The Utilization of Systematic Reviews and Meta-Analyses in Stroke Guidelines
Abstract
1. Introduction
2. Methods
2.1. Literature Search, Screening, and Data Extraction
2.2. Statistical Analysis
3. Results
3.1. Summary of the Included Guidelines
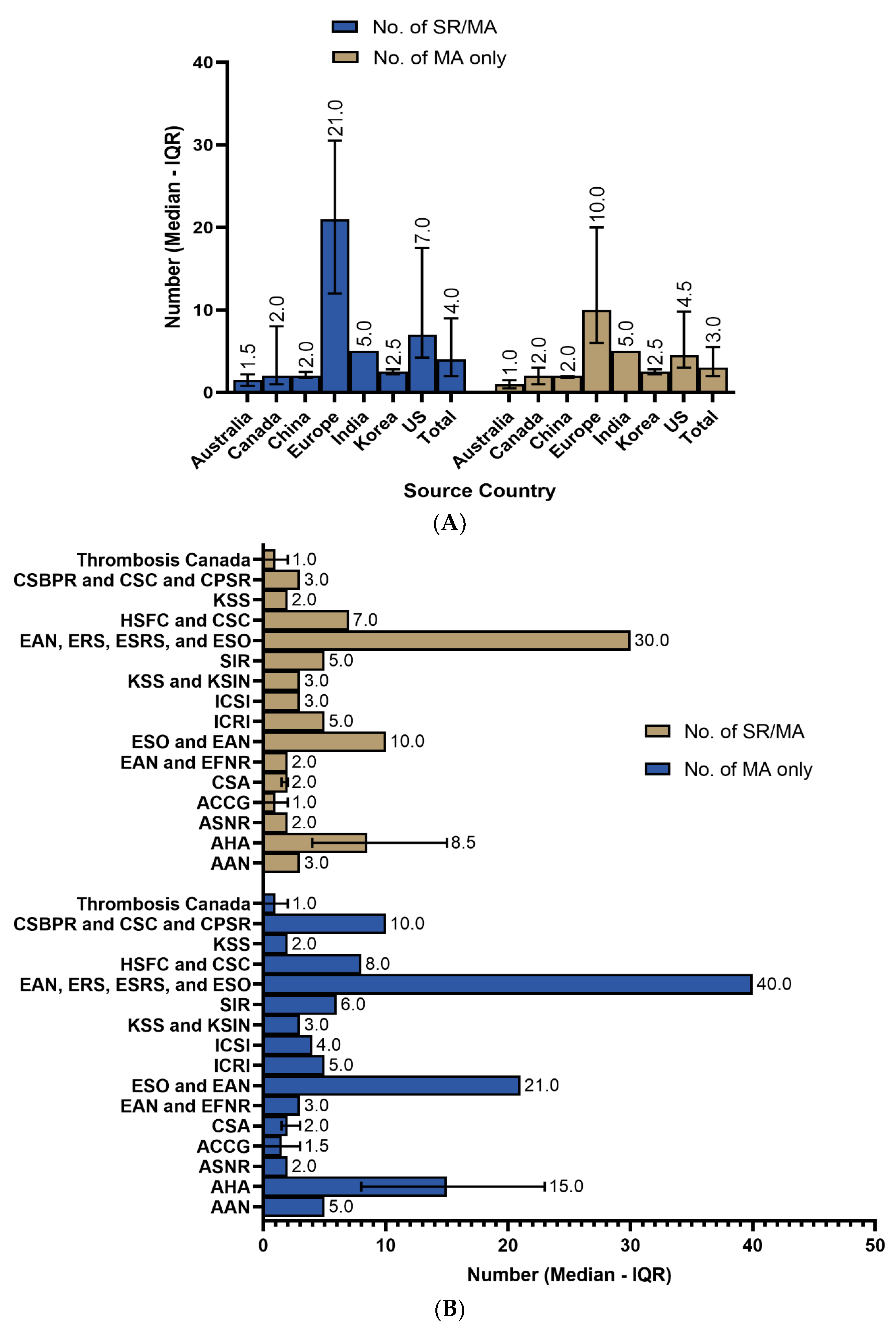
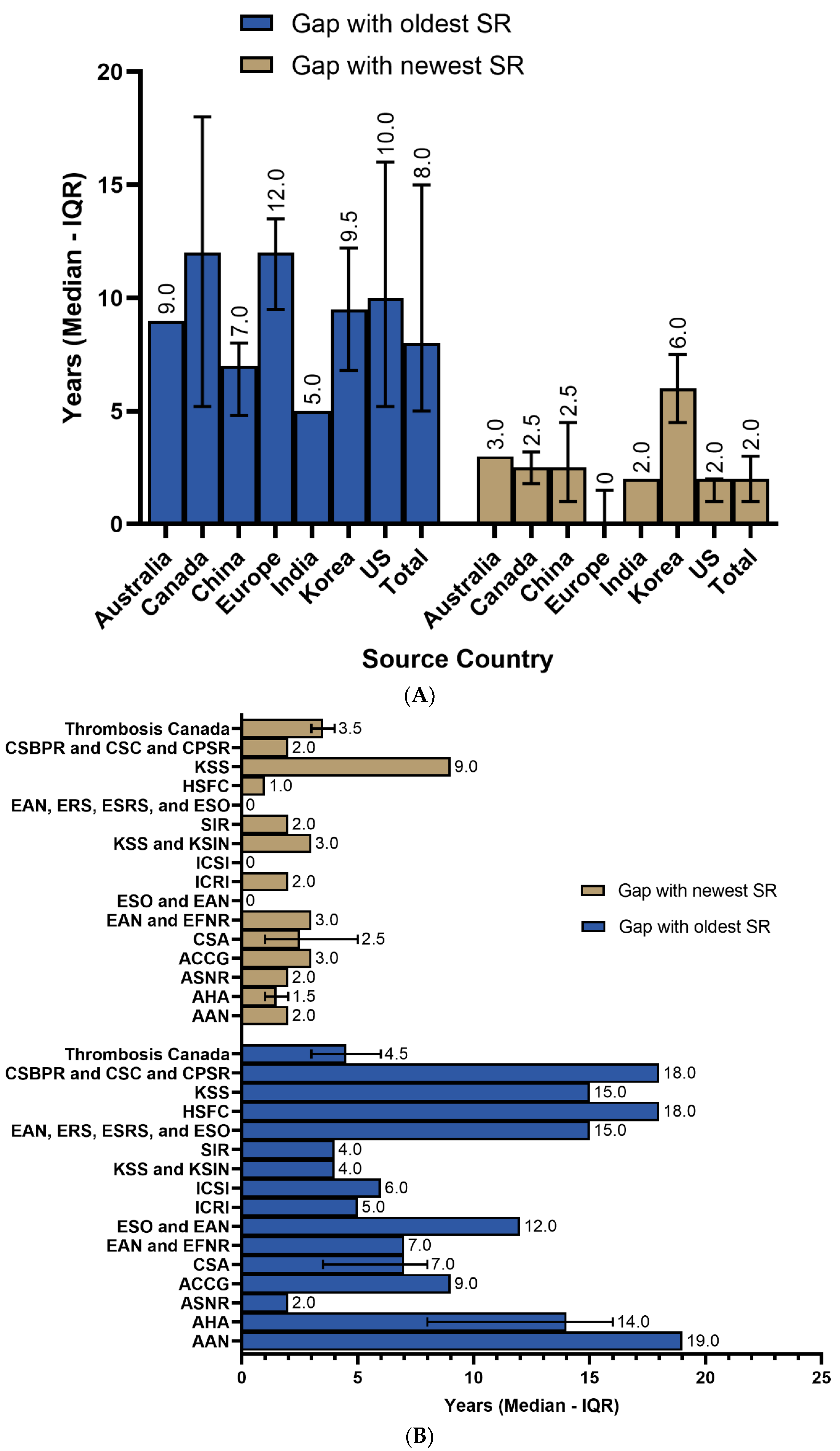
3.2. Systematic Reviews’ Utilization
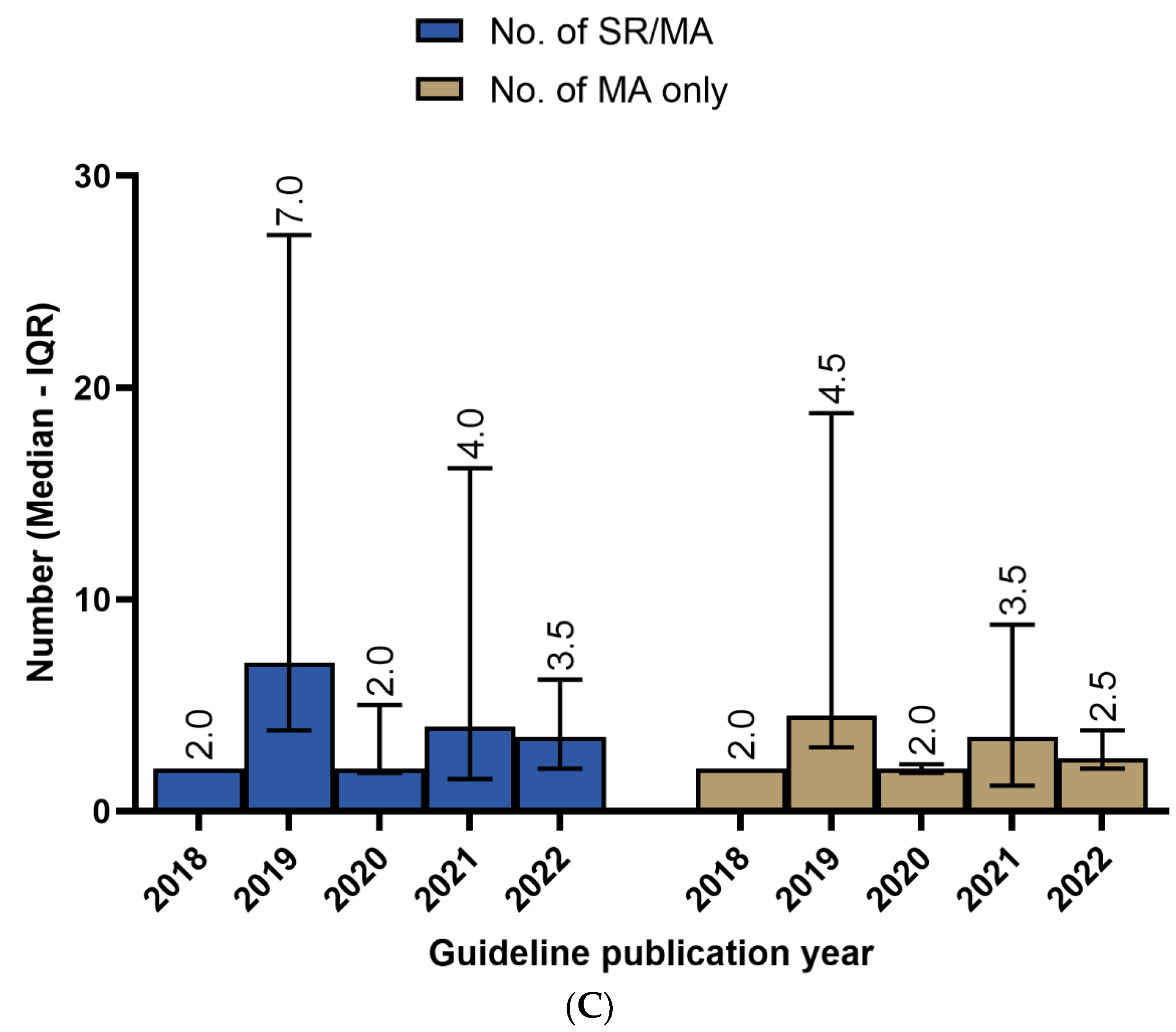
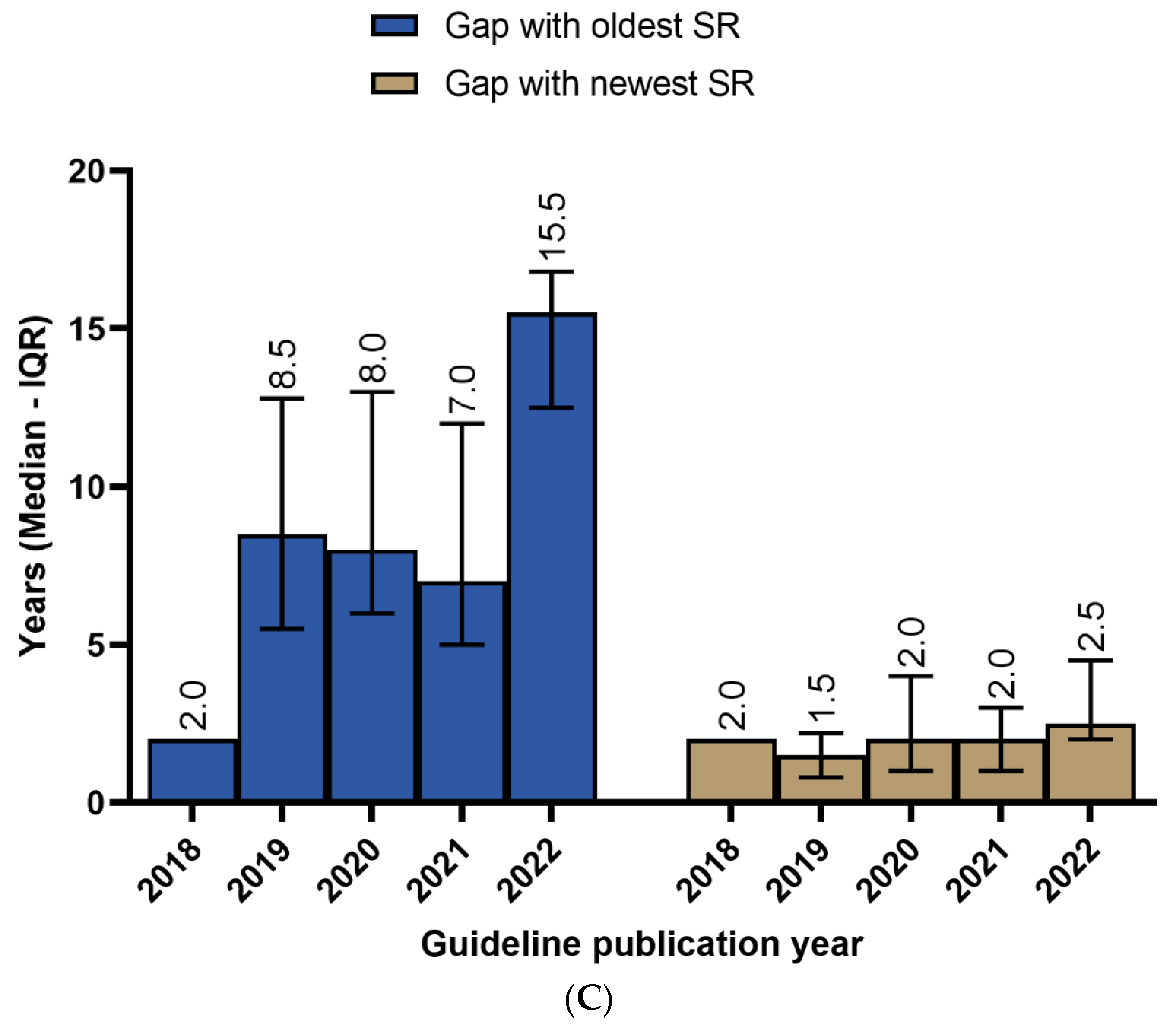
3.3. Recency of SRs/MAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Source Country | Overall (N = 27) |
|---|---|
| Australia | 2 (7.4%) |
| Canada | 5 (19%) |
| China | 4 (15%) |
| Europe | 3 (11%) |
| India | 1 (3.7%) |
| Korea | 2 (7.4%) |
| US | 10 (37%) |
| Guideline Publication Year | |
| 2018 | 1 (3.7%) |
| 2019 | 8 (30%) |
| 2020 | 8 (30%) |
| 2021 | 6 (22%) |
| 2022 | 4 (15%) |
| Issuing Body | |
| AAN | 1 (3.7%) |
| AHA | 6 (22%) |
| ASNR | 1 (3.7%) |
| ACCG | 2 (7.4%) |
| CSA | 4 (15%) |
| EAN and EFNR | 1 (3.7%) |
| ESO and EAN | 1 (3.7%) |
| ICRI | 1 (3.7%) |
| ICSI | 1 (3.7%) |
| KSS and KSIN | 1 (3.7%) |
| SIR | 1 (3.7%) |
| CSBPR, CSC, and CPSR | 1 (3.7%) |
| Thrombosis Canada | 3 (11%) |
| EAN, ERS, ESRS, and ESO | 1 (3.7%) |
| HSFC and CSC | 1 (3.7%) |
| KSS | 1 (3.7%) |
| Variable | Median | IQR |
|---|---|---|
| Number of systematic reviews and/or meta-analyses | 4.0 | 2–10 |
| Number of meta-analyses only | 3.0 | 2–6 |
| Gap with oldest (years) | 8.0 | 5–15 |
| Gap with newest (years) | 2.0 | 1–3 |
References
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Patel, R.A.; McMullen, P.W. Neuroprotection in the treatment of acute ischemic stroke. Prog. Cardiovasc. Dis. 2017, 59, 542–548. [Google Scholar] [CrossRef]
- Kadir, R.R.A.; Bayraktutan, U. Urokinase plasminogen activator: A potential thrombolytic agent for ischaemic stroke. Cell. Mol. Neurobiol. 2020, 40, 347–355. [Google Scholar] [CrossRef]
- Baird, A.E.; Jackson, R.; Jin, W. Tenecteplase for Acute Ischemic Stroke Treatment; Thieme Medical Publishers, Inc.: Ottawa, ON, Canada, 2021; pp. 28–38. [Google Scholar]
- Albright, D.; Alunday, R.; Schaller, E.; Tran, H.Q.; Crandall, C.S. Evaluating Target: Stroke guideline implementation on assessment and treatment times for patients with suspected stroke. Am. J. Emerg. Med. 2021, 42, 143–149. [Google Scholar] [CrossRef]
- Institute of Medicine Committee on Standards for Developing Trustworthy Clinical Practice G. Clinical Practice Guidelines We Can Trust; Graham, R., Mancher, M., Miller Wolman, D., Greenfield, S., Steinberg, E., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. Evid.-Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef]
- Pielenz, C.; Schneider, M.; Salveridou-Hof, E.; Flick, M.; Gaigl, G.; Khorikian-Ghazari, N.; Güler, D.; Halms, T.; Kapfhammer, A.; Lorenz, C.; et al. From conventional to living guidelines-faster updates for better informed guidance? A scoping review. Z Evid. Fortbild. Qual. Gesundhwes. 2022, 174, 20–31. [Google Scholar] [CrossRef]
- Akl, E.A.; Meerpohl, J.J.; Elliott, J.; Kahale, L.A.; Schünemann, H.J. Living systematic reviews: 4. Living guideline recommendations. J. Clin. Epidemiol. 2017, 91, 47–53. [Google Scholar] [CrossRef]
- Hill, K.; English, C.; Campbell, B.C.V.; McDonald, S.; Pattuwage, L.; Bates, P.; Lassig, C.; Turner, T. Feasibility of national living guideline methods: The Australian Stroke Guidelines. J. Clin. Epidemiol. 2022, 142, 184–193. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.2.1; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.r-project.org/ (accessed on 12 December 2023).
- Rcmdr: R Commander. Version 2.6-2. 2020. Available online: https://CRAN.R-project.org/package=Rcmdr (accessed on 22 March 2020).
- Vale, C.L.; Rydzewska, L.H.; Rovers, M.M.; Emberson, J.R.; Gueyffier, F.; Stewart, L.A. Uptake of systematic reviews and meta-analyses based on individual participant data in clinical practice guidelines: Descriptive study. BMJ 2015, 350, h1088. [Google Scholar] [CrossRef]
- Guerra-Farfan, E.; Garcia-Sanchez, Y.; Jornet-Gibert, M.; Nuñez, J.H.; Balaguer-Castro, M.; Madden, K. Clinical practice guidelines: The good, the bad, and the ugly. Injury 2023, 54 (Suppl. S3), S26–S29. [Google Scholar] [CrossRef]
- Tao, C.; Nogueira, R.G.; Zhu, Y.; Sun, J.; Han, H.; Yuan, G.; Wen, C.; Zhou, P.; Chen, W.; Zeng, G.; et al. Trial of Endovascular Treatment of Acute Basilar-Artery Occlusion. N. Engl. J. Med. 2022, 387, 1361–1372. [Google Scholar] [CrossRef]
- Jovin, T.G.; Li, C.; Wu, L.; Wu, C.; Chen, J.; Jiang, C.; Shi, Z.; Gao, Z.; Song, C.; Chen, W.; et al. Trial of Thrombectomy 6 to 24 Hours after Stroke Due to Basilar-Artery Occlusion. N. Engl. J. Med. 2022, 387, 1373–1384. [Google Scholar] [CrossRef]
- Adusumilli, G.; Kobeissi, H.; Ghozy, S.; Hardy, N.; Kallmes, K.M.; Hutchison, K.; Kallmes, D.F.; Brinjikji, W.; Albers, G.W.; Heit, J.J. Endovascular thrombectomy after acute ischemic stroke of the basilar artery: A meta-analysis of four randomized controlled trials. J. NeuroInterv. Surg. 2022, 15, e446–e451. [Google Scholar] [CrossRef]
- Hoffmann, F.; Allers, K.; Rombey, T.; Helbach, J.; Hoffmann, A.; Mathes, T.; Pieper, D. Nearly 80 systematic reviews were published each day: Observational study on trends in epidemiology and reporting over the years 2000–2019. J. Clin. Epidemiol. 2021, 138, 1–11. [Google Scholar] [CrossRef]
- Radu, R.A.; Gascou, G.; Machi, P.; Capirossi, C.; Costalat, V.; Cagnazzo, F. Current and future trends in acute ischemic stroke treatment: Direct-to-angiography suite, middle vessel occlusion, large core, and minor strokes. Eur. J. Radiol. Open 2023, 11, 100536. [Google Scholar] [CrossRef]
- Robbins, B.T.; Howington, G.T.; Swafford, K.; Zummer, J.; Woolum, J.A. Advancements in the management of acute ischemic stroke: A narrative review. J. Am. Coll. Emerg. Physicians Open 2023, 4, e12896. [Google Scholar] [CrossRef]
- Shekelle, P.; Woolf, S.; Grimshaw, J.M.; Schünemann, H.J.; Eccles, M.P. Developing clinical practice guidelines: Reviewing, reporting, and publishing guidelines; updating guidelines; and the emerging issues of enhancing guideline implementability and accounting for comorbid conditions in guideline development. Implement. Sci. 2012, 7, 62. [Google Scholar] [CrossRef]
- Kastner, M.; Bhattacharyya, O.; Hayden, L.; Makarski, J.; Estey, E.; Durocher, L.; Chatterjee, A.; Perrier, L.; Graham, I.D.; Straus, S.E.; et al. Guideline uptake is influenced by six implementability domains for creating and communicating guidelines: A realist review. J. Clin. Epidemiol. 2015, 68, 498–509. [Google Scholar] [CrossRef]
- Qaseem, A.; Forland, F.; Macbeth, F.; Ollenschläger, G.; Phillips, S.; van der Wees, P. Guidelines International Network: Toward international standards for clinical practice guidelines. Ann. Intern. Med. 2012, 156, 525–531. [Google Scholar] [CrossRef]
- Lunny, C.; Ramasubbu, C.; Puil, L.; Liu, T.; Gerrish, S.; Salzwedel, D.M.; Mintzes, B.; Wright, J.M. Over half of clinical practice guidelines use non-systematic methods to inform recommendations: A methods study. PLoS ONE 2021, 16, e0250356. [Google Scholar] [CrossRef]
- Hutchinson, N.; Moyer, H.; Zarin, D.A.; Kimmelman, J. The proportion of randomized controlled trials that inform clinical practice. eLife 2022, 11, e79491. [Google Scholar] [CrossRef]
- Hariton, E.; Locascio, J.J. Randomised controlled trials—the gold standard for effectiveness research: Study design: Randomised controlled trials. Bjog 2018, 125, 1716. [Google Scholar] [CrossRef]
- Tricoci, P.; Allen, J.M.; Kramer, J.M.; Califf, R.M.; Smith, S.C., Jr. Scientific evidence underlying the ACC/AHA clinical practice guidelines. Jama 2009, 301, 831–841. [Google Scholar] [CrossRef]
- da Costa, B.R.; Jüni, P. Systematic reviews and meta-analyses of randomized trials: Principles and pitfalls. Eur. Heart J. 2014, 35, 3336–3345. [Google Scholar] [CrossRef]
- Page, M.J.; Shamseer, L.; Tricco, A.C. Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst. Rev. 2018, 7, 32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghozy, S.; Kobeissi, H.; Amoukhteh, M.; Kadirvel, R.; Brinjikji, W.; Rabinstein, A.A.; Carpenter, C.R.; Kallmes, D.F. The Utilization of Systematic Reviews and Meta-Analyses in Stroke Guidelines. Brain Sci. 2024, 14, 728. https://doi.org/10.3390/brainsci14070728
Ghozy S, Kobeissi H, Amoukhteh M, Kadirvel R, Brinjikji W, Rabinstein AA, Carpenter CR, Kallmes DF. The Utilization of Systematic Reviews and Meta-Analyses in Stroke Guidelines. Brain Sciences. 2024; 14(7):728. https://doi.org/10.3390/brainsci14070728
Chicago/Turabian StyleGhozy, Sherief, Hassan Kobeissi, Melika Amoukhteh, Ramanathan Kadirvel, Waleed Brinjikji, Alejandro A. Rabinstein, Christopher R. Carpenter, and David F. Kallmes. 2024. "The Utilization of Systematic Reviews and Meta-Analyses in Stroke Guidelines" Brain Sciences 14, no. 7: 728. https://doi.org/10.3390/brainsci14070728
APA StyleGhozy, S., Kobeissi, H., Amoukhteh, M., Kadirvel, R., Brinjikji, W., Rabinstein, A. A., Carpenter, C. R., & Kallmes, D. F. (2024). The Utilization of Systematic Reviews and Meta-Analyses in Stroke Guidelines. Brain Sciences, 14(7), 728. https://doi.org/10.3390/brainsci14070728







