Association of TRPV1 and the SIRT3/SOD2 Signaling Pathway in Mononuclear Cells and Astrocyte-Derived Extracellular Vesicles in Patients with Schizophrenia
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Clinical Data Collection
2.3. Extraction of PBMCs
2.4. Extraction of ADEs
2.5. Western Blot
2.6. Identification of ADEs
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Identification of Exosomes
3.3. Differences in the TRPV1 Expressions and Partial Oxidative Stress Indicators in the PBMCs and ADEs
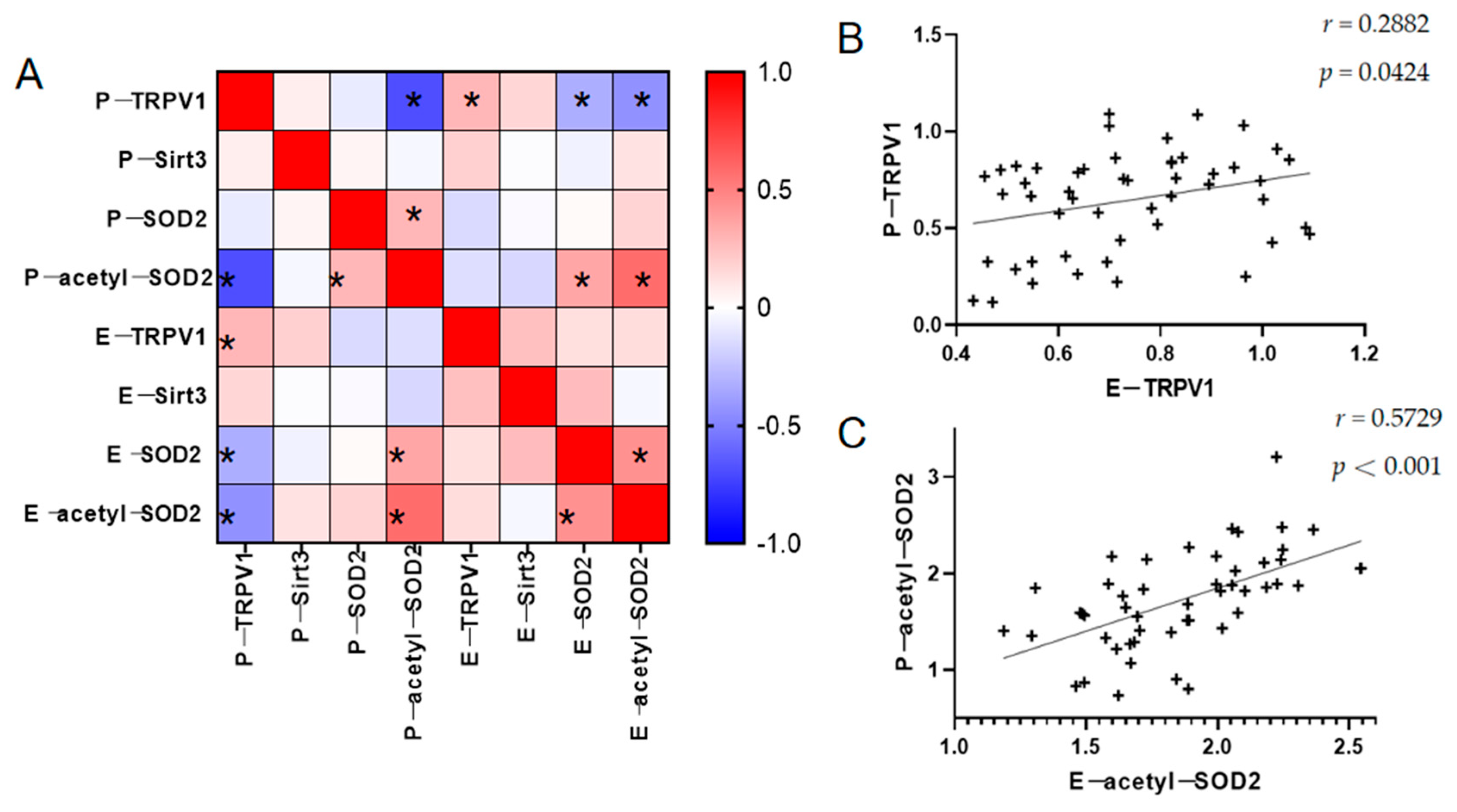
3.4. Relationship Between TRPV1 and the Partial Oxidative Stress Indicators in the PBMCs and ADEs
3.5. Relationship Between the TRPV1 Expressions and the Partial Oxidative Stress Indicators in the PBMCs and ADEs with Clinical Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Wang, Y.; Wang, H.; Liu, Z.; Yu, X.; Yan, J.; Yu, Y.; Kou, C.; Xu, X.; Lu, J.; et al. Prevalence of mental disorders in China: A cross-sectional epidemiological study. Lancet Psychiatry 2019, 6, 211–224. [Google Scholar]
- Zhang, H.; Wang, Y.; Hu, Y.; Zhu, Y.; Zhang, T.; Wang, J.; Ma, K.; Shi, C.; Yu, X.; Li, C. Meta-analysis of cognitive function in Chinese first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) profile of impairment. Gen. Psychiatry 2019, 32, e100043. [Google Scholar]
- Lin, P.; Sun, J.; Lou, X.; Li, D.; Shi, Y.; Li, Z.; Ma, P.; Chen, S.; Jin, W.; Liu, S.; et al. Consensus on potential biomarkers developed for use in clinical tests for schizophrenia. Gen. Psychiatry 2022, 35, e100685. [Google Scholar]
- Sotiropoulos, M.G.; Poulogiannopoulou, E.; Delis, F.; Dalla, C.; Antoniou, K.; Kokras, N. Innovative screening models for the discovery of new schizophrenia drug therapies: An integrated approach. Expert. Opin. Drug Discov. 2021, 16, 791–806. [Google Scholar]
- Iglesias, L.P.; Aguiar, D.C.; Moreira, F.A. TRPV1 blockers as potential new treatments for psychiatric disorders. Behav. Pharmacol. 2022, 33, 2–14. [Google Scholar]
- Ngoc, K.H.; Kecskés, A.; Kepe, E.; Nabi, L.; Keeble, J.; Borbély, É.; Helyes, Z. Expression of the Transient Receptor Potential Vanilloid 1 ion channel in the supramammillary nucleus and the antidepressant effects of its antagonist AMG9810 in mice. Eur. Neuropsychopharmacol. 2023, 73, 96–107. [Google Scholar]
- Kirkedal, C.; Wegener, G.; Moreira, F.; Joca, S.R.L.; Liebenberg, N. A dual inhibitor of FAAH and TRPV1 channels shows dose-dependent effect on depression-like behaviour in rats. Acta Neuropsychiatr. 2017, 29, 324–329. [Google Scholar]
- Escelsior, A.; Sterlini, B.; Belvederi Murri, M.; Valente, P.; Amerio, A.; Brozolo, M.R.; Silva, B.P.; Amore, M. Transient receptor potential vanilloid 1 antagonism in neuroinflammation, neuroprotection and epigenetic regulation: Potential therapeutic implications for severe psychiatric disorders treatment. Psychiatr. Genet. 2020, 30, 39–48. [Google Scholar]
- Huang, J.; Huang, H.; Liu, M.; Yang, W.; Wang, H. Involvement of the TRPV1 receptor and the endocannabinoid system in schizophrenia. Brain Res. Bull. 2024, 215, 111007. [Google Scholar]
- Talebi, F.; Ghorbani, S.; Alizadeh, L.; Akhlaghi, F.; Sadat Moeeni, S.; Karimzadeh, F. Alteration in Neuregulin 1/ERbB4 in Absence Epilepsy: Regulatory Effect on TRPV1 Expression. Basic. Clin. Neurosci. 2022, 13, 777–788. [Google Scholar]
- Xu, S.; Hao, K.; Xiong, Y.; Xu, R.; Huang, H.; Wang, H. Capsaicin alleviates neuronal apoptosis and schizophrenia-like behavioral abnormalities induced by early life stress. Schizophrenia 2023, 9, 77. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, P.; Yuan, L.; Geng, Z.; Li, B.; Zhang, B. Neuroprotective effects of TRPV1 by targeting GDF11 in the Mpp+/MPTP-induced Parkinson’s disease model. Biochem. Biophys. Res. Commun. 2022, 623, 104–110. [Google Scholar] [CrossRef]
- Wang, W.; Sun, T. Impact of TRPV1 on Pathogenesis and Therapy of Neurodegenerative Diseases. Molecules 2024, 29, 181. [Google Scholar] [CrossRef]
- Cy, C.; Dz, L.; Jc, P.; Mc, K.; Yc, H.; Ws, L. Not Just a Bystander: The Emerging Role of Astrocytes and Research Tools in Studying Cognitive Dysfunctions in Schizophrenia. Int. J. Mol. Sci. 2021, 22, 5343. [Google Scholar] [CrossRef]
- Gao, P.; Jiang, Y.; Wu, H.; Sun, F.; Li, Y.; He, H.; Wang, B.; Lu, Z.; Hu, Y.; Wei, X.; et al. Inhibition of Mitochondrial Calcium Overload by SIRT3 Prevents Obesity- or Age-Related Whitening of Brown Adipose Tissue. Diabetes 2020, 69, 165–180. [Google Scholar] [CrossRef]
- Hao, K.; Chen, F.; Xu, S.; Xiong, Y.; Xu, R.; Huang, H.; Shu, C.; Wang, H.; Wang, G.; Reynolds, G.P. The role of SIRT3 in mediating the cognitive deficits and neuroinflammatory changes associated with a developmental animal model of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 130, 110914. [Google Scholar]
- Schober, A.L.; Wicki-Stordeur, L.E.; Murai, K.K.; Swayne, L.A. Foundations and implications of astrocyte heterogeneity during brain development and disease. Trends Neurosci. 2022, 45, 692–703. [Google Scholar] [CrossRef]
- Raghavan, V. Role of exosomes in psychiatric disorders. Asian J. Psychiatry 2017, 28, 78–79. [Google Scholar] [CrossRef]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Keefe, R.S.E.; Goldberg, T.E.; Harvey, P.D.; Gold, J.M.; Poe, M.P.; Coughenour, L. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res. 2004, 68, 283–297. [Google Scholar] [CrossRef]
- Jia, L.; Qiu, Q.; Zhang, H.; Chu, L.; Du, Y.; Zhang, J.; Zhou, C.; Liang, F.; Shi, S.; Wang, S.; et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimer’s Dement. 2019, 15, 1071–1080. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Yang, M.; Liu, J.; Zhang, Y.; Liu, D.; Zhang, X. Variations of plasma oxidative stress levels in male patients with chronic schizophrenia. Correlations with psychopathology and matrix metalloproteinase-9: A case-control study. BMC Psychiatry 2024, 24, 20. [Google Scholar] [CrossRef]
- Asevedo, E.; Gadelha, A.; Noto, C.; Mansur, R.B.; Zugman, A.; Belangero, S.I.; Berberian, A.A.; Scarpato, B.S.; Leclerc, E.; Teixeira, A.L.; et al. Impact of peripheral levels of chemokines, BDNF and oxidative markers on cognition in individuals with schizophrenia. J. Psychiatr. Res. 2013, 47, 1376–1382. [Google Scholar] [CrossRef]
- Fraguas, D.; Diaz-Caneja, C.M.; Ayora, M.; Hernández-Álvarez, F.; Rodríguez-Quiroga, A.; Recio, S.; Leza, J.C.; Arango, C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis (Publication with Expression of Concern). Schizophr. Bull. 2019, 45, 742–751. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Xie, Y.; Deng, Q.; Guo, M.; Li, X.; Xian, D.; Zhong, J. Proanthocyanidins: A novel approach to Henoch-Schonlein purpura through balancing immunity and arresting oxidative stress via TLR4/MyD88/NF-κB signaling pathway (Review). Exp. Ther. Med. 2023, 25, 300. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie Restriction Reduces Oxidative Stress by SIRT3-Mediated SOD2 Activation. Cell Metabolism 2010, 12, 662–667. [Google Scholar]
- Tao, R.; Coleman, M.C.; Pennington, J.D.; Ozden, O.; Park, S.-H.; Jiang, H.; Kim, H.-S.; Flynn, C.R.; Hill, S.; McDonald, W.H.; et al. Sirt3-Mediated Deacetylation of Evolutionarily Conserved Lysine 122 Regulates MnSOD Activity in Response to Stress. Mol. Cell. 2010, 40, 893–904. [Google Scholar]
- Ansari, A.; Rahman, M.S.; Saha, S.K.; Saikot, F.K.; Deep, A.; Kim, K.H. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 2017, 16, 4–16. [Google Scholar] [CrossRef]
- Tao, R.; Vassilopoulos, A.; Parisiadou, L.; Yan, Y.; Gius, D. Regulation of MnSOD enzymatic activity by Sirt3 connects the mitochondrial acetylome signaling networks to aging and carcinogenesis. Antioxid. Redox Signal. 2014, 20, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Bao, W.; Gao, Y.; Chen, J.; Song, G. Honokiol improves cognitive impairment in APP/PS1 mice through activating mitophagy and mitochondrial unfolded protein response. Chem. Biol. Interact. 2022, 351, 109741. [Google Scholar]
- Luo, H.; Peng, C.; Xu, X.; Peng, Y.; Shi, F.; Li, Q.; Dong, J.; Chen, M. The Protective Effects of Mogroside V Against Neuronal Damages by Attenuating Mitochondrial Dysfunction via Upregulating Sirtuin3. Mol. Neurobiol. 2022, 59, 2068–2084. [Google Scholar] [PubMed]
- Shen, Y.; Wu, Q.; Shi, J.; Zhou, S. Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson’s disease. Biomed. Pharmacother. 2020, 132, 110928. [Google Scholar]
- You, I.J.; Hong, S.I.; Ma, S.X.; Nguyen, T.L.; Kwon, S.H.; Lee, S.Y.; Jang, C.G. Transient receptor potential vanilloid 1 mediates cocaine reinstatement via the D1 dopamine receptor in the nucleus accumbens. J. Psychopharmacol. 2019, 33, 1491–1500. [Google Scholar]
- Tokumitsu, H.; Sakagami, H. Molecular Mechanisms Underlying Ca2+/Calmodulin-Dependent Protein Kinase Signal Transduction. Int. J. Mol. Sci. 2022, 23, 11025. [Google Scholar] [CrossRef]
- Öz, A.; Çinar, R.; Naziroğlu, M. TRPV1 stimulation increased oxidative neurotoxicity and apoptosis in the glia cell membrane but not in the perinuclear area: An evidence of TRPV1 subtype. Metab. Brain Dis. 2022, 37, 2291–2304. [Google Scholar]
- Kong, W.L.; Peng, Y.Y.; Peng, B.W. Modulation of neuroinflammation: Role and therapeutic potential of TRPV1 in the neuro-immune axis. Brain Behav. Immun. 2017, 64, 354–366. [Google Scholar]
- Baek, J.Y.; Jeong, J.Y.; Kim, K.I.; Won, S.-Y.; Chung, Y.C.; Nam, J.H.; Cho, E.J.; Ahn, T.-B.; Bok, E.; Shin, W.-H.; et al. Inhibition of Microglia-Derived Oxidative Stress by Ciliary Neurotrophic Factor Protects Dopamine Neurons In Vivo from MPP+ Neurotoxicity. Int. J. Mol. Sci. 2018, 19, 3543. [Google Scholar] [CrossRef]
- Zhang, X.; El Demerdash, N.; Falck, J.R.; Munnuri, S.; Koehler, R.C.; Yang, Z.J. The contribution of TRPV1 channel to 20-HETE-Aggravated ischemic neuronal injury. Prostaglandins Other Lipid Mediat. 2018, 137, 63–68. [Google Scholar]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [PubMed]
- Catts, V.S.; Wong, J.; Fillman, S.G.; Fung, S.J.; Weickert, C.S. Increased expression of astrocyte markers in schizophrenia: Association with neuroinflammation. Aust. N. Z. J. Psychiatry 2014, 48, 722–734. [Google Scholar]
- Panatier, A.; Theodosis, D.T.; Mothet, J.P.; Touquet, B.; Pollegioni, L.; Poulain, D.A.; Oliet, S.H. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 2006, 125, 775–784. [Google Scholar]
- de Oliveira Figueiredo, E.C.; Cali, C.; Petrelli, F.; Bezzi, P. Emerging evidence for astrocyte dysfunction in schizophrenia. Glia 2022, 70, 1585–1604. [Google Scholar] [PubMed]
- Williams, M.R.; Hampton, T.; Pearce, R.K.B.; Hirsch, S.R.; Ansorge, O.; Thom, M.; Maier, M. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur. Arch. Psych. Clin. Neurosci. 2013, 263, 41–52. [Google Scholar]
- Notter, T. Astrocytes in schizophrenia. Brain Neurosci. Adv. 2021, 5, 23982128211009148. [Google Scholar]
- Goetzl, E.J.; Wolkowitz, O.M.; Srihari, V.H.; Reus, V.I.; Goetzl, L.; Kapogiannis, D.; Heninger, G.R.; Mellon, S.H. Abnormal levels of mitochondrial proteins in plasma neuronal extracellular vesicles in major depressive disorder. Mol. Psychiatry 2021, 26, 7355–7362. [Google Scholar]
- Mustapic, M.; Eitan, E.; Werner, J.K., Jr.; Berkowitz, S.T.; Lazaropoulos, M.P.; Tran, J.; Goetzl, E.J.; Kapogiannis, D. Plasma extracellular vesicles enriched for neuronal origin: A potential window into brain pathologic processes. Front. Neurosci. 2017, 11, 278. [Google Scholar]
- Xie, X.H.; Xu, S.X.; Yao, L.; Chen, M.M.; Zhang, H.; Wang, C.; Nagy, C.; Liu, Z. Altered in vivo early neurogenesis traits in patients with depression: Evidence from neuron-derived extracellular vesicles and electroconvulsive therapy. Brain Stimul. 2024, 17, 19–28. [Google Scholar]
- Peres, F.F.; Levin, R.; Almeida, V.; Zuardi, A.W.; Hallak, J.E.; Crippa, J.A.; Abilio, V.C. Cannabidiol, among Other Cannabinoid Drugs, Modulates Prepulse Inhibition of Startle in the SHR Animal Model: Implications for Schizophrenia Pharmacotherapy. Front. Pharmacol. 2016, 7, 303. [Google Scholar]
- Ali, A.M.; Kunugi, H. Bee honey protects astrocytes against oxidative stress: A preliminary in vitro investigation. Neuropsychopharmacol. Rep. 2019, 39, 312–314. [Google Scholar] [PubMed]
- Piciu, F.; Balas, M.; Badea, M.A.; Cucu, D. TRP Channels in Tumoral Processes Mediated by Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1327. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, E.A.; Dmitrieva, E.M.; Parshukova, D.A.; Kazantseva, D.V.; Vasilieva, A.R.; Smirnova, L.P. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxid. Med. Cell Longev. 2021, 2021, 8881770. [Google Scholar] [PubMed]
- Do, K.Q.; Cabungcal, J.H.; Frank, A.; Steullet, P.; Cuenod, M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 2009, 19, 220–230. [Google Scholar]
- Kulak, A.; Steullet, P.; Cabungcal, J.H.; Werge, T.; Ingason, A.; Cuenod, M.; Do, K.Q. Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: Insights from animal models. Antioxid. Redox Signal. 2013, 18, 1428–1443. [Google Scholar]
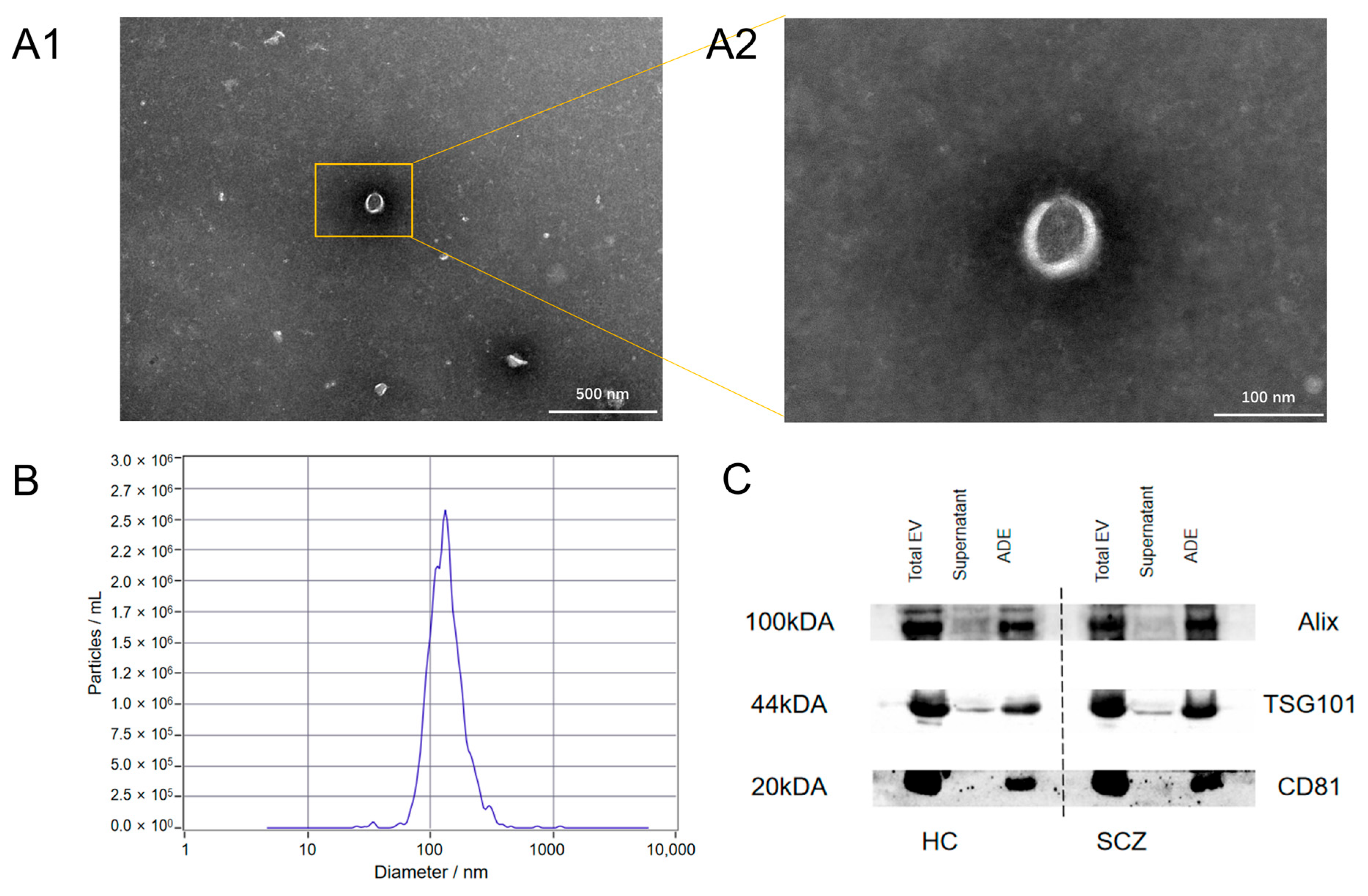

| Parameters | HC (n = 50) | SCZ (n = 50) | t/χ2/F | p |
|---|---|---|---|---|
| Sex (female/male) | 25/25 | 24/26 | 0.198 | 0.843 |
| Age (years) | 24.09 ± 4.15 | 24.74 ± 6.07 | 1.391 | 0.167 |
| Years of education (years) | 16.61 ± 1.35 | 12.18 ± 3.35 | 7.911 | <0.0001 |
| BMI (kg/m2) | 21.69 ± 2.72 | 22.83 ± 3.73 | 1.940 | 0.055 |
| Duration (months) | - | 48 (24, 96) | - | - |
| Number of episodes | - | 2 (1, 4) | - | - |
| PANSS scores | ||||
| Positive symptoms | - | 20.01 ± 4.82 | - | - |
| Negative symptoms | - | 17.99 ± 4.71 | - | - |
| General psychopathology | - | 38.25 ± 7.61 | - | - |
| Total | - | 83.04 ± 14.38 | - | - |
| BACS | ||||
| Verbal memory test (VM) | 53.03 ± 8.19 | 38.80 ± 11.00 | 7.655 | <0.0001 |
| Digit sequencing test (DS) | 25.70 ± 1.93 | 20.78 ± 4.40 | 7.352 | <0.0001 |
| Token motor task (TM) | 92.84 ± 8.01 | 66.86 ± 18.08 | 9.421 | <0.0001 |
| Category fluency (CF) | 28.56 ± 7.73 | 20.58 ± 6.58 | 6.245 | <0.0001 |
| Word fluency (WF) | 16.28 ± 4.80 | 12.06 ± 4.40 | 4.583 | <0.0001 |
| Symbol coding (SC) | 69.46 ± 9.00 | 51.84 ± 16.39 | 6.875 | <0.0001 |
| Tower of London (TL) | 19.50 ± 1.74 | 16.46 ± 4.48 | 4.593 | <0.0001 |
| Total score | 0 ± 1 | −1.91 ± 0.99 | 2.465 | <0.0001 |
| Parameters | PBMCs | ADEs | ||||||
|---|---|---|---|---|---|---|---|---|
| TRPV1 | Sirt3 | SOD2 | acetyl-SOD2 | TRPV1 | Sirt3 | SOD2 | acetyl-SOD2 | |
| PANSS | ||||||||
| Total score | −0.0263 | 0.2779 | 0.2959 * | 0.0397 | −0.1799 | 0.0588 | −0.0460 | 0.1371 |
| Positive symptoms | 0.3145 *# | −0.4231 **# | 0.0031 | −0.2458 | 0.0751 | −0.3137 * | −0.0788 | 0.0505 |
| Negative symptoms | −0.2991 **# | −0.0314 | 0.2654 | 0.2822 *# | −0.3255 *# | −0.2643 | −0.0331 | 0.1713 |
| General psychopathology | −0.1151 | 0.2418 | 0.3061 *# | 0.08187 | −0.1961 | 0.1088 | 0.0087 | 0.1436 |
| BACS | ||||||||
| VB | 0.5532 ****# | 0.0833 | −0.1334 | −0.3733 ** | 0.6825 ***# | −0.0943 | −0.0505 | −0.0641 |
| DS | 0.1943 | 0.2214 | 0.0140 | −0.0622 | 0.3354 * | −0.0855 | −0.0985 | 0.0033 |
| TM | 0.6903 ****# | −0.0194 | −0.0566 | −0.3794 **# | 0.0729 | −0.1075 | −0.1883 | −0.2403 |
| CF | 0.6681 ****# | −0.0131 | −0.1118 | −0.6618 ****# | 0.212 | 0.0922 | −0.1669 | −0.2969 * |
| WF | 0.5791 ****# | 0.0602 | −0.0290 | −0.4173 **# | 0.1972 | −0.1590 | −0.3218 * | −0.3432 * |
| SC | 0.3038 * | −0.1401 | 0.2108 | −0.278 | −0.2011 | 0.1161 | −0.2709 | −0.2889 * |
| TL | 0.4753 ***# | 0.1696 | 0.0306 | −0.3054 * | −0.0109 | 0.1191 | −0.1914 | −0.2252 |
| Total score | 0.8658 ****# | 0.0447 | −0.0551 | −0.6401 ****# | 0.2929 * | 0.0611 | −0.3042 * | −0.3986 **# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Liu, H.; Shu, C.; Li, Y.; Wang, S.; Xiong, Y.; Chen, F.; Wang, X.; Huang, H.; Liu, Z.; et al. Association of TRPV1 and the SIRT3/SOD2 Signaling Pathway in Mononuclear Cells and Astrocyte-Derived Extracellular Vesicles in Patients with Schizophrenia. Brain Sci. 2025, 15, 339. https://doi.org/10.3390/brainsci15040339
Xu R, Liu H, Shu C, Li Y, Wang S, Xiong Y, Chen F, Wang X, Huang H, Liu Z, et al. Association of TRPV1 and the SIRT3/SOD2 Signaling Pathway in Mononuclear Cells and Astrocyte-Derived Extracellular Vesicles in Patients with Schizophrenia. Brain Sciences. 2025; 15(4):339. https://doi.org/10.3390/brainsci15040339
Chicago/Turabian StyleXu, Rui, Hao Liu, Chang Shu, Yuan Li, Shijing Wang, Ying Xiong, Fashuai Chen, Xiaowei Wang, Huan Huang, Zhongchun Liu, and et al. 2025. "Association of TRPV1 and the SIRT3/SOD2 Signaling Pathway in Mononuclear Cells and Astrocyte-Derived Extracellular Vesicles in Patients with Schizophrenia" Brain Sciences 15, no. 4: 339. https://doi.org/10.3390/brainsci15040339
APA StyleXu, R., Liu, H., Shu, C., Li, Y., Wang, S., Xiong, Y., Chen, F., Wang, X., Huang, H., Liu, Z., Wang, G., & Wang, H. (2025). Association of TRPV1 and the SIRT3/SOD2 Signaling Pathway in Mononuclear Cells and Astrocyte-Derived Extracellular Vesicles in Patients with Schizophrenia. Brain Sciences, 15(4), 339. https://doi.org/10.3390/brainsci15040339






