Repetitive Gamma-tACS Improves the Reaction Times of Healthy Young Adults in a Visuospatial Working Memory Task: A Randomized Study
Abstract
1. Introduction
1.1. WM and Neural Oscillations
1.2. Neuromodulation and WM
| Palm et al., 2014 [22] | |
| Band frequency (tACS): | Gamma (40 Hz)-tACS |
| Study design: | Between-subjects (active/sham) |
| Number of active session(s): | 1 |
| Region(s) of stimulation: | Bilateral frontal cortex (F3 and F4) |
| Participant characteristics: | Healthy volunteers and major depression patients |
| Task(s)—online/offline: | n-Back task (2-back and 3-back)—online |
| Significant results: | No effects independent of retention load |
| DOI: | 10.1016/j.neucli.2022.03.002 |
| Hoy et al., 2015 [21] | |
| Band frequency (tACS): | 3 sessions: gamma(40 Hz)-tACS + tDCS + sham |
| Study design: | Within subjects (active/tDCS/sham) |
| Number of active session(s): | 2 (one tACS + one tDCS) |
| Region(s) of stimulation: | Left frontal cortex (F3) |
| Participant characteristics: | Young adults |
| Task(s)—online/offline: | 2-back (online and offline) 3-back (offline) |
| Significant results: | d-prime: For γ-tACS only, larger improvement in 3-back than 2-back. RT: no effects. |
| DOI: | 10.1016/j.bandc.2015.11.002 |
| Kvašňák et al., 2018 [23] | |
| Band frequency (tACS): | Gamma (40 Hz)-tACS |
| Study design: | Between-subjects (active/sham) |
| Number of active session(s): | 1 |
| Region(s) of stimulation: | Bilateral frontal cortex (F3 and F4) |
| Participant characteristics: | Young adults |
| Task(s)—online/offline: | Visual WM task (Luck and Vogel paradigm)—offline |
| Significant results: | None |
| DOI: | 10.3390/bs13010039 |
| Pahor et al., 2018 [24] | |
| Band frequency (tACS): | Theta-tACS or gamma-tACS |
| Study design: | Within-subjects (theta/gamma/sham) Between-subjects (regions of stimulation) |
| Number of active session(s): | 2 (one theta-tACS + one gamma-tACS) |
| Region(s) of stimulation: | 4 groups: bilateral parietal (P3-P4), left fronto-parietal (F3-P3), right fronto-parietal (F4-P4), bilateral frontal (F3-F4) |
| Participant characteristics: | Young adults |
| Task(s)—online/offline: | Change detection tasks (figural and verbal)—offline. N-back tasks (figural and verbal variants of 2- and 3-back tests)—offline |
| Significant results: | No behavioral effects. Offline: active theta-tACS increased P3 component during n-back tasks in the bilateral parietal and right fronto-parietal protocols. |
| DOI: | 10.3389/fnhum.2017.00651 |
| Grover et al., 2022 [29] | |
| Band frequency (tACS): | Gamma (60 Hz)-HD-tACS or theta (4 Hz)-HD-tACS |
| Study design: | Between-subjects (active/sham) |
| Number of active session(s): | 4 (day 1, 2, 3 and 4) |
| Region(s) of stimulation: | DLPFC (AF3) or IPL (CP5) |
| Participant characteristics: | Old adults (69–88 ys old) |
| Task(s)—online/offline: | Free recall task (online for stimulation days, offline at baseline pre-stimulation and one month follow-up) |
| Significant results: | Theta-frequency in IPL improved WM on day 3 and 4 and 1 month after intervention. Gamma-frequency in DLPFC improved LTM on days 2–4 and 1 month after intervention. |
| DOI: | 10.1038/s41593-022-01132-3 |
| Abubaker et al., 2024 [25] | |
| Band frequency (tACS): | Theta (6 Hz)–gamma (80 Hz) peak coupled HD-tACS |
| Study design: | Within subjects (active/sham) |
| Number of active session(s): | 1 |
| Region(s) of stimulation: | Left frontal cortex (F3) |
| Participant characteristics: | Old adults |
| Task(s)—online/offline: | Visuospatial WM task, Sternberg task, Flanker task, DSST, WCST (online) |
| Significant results: | Decrease in ACC and RTs on the 10- and 14-item Sternberg tasks. Increase in RTs on the DSST. |
| DOI: | 10.1186/s13041-024-01149-8 |
| Al Qasem et al., 2024 [26] | |
| Band frequency (tACS): | Theta (6 Hz)–gamma (80 Hz) peak coupled HD-tACS |
| Study design: | Within subjects (active/sham) |
| Number of active session(s): | 1 |
| Region(s) of stimulation: | Left frontal cortex (F3) |
| Participant characteristics: | Young adults |
| Task(s)—online/offline: | Visuospatial WM task, Sternberg task, Flanker task, DSST, WCST (online) |
| Significant results: | Increase in ACC only on the 14-item Sternberg task. |
| DOI: | 10.1186/s13041-024-01142-1 |
1.3. Study Rationale and Aims
1.4. Hypotheses
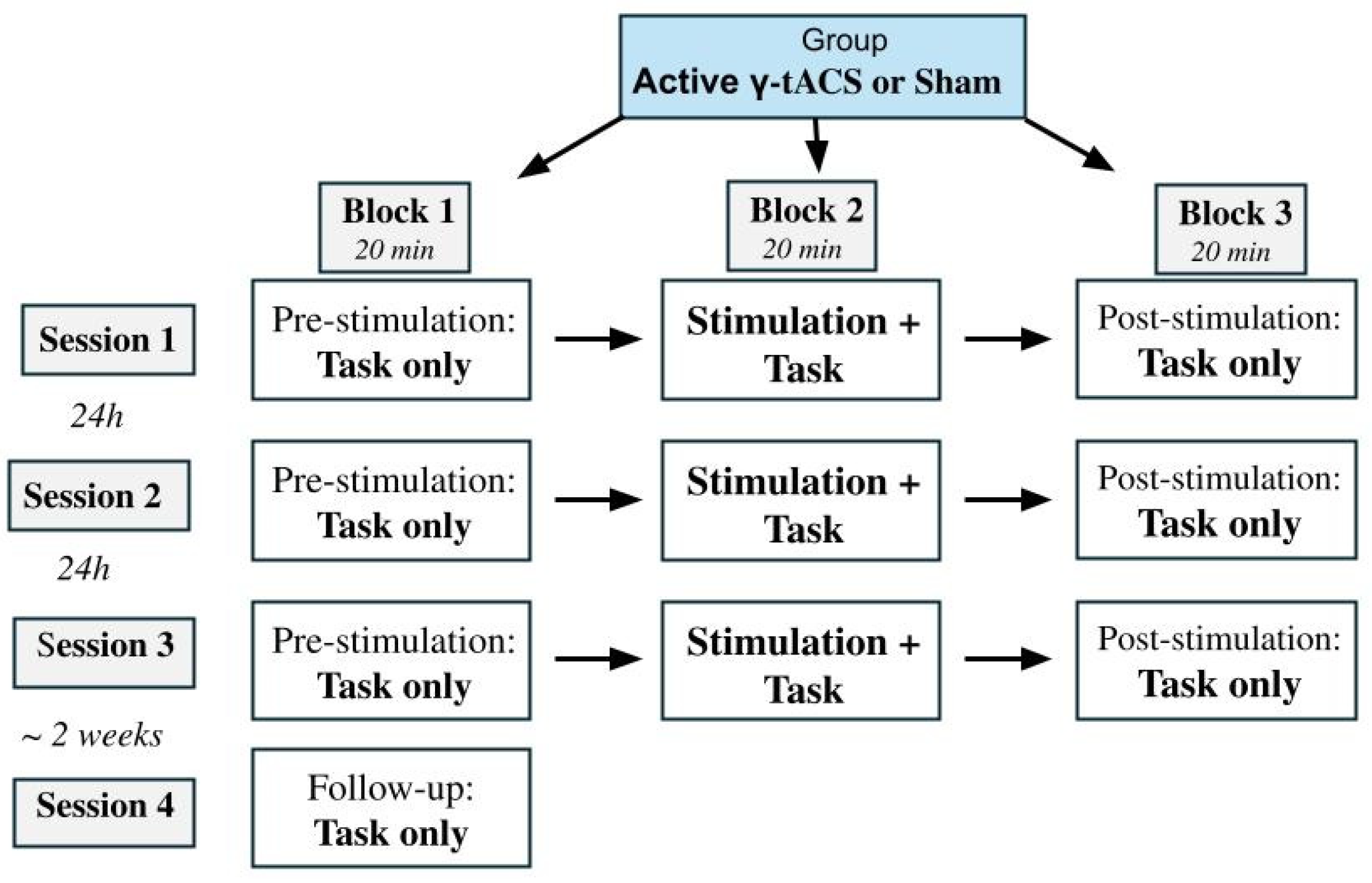
2. Materials and Methods
2.1. Participants
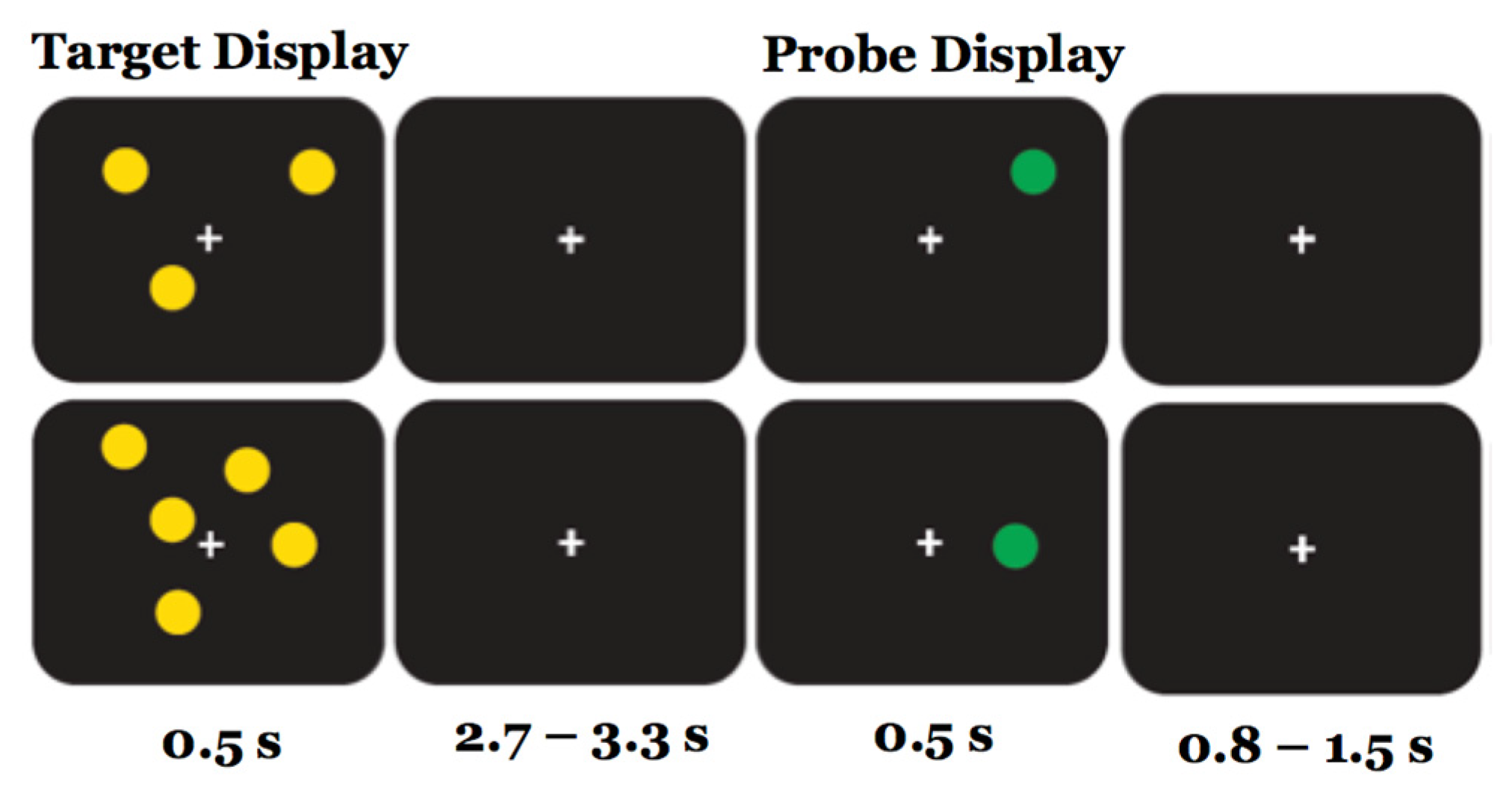
2.2. Experimental Task
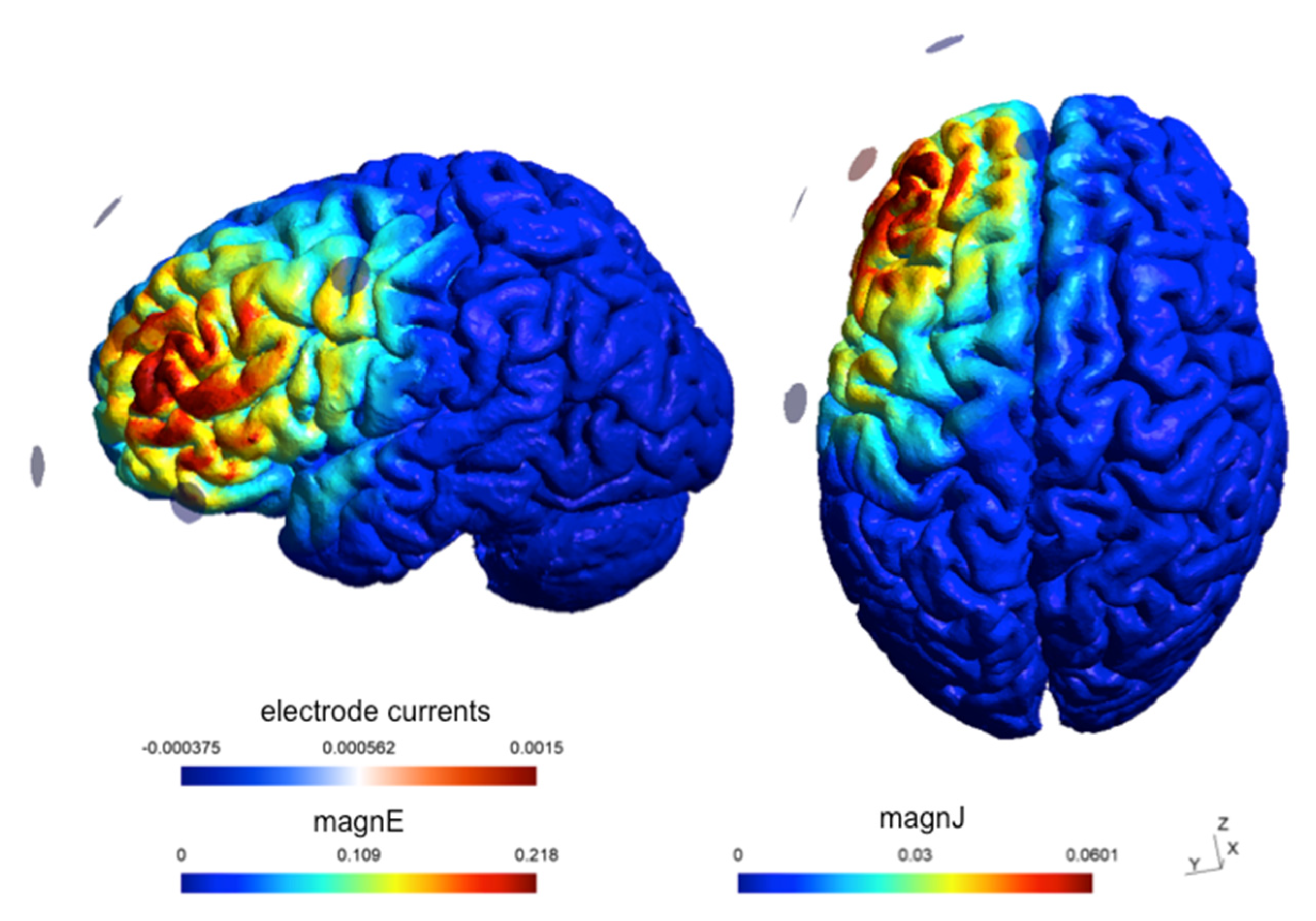
2.3. HD-tACS
2.4. Statistical Analysis
3. Results
3.1. Demographic Variables and Descriptive Statistics
3.1.1. Short-Term Effects of Stimulation
Accuracy
Reaction Times (RTs)
3.2. Mixed Models Results
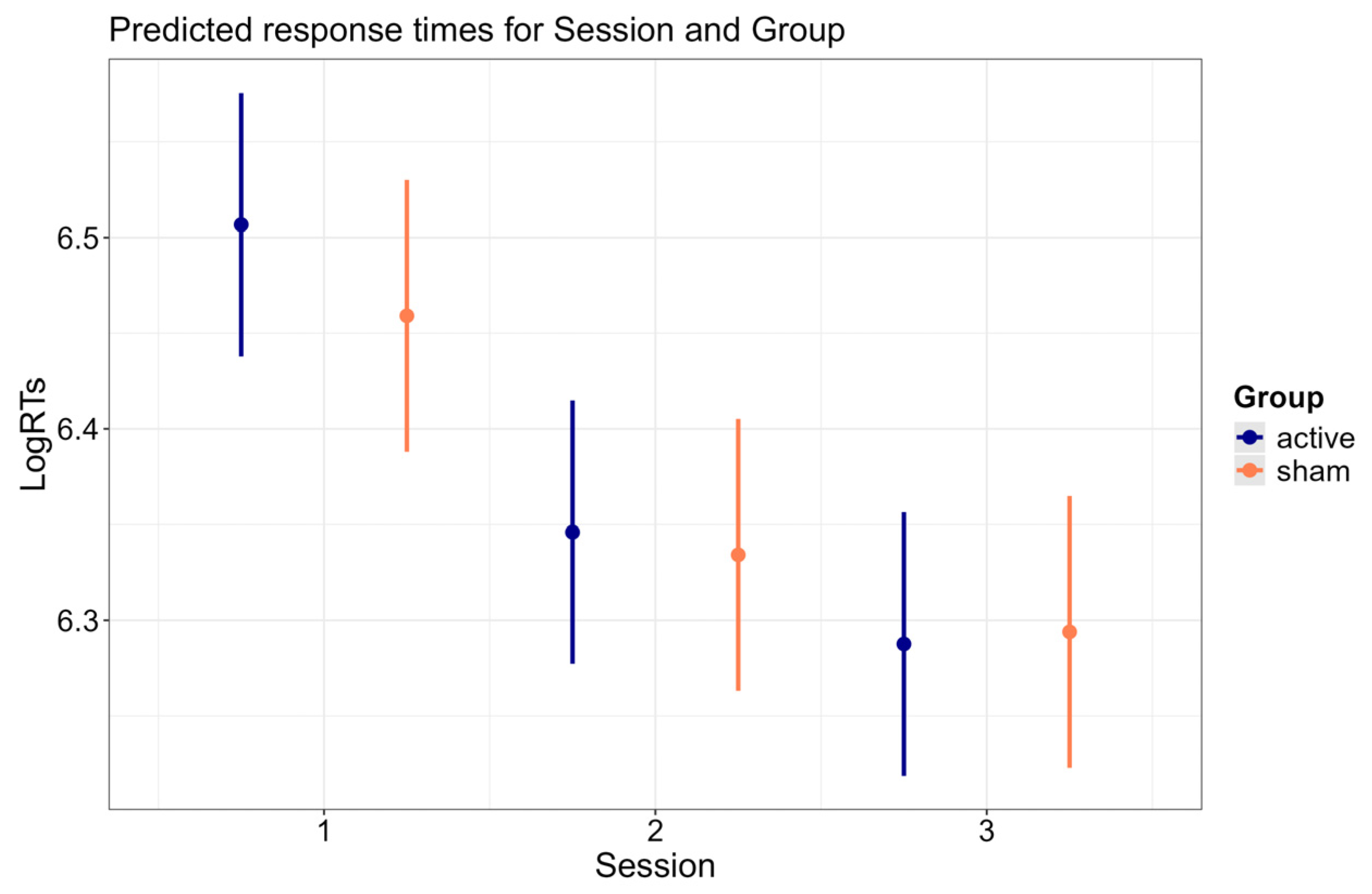
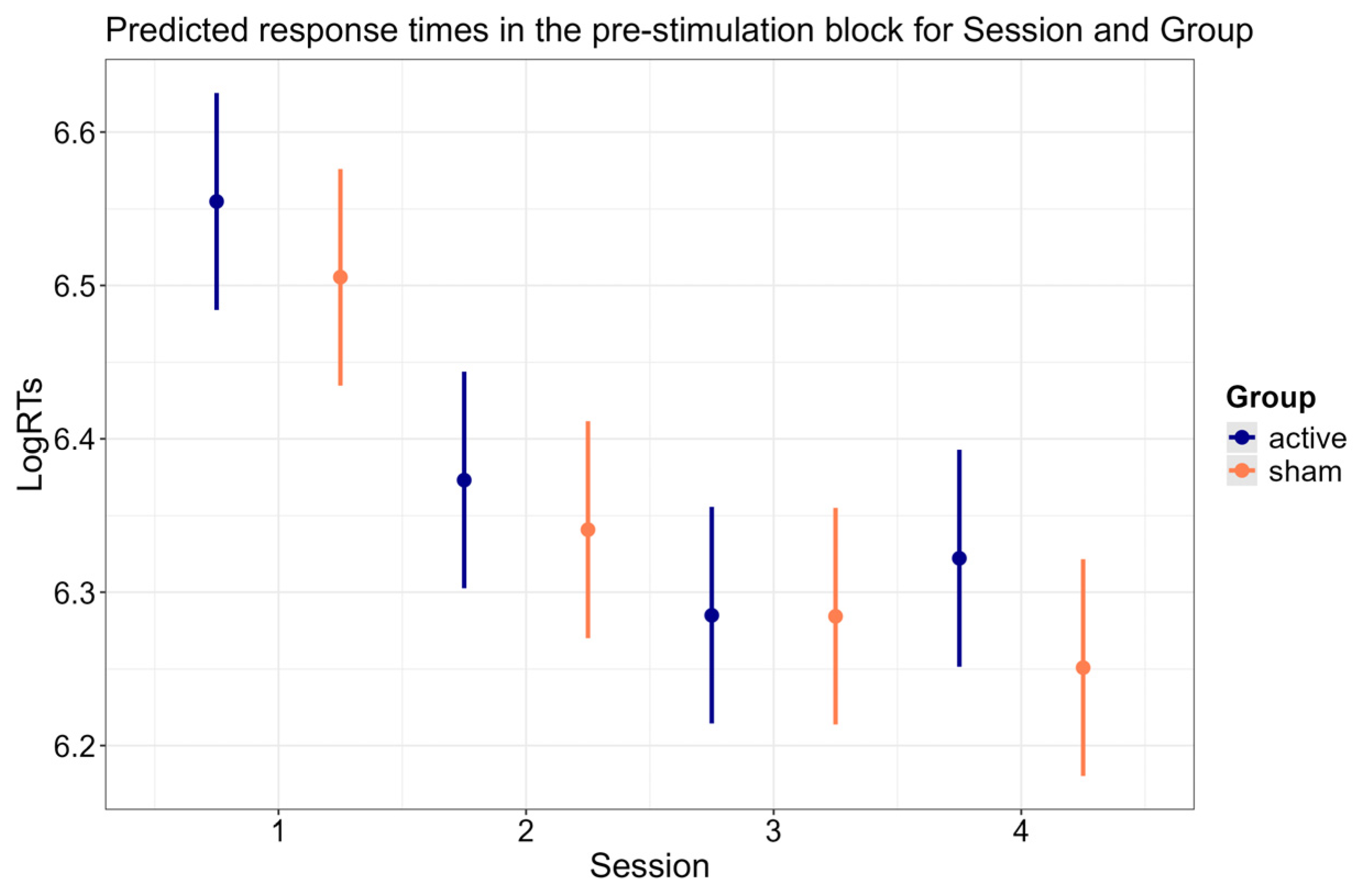
3.2.1. Session × Group Interaction
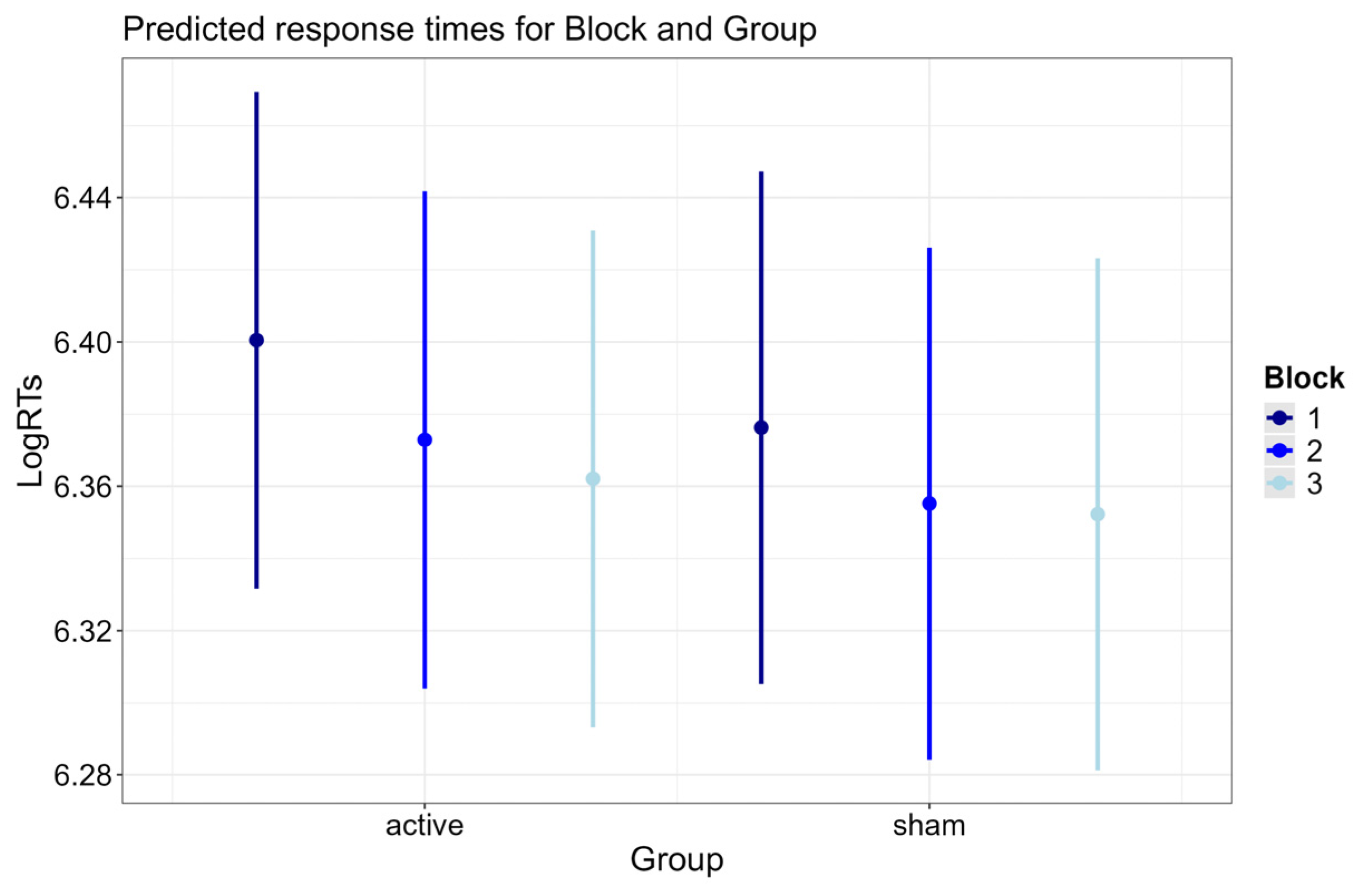
3.2.2. Block × Group Interaction
3.2.3. Block × Session × Group Interaction
3.3. Long-Term Effects of Stimulation
3.3.1. Accuracy
3.3.2. Reaction Times (RTs)
3.4. Session × Group Interaction
4. Discussion
4.1. Effects on RTs and Not Accuracy
4.2. Short-Term Effects of γ-tACS Repetition (24 h)
4.3. Short-Term Effects of Online vs. Offline γ-tACS (30 min)
4.4. Short-Term Effects of WM Load
4.5. Long-Term Effects of γ-tACS
4.6. Caveats and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beliaeva, V.; Savvateev, I.; Zerbi, V.; Polania, R. Toward integrative approaches to study the causal role of neural oscillations via transcranial electrical stimulation. Nat. Commun. 2021, 12, 2243. [Google Scholar] [CrossRef] [PubMed]
- Polanía, R.; Nitsche, M.A.; Ruff, C.C. Studying and modifying brain function with non-invasive brain stimulation. Nat. Neurosci. 2018, 21, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Vöröslakos, M.; Kronberg, G.; Henin, S.; Krause, M.R.; Huang, Y.; Opitz, A.; Mehta, A.; Pack, C.C.; Krekelberg, B.; et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat. Commun. 2018, 9, 5092. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Nguyen, J.A.; Reinhart, R.M.G. Synchronizing Brain Rhythms to Improve Cognition. Annu. Rev. Med. 2021, 72, 29–43. [Google Scholar] [CrossRef]
- Reinhart, R.M.G.; Nguyen, J.A. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat. Neurosci. 2019, 22, 820–827. [Google Scholar] [CrossRef]
- Glahn, D.; Kim, J.; Cohen, M.; Poutanen, V.-P.; Therman, S.; Bava, S.; Van Erp, T.; Manninen, M.; Huttunen, M.; Lönnqvist, J. Maintenance and manipulation in spatial working memory: Dissociations in the prefrontal cortex. Neuroimage 2002, 17, 201–213. [Google Scholar] [CrossRef]
- Cannon, T.D.; Glahn, D.C.; Kim, J.; Van Erp, T.G.M.; Karlsgodt, K.; Cohen, M.S.; Nuechterlein, K.H.; Bava, S.; Shirinyan, D. Dorsolateral Prefrontal Cortex Activity During Maintenance and Manipulation of Information in Working Memory in Patients with Schizophrenia. Arch. Gen. Psychiatry 2005, 62, 1071–1080. [Google Scholar] [CrossRef]
- Roux, F.; Uhlhaas, P.J. Working memory and neural oscillations: α-γ versus θ-γ codes for distinct WM information? Trends Cogn. Sci. 2014, 18, 16–25. [Google Scholar] [CrossRef]
- Palva, J.M.; Monto, S.; Kulashekhar, S.; Palva, S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc. Nat. Acad. Sci. USA 2010, 107, 7580–7585. [Google Scholar] [CrossRef]
- Bhattacharya, J.; Petsche, H.; Feldmann, U.; Rescher, B. EEG Gamma-Band Phase Synchronization between Posterior and Frontal Cortex during Mental Rotation in Humans. Neurosci. Lett. 2001, 311, 29–32. [Google Scholar] [CrossRef]
- Gruber, T.; Müller, M.M.; Keil, A.; Elbert, T. Selective Visual-Spatial Attention Alters Induced Gamma Band Responses in the Human EEG. Clin. Neurophysiol. 1999, 110, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, X.; Lv, Y.; Sun, J.; Tong, S. Mental Rotation Process for Mirrored and Identical Stimuli: A Beta-Band ERD Study. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 4948–4951. [Google Scholar] [CrossRef]
- Tallon-Baudry, C. Oscillatory Synchrony and Human Visual Cognition. J. Physiol. 2003, 97, 355–363. [Google Scholar] [CrossRef] [PubMed]
- von Stein, A.; Sarnthein, J. Different Frequencies for Different Scales of Cortical Integration: From Local Gamma to Long Range Alpha/Theta Synchronization. Int. J. Psychophysiol. 2000, 38, 301–313. [Google Scholar] [CrossRef]
- Coccaro, A.; Di Bono, M.G.; Maffei, A.; Orefice, C.; Lievore, R.; Mammarella, I.; Liotti, M. Resting state dynamic reconfiguration of spatial attention cortical networks and visuospatial functioning in non-verbal learning disability (NVLD): A HD-EEG Investigation. Brain Sci. 2023, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.C.; Hoy, K.E.; Enticott, P.G.; Daskalakis, Z.J.; Fitzgerald, P.B. Improving working memory: The effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 2011, 4, 84–89. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Vanderhasselt, M.A. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain Cogn. 2014, 86, 1–9. [Google Scholar] [CrossRef]
- Veldema, J.; Gharabaghi, A.; Jansen, P. Non-invasive brain stimulation in modulation of mental rotation ability: A systematic review and meta-analysis. Eur. J. Neurosci. 2021, 54, 7493–7512. [Google Scholar] [CrossRef]
- Hill, A.T.; Fitzgerald, P.B.; Hoy, K.E. Effects of Anodal Transcranial Direct Current Stimulation on Working Memory: A Systematic Review and Meta-Analysis of Findings From Healthy and Neuropsychiatric Population. Brain Stimul. 2016, 9, 197–208. [Google Scholar] [CrossRef]
- Grover, S.; Fayzullina, R.; Bullard, B.M.; Levina, V.; Reinhart, R.M. A meta-analysis suggests that tACS improves cognition in healthy, aging, and psychiatric populations. Sci. Transl. Med. 2023, 15, eabo2044. [Google Scholar] [CrossRef]
- Hoy, K.E.; Bailey, N.; Arnold, S.; Windsor, K.; John, J.; Daskalakis, Z.J.; Fitzgerald, P.B. The effect of γ-tACS on working memory performance in healthy controls. Brain Cogn. 2015, 101, 51–56. [Google Scholar] [CrossRef]
- Palm, U.; Baumgartner, C.; Hoffmann, L.; Padberg, F.; Hasan, A.; Strube, W.; Papazova, I. Single session gamma transcranial alternating stimulation does not modulate working memory in depressed patients and healthy controls. Neurophysiol. Clin. 2022, 52, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Kvašňák, E.; Magyarová, E.; Domankuš, M.; Tesař, M.; Kymplová, J.; Fetissov, V.; Abubaker, M.; Al Qasem, W. 10 Minutes Frontal 40 Hz tACS-Effects on Working Memory Tested by Luck-Vogel Task. Behav. Sci. 2022, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Pahor, A.; Jaušovec, N. The Effects of Theta and Gamma tACS on Working Memory and Electrophysiology. Front. Hum. Neurosci. 2018, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Al Qasem, W.; Abubaker, M.; Pilátová, K.; Ježdík, P.; Kvašňák, E. Improving working memory by electrical stimulation and cross-frequency coupling. Mol. Brain 2024, 17, 72. [Google Scholar] [CrossRef]
- Abubaker, M.; Al Qasem, W.; Pilátová, K.; Ježdík, P.; Kvašňák, E. Theta-gamma-coupling as predictor of working memory performance in young and elderly healthy people. Mol. Brain 2024, 17, 74. [Google Scholar] [CrossRef]
- Kehler, L.; Francisco, C.O.; Uehara, M.A.; Moussavi, Z. The effect of transcranial alternating current stimulation (tACS) on cognitive function in older adults with dementia. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2020; pp. 3649–3653. [Google Scholar] [CrossRef]
- Moussavi, Z.; Kimura, K.; Kehler, L.; de Oliveira Francisco, C.; Lithgow, B. A Novel Program to improve cognitive function in individuals with dementia using Transcranial Alternating Current Stimulation (tACS) and tutored Cognitive exercises. Front. Aging 2021, 2, 632545. [Google Scholar] [CrossRef]
- Grover, S.; Wen, W.; Viswanathan, V.; Gill, C.T.; Reinhart, R.M.G. Long-lasting, dissociable improvements in working memory and long-term memory in older adults with repetitive neuromodulation. Nat. Neurosci. 2022, 25, 1237–1246. [Google Scholar] [CrossRef]
- Okamoto, M.; Dan, H.; Sakamoto, K.; Takeo, K.; Shimizu, K.; Kohno, S.; Oda, I.; Isobe, S.; Suzuki, T.; Kohyama, K.; et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 2014, 21, 99–111. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P.; Bolker, M.B. Package “lme4”. Convergence 2015, 12, 2. [Google Scholar]
- Voeten, C.C. Buildmer: Stepwise Elimination and Term Reordering for Mixed-Effects Regression. R Package Version 2.4. 2024. Available online: https://CRAN.R-project.org/package=buildmer (accessed on 15 February 2025).
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Proceedings of the Second International Symposium on Information Theory; Petrov, B.N., Caski, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Bozdogan, H. Model selection and Akaike’s information criterion (AIC): The general theory and its analytical extensions. Psychometrika 1987, 52, 345–370. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Newcastle upon Tyne, UK, 2018. [Google Scholar]
- Brehm, L.; Alday, P.M. Contrast coding choices in a decade of mixed models. J. Mem. Lang. 2022, 125, 104334. [Google Scholar] [CrossRef]
- Lenth, R.V.; Banfai, B.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; et al. Emmeans: Estimated Marginal Means, aka Least-Squares Means. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 15 June 2023).
- Bonferroni, C. Teoria Statistica delle Classi e Calcolo delle Probabilità; Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali Di Firenze: Firenze, Italia, 1936; Volume 8, pp. 3–62. [Google Scholar]
- Clancy, K.J.; Baisley, S.K.; Albizu, A.; Kartvelishvili, N.; Ding, M.; Li, W. Lasting connectivity increase and anxiety reduction via transcranial alternating current stimulation. Soc. Cognit. Affect. Neurosci. 2018, 13, 1305–1316. [Google Scholar] [CrossRef]
| Participant Variables | Active | Sham | Comparison |
|---|---|---|---|
| N | 18 | 17 | |
| Age (years) | 21.61 ± 1.29 | 24.71 ± 6.72 | ns * |
| Education (years) | 16.06 ± 1.26 | 17 ± 2.45 | ns * |
| Male (N, %) | 2 (11%) | 5 (29%) | ns $ |
| Right-Handed (N, %) | 17 (94%) | 13 (76%) | ns $ |
| Active | Sham | |||||
|---|---|---|---|---|---|---|
| Block 1 | Block 2 | Block 3 | Block 1 | Block 2 | Block 3 | |
| Session 1 | 0.85 ± 0.05 | 0.84 ± 0.06 | 0.84 ± 0.08 | 0.85 ± 0.07 | 0.85 ± 0.08 | 0.86 ± 0.09 |
| Session 2 | 0.85 ± 0.08 | 0.84 ± 0.07 | 0.85 ± 0.07 | 0.85 ± 0.09 | 0.86 ± 0.08 | 0.85 ± 0.10 |
| Session 3 | 0.87 ± 0.07 | 0.85 ± 0.08 | 0.84 ± 0.08 | 0.87 ± 0.08 | 0.85 ± 0.07 | 0.86 ± 0.09 |
| Follow-up | 0.86 ± 0.07 | 0.86 ± 0.09 | ||||
| Active | Sham | |||||
|---|---|---|---|---|---|---|
| Block 1 | Block 2 | Block 3 | Block 1 | Block 2 | Block 3 | |
| Session 1 | 726 ± 212 | 690 ± 209 | 663 ± 200 | 691 ± 217 | 653 ± 215 | 647 ± 210 |
| Session 2 | 607 ± 190 | 590 ± 191 | 585 ± 190 | 589 ± 193 | 586 ± 190 | 581 ± 188 |
| Session 3 | 558 ± 176 | 559 ± 189 | 568 ± 194 | 559 ± 177 | 564 ± 188 | 569 ± 184 |
| Follow-up | 580 ± 182 | 540 ± 171 | ||||
| Chisq | Df | p-Value | |
|---|---|---|---|
| Intercept | 430.545 | 1 | <0.001 |
| WM Load | 1147.786 | 3 | <0.001 |
| Block | 18.983 | 2 | <0.001 |
| Session | 9.152 | 2 | 0.010 |
| WM Load × Block | 29.120 | 6 | <0.001 |
| Block × Session | 8.021 | 4 | 0.091 |
| Chisq | Df | p-Value | |
|---|---|---|---|
| Intercept | 64,099.766 | 1 | <0.001 |
| WM Load | 6237.970 | 3 | <0.001 |
| Session | 5154.541 | 2 | <0.001 |
| Block | 155.666 | 2 | <0.001 |
| Group | 0.122 | 1 | 0.727 |
| Session × Block | 225.740 | 4 | <0.001 |
| Session × Group | 97.406 | 2 | <0.001 |
| WM Load × Block | 56.954 | 6 | <0.001 |
| WM Load × Session | 53.370 | 6 | <0.001 |
| Block × Group | 6.681 | 2 | 0.035 |
| Block × Session × Group | 8.741 | 4 | 0.068 |
| Chisq | Df | p-Value | |
|---|---|---|---|
| Intercept | 465.179 | 1 | <0.001 |
| WM Load | 513.608 | 3 | <0.001 |
| Session | 23.283 | 3 | <0.001 |
| Chisq | Df | p-Value | |
|---|---|---|---|
| Intercept | 63,235.497 | 1 | <0.001 |
| Session | 3670.141 | 3 | <0.001 |
| WM Load | 3383.867 | 3 | <0.001 |
| Group | 0.576 | 1 | 0.448 |
| Session × Group | 61.837 | 3 | <0.001 |
| Session × WM Load | 30.470 | 9 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosato, M.; Sala, M.; Coccaro, A.; Cutini, S.; Liotti, M. Repetitive Gamma-tACS Improves the Reaction Times of Healthy Young Adults in a Visuospatial Working Memory Task: A Randomized Study. Brain Sci. 2025, 15, 343. https://doi.org/10.3390/brainsci15040343
Rosato M, Sala M, Coccaro A, Cutini S, Liotti M. Repetitive Gamma-tACS Improves the Reaction Times of Healthy Young Adults in a Visuospatial Working Memory Task: A Randomized Study. Brain Sciences. 2025; 15(4):343. https://doi.org/10.3390/brainsci15040343
Chicago/Turabian StyleRosato, Miriam, Marco Sala, Ambra Coccaro, Simone Cutini, and Mario Liotti. 2025. "Repetitive Gamma-tACS Improves the Reaction Times of Healthy Young Adults in a Visuospatial Working Memory Task: A Randomized Study" Brain Sciences 15, no. 4: 343. https://doi.org/10.3390/brainsci15040343
APA StyleRosato, M., Sala, M., Coccaro, A., Cutini, S., & Liotti, M. (2025). Repetitive Gamma-tACS Improves the Reaction Times of Healthy Young Adults in a Visuospatial Working Memory Task: A Randomized Study. Brain Sciences, 15(4), 343. https://doi.org/10.3390/brainsci15040343






