.; Zhou, F.M. Presynaptic serotonergic gating of the subthalamonigral glutamatergic projection. J. Neurosci. 2013, 33, 4875–4885. [Google Scholar] [PubMed]
- Li, Y.W.; Bayliss, D.A. Presynaptic inhibition by 5-HT1B receptors of glutamatergic synaptic inputs onto serotonergic caudal raphe neurones in rat. J. Physiol. 1998, 510, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Sari, Y. Serotonin1B receptors: From protein to physiological function and behavior. Neurosci. Biobehav. Rev. 2004, 28, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Argañaraz, C.V.; Adjimann, T.S.; Perissinotti, P.P.; Soiza-Reilly, M. Selective refinement of glutamate and GABA synapses on dorsal raphe 5-HT neurons during postnatal life. Development 2022, 149, dev201121. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.; Bueno, E.; Kentros, C.; Vega-Saenz de Miera, E.; Chow, A.; Hillman, D.; Chen, S.; Zhu, L.; Wu, M.B.; Wu, X.; et al. G-protein-gated inward rectifier K+ channel proteins (GIRK1) are present in the soma and dendrites as well as in nerve terminals of specific neurons in the brain. J. Neurosci. 1996, 16, 1990–2001. [Google Scholar] [PubMed]
- Michaeli, A.; Yaka, R. Dopamine inhibits GABA(A) currents in ventral tegmental area dopamine neurons via activation of presynaptic G-protein coupled inwardly-rectifying potassium channels. Neuroscience 2010, 165, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.P.; Sexton, T.J.; Neumaier, J.F. Antidepressant-induced regulation of 5-HT(1b) mRNA in rat dorsal raphe nucleus reverses rapidly after drug discontinuation. J. Neurosci. Res. 2000, 61, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, J.F.; Root, D.C.; Hamblin, M.W. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology 1996, 15, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.E.; Shalom, G.; Ran, A.; Gur, E.; Van de Kar, L.D. Chronic fluoxetine-induced desensitization of 5-HT1A and 5-HT1B autoreceptors: Regional differences and effects of WAY-100635. Eur. J. Pharmacol. 2004, 486, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tiger, M.; Varnäs, K.; Okubo, Y.; Lundberg, J. The 5-HT(1B) receptor—A potential target for antidepressant treatment. Psychopharmacology 2018, 235, 1317–1334. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, R.A.; Hiroi, R.; Mackenzie, S.M.; Robin, N.C.; Cohn, A.; Kim, J.J.; Neumaier, J.F. Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior. Biol. Psychiatry 2011, 69, 780–787. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, R.A.; Neumaier, J.F. Regulation of dorsal raphe nucleus function by serotonin autoreceptors: A behavioral perspective. J. Chem. Neuroanat. 2011, 41, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Diaz, A.; del Olmo, E.; Pazos, A. Chronic fluoxetine induces opposite changes in G protein coupling at pre and postsynaptic 5-HT1A receptors in rat brain. Neuropharmacology 2003, 44, 93–101. [Google Scholar] [CrossRef]
- Cornelisse, L.N.; Van der Harst, J.E.; Lodder, J.C.; Baarendse, P.J.; Timmerman, A.J.; Mansvelder, H.D.; Spruijt, B.M.; Brussaard, A.B. Reduced 5-HT1A- and GABAB receptor function in dorsal raphe neurons upon chronic fluoxetine treatment of socially stressed rats. J. Neurophysiol. 2007, 98, 196–204. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Q.; Muma, N.A.; Van de Kar, L.D. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: Reductions in hypothalamic and midbrain Gi and G(o) proteins and in neuroendocrine responses to a 5-HT1A agonist. J. Pharmacol. Exp. Ther. 1996, 279, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Muma, N.A.; Battaglia, G.; Van de Kar, L.D. A desensitization of hypothalamic 5-HT1A receptors by repeated injections of paroxetine: Reduction in the levels of G(i) and G(o) proteins and neuroendocrine responses, but not in the density of 5-HT1A receptors. J. Pharmacol. Exp. Ther. 1997, 282, 1581–1590. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).