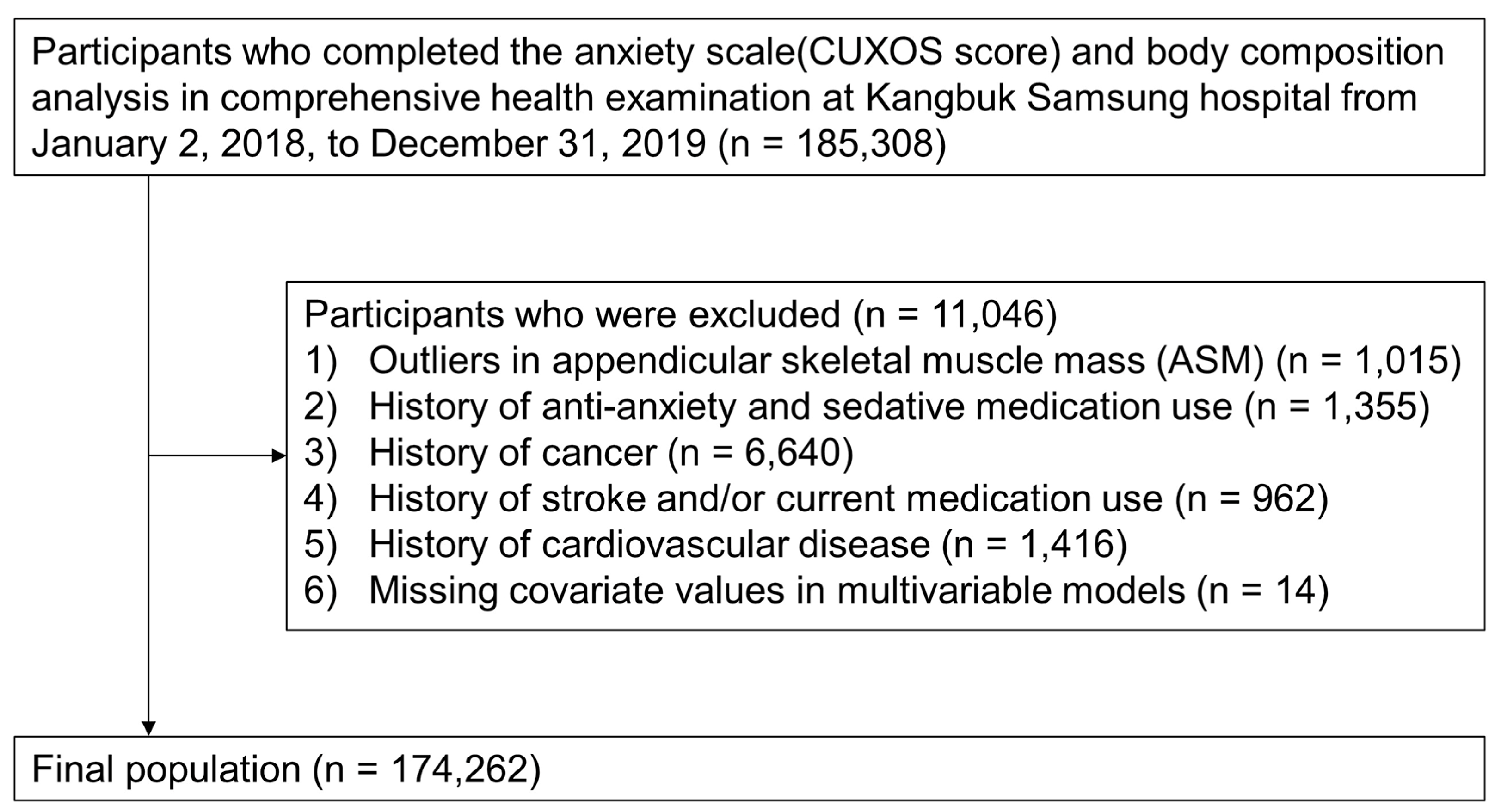
Exploring the Association between Elevated Anxiety Symptoms and Low Skeletal Muscle Mass among Asymptomatic Adults: A Population-Based Study in Republic of Korea
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association between Severity of Low Skeletal Muscle Mass and Anxiety Symptoms (CUXOS Score)
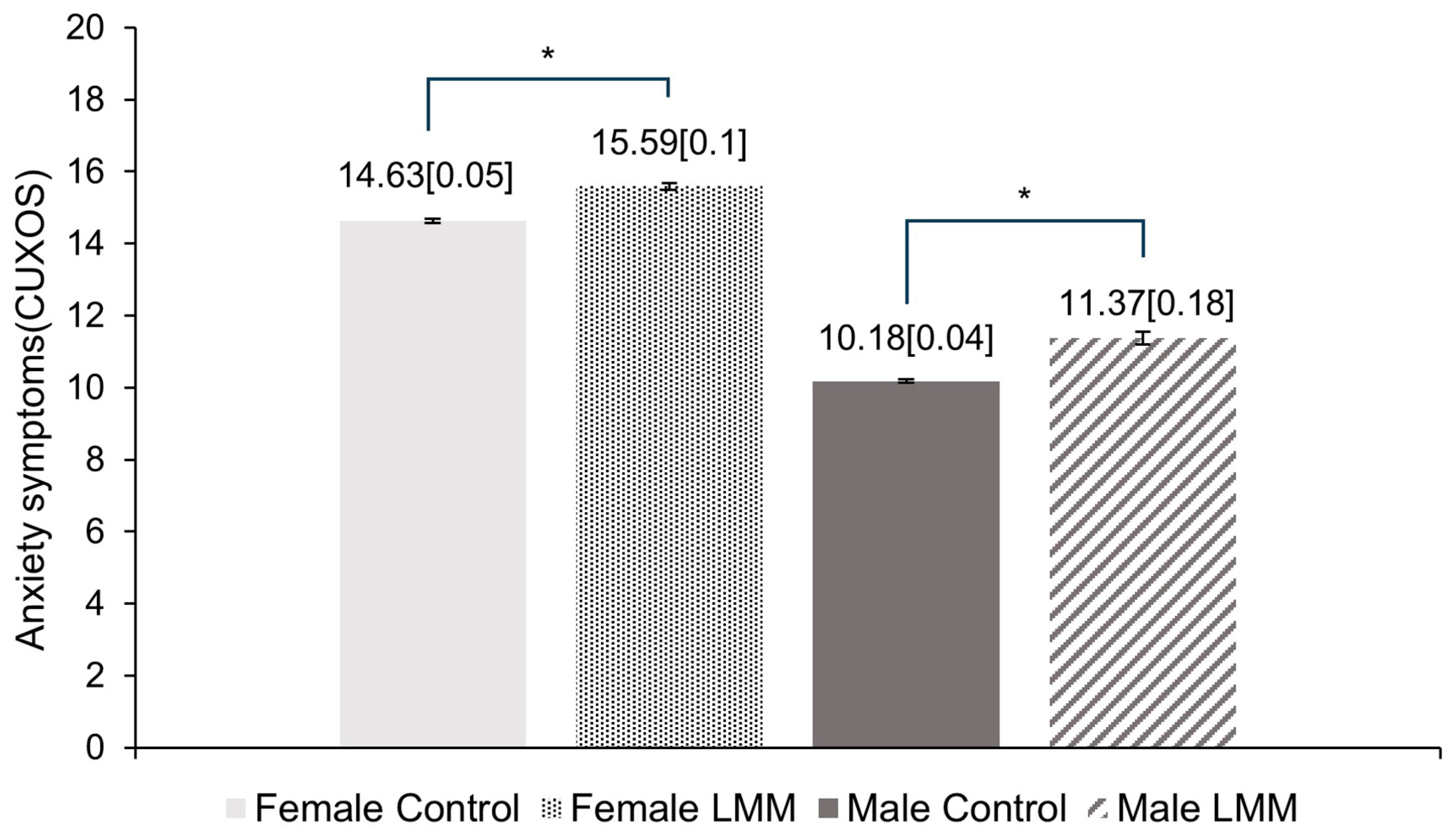
3.3. Comparison of Anxiety Level between the Control Group and Sarcopenia Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Alajos, P.; Peter, H.J.; Szilard, V.; Gabriella, P. Sarcopenia-2021 Pathophysiology, diagnosis, therapy. Orvosi Hetil. 2021, 162, 3–12. [Google Scholar]
- Borges, T.C.; Gomes, T.L.N.; Pimentel, G.D. Sarcopenia as a predictor of nutritional status and comorbidities in hospitalized patients with cancer: A cross-sectional study. Nutrition 2020, 73, 110703. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, J.; Geerlings, M.A.; Reijnierse, E.M.; Phassouliotis, C.; Lim, W.K.; Maier, A.B. Prevalence of sarcopenia as a comorbid disease: A systematic review and meta-analysis. Exp. Gerontol. 2020, 131, 110801. [Google Scholar] [CrossRef] [PubMed]
- Chindapasirt, J. Sarcopenia in cancer patients. Asian Pac. J. Cancer Prev. 2016, 16, 8075–8077. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Iwata, K.; Yoshimura, Y.; Shinoda, T.; Inagaki, Y.; Ohya, S.; Yamada, K.; Oyanagi, K.; Maekawa, Y.; Honda, A. Low muscle mass is associated with walking function in patients with acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105259. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Hsu, T.-H.; Wu, W.-T.; Huang, K.-C.; Han, D.-S. Association between sarcopenia and cognitive impairment: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164.e7–1164.e15. [Google Scholar] [CrossRef]
- Firth, J.; Firth, J.A.; Stubbs, B.; Vancampfort, D.; Schuch, F.B.; Hallgren, M.; Veronese, N.; Yung, A.R.; Sarris, J. Association Between Muscular Strength and Cognition in People With Major Depression or Bipolar Disorder and Healthy Controls. JAMA Psychiatry 2018, 75, 740–746. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Firth, J.A.; Large, M.; Rosenbaum, S.; Hallgren, M.; Ward, P.B.; Sarris, J.; Yung, A.R. Grip strength is associated with cognitive performance in schizophrenia and the general population: A UK biobank study of 476559 participants. Schizophr. Bull. 2018, 44, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, S.K.; Lee, D.R.; Lee, J. The relationship between handgrip strength and cognitive function in elderly Koreans over 8 years: A prospective population-based study using Korean longitudinal study of ageing. Korean J. Fam. Med. 2019, 40, 9. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Sui, S.X.; West, E.C.; Holloway-Kew, K.L.; Hyde, N.K.; Stuart, A.L.; Gaston, J.; Williams, L.J. Operational definitions of sarcopenia should consider depressive symptoms. JCSM Clin. Rep. 2021, 6, 62–68. [Google Scholar] [CrossRef]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing 2017, 46, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.; Gomez-Baya, D.; Peralta, M.; Frasquilho, D.; Santos, T.; Martins, J.; Ferrari, G.; Gaspar de Matos, M. The Effect of Muscular Strength on Depression Symptoms in Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 5674. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, N.; Yamamoto, Y.; Takegami, M.; Yamazaki, S.; Onishi, Y.; Sekiguchi, M.; Otani, K.; Konno, S.-I.; Kikuchi, S.-I.; Fukuhara, S. Association between hand-grip strength and depressive symptoms: Locomotive Syndrome and Health Outcomes in Aizu Cohort Study (LOHAS). Age Ageing 2015, 44, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.; Cyrino, E.S.; Antunes, M.; Santos, D.A.; Sardinha, L.B. Sarcopenia and physical independence in older adults: The independent and synergic role of muscle mass and muscle function. J. Cachexia Sarcopenia Muscle 2017, 8, 245–250. [Google Scholar] [CrossRef]
- Liu, C.-J.; Shiroy, D.M.; Jones, L.Y.; Clark, D.O. Systematic review of functional training on muscle strength, physical functioning, and activities of daily living in older adults. Eur. Rev. Aging Phys. Act. 2014, 11, 95–106. [Google Scholar] [CrossRef]
- Lynch, G.S.; Schertzer, J.D.; Ryall, J.G. Therapeutic approaches for muscle wasting disorders. Pharmacol. Ther. 2007, 113, 461–487. [Google Scholar] [CrossRef]
- Deng, H.-Y.; Zha, P.; Peng, L.; Hou, L.; Huang, K.-L.; Li, X.-Y. Preoperative sarcopenia is a predictor of poor prognosis of esophageal cancer after esophagectomy: A comprehensive systematic review and meta-analysis. Dis. Esophagus 2019, 32, doy115. [Google Scholar] [CrossRef]
- Harimoto, N.; Shirabe, K.; Yamashita, Y.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. J. Br. Surg. 2013, 100, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shen, Y.; Tan, L.; Li, W. Prognostic Value of Sarcopenia in Lung Cancer: A Systematic Review and Meta-analysis. Chest 2019, 156, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, T.; Kondo, S.; Saito, T.; Inoue, T.; Otake, K.; Misu, S.; Sakai, H.; Ono, R.; Tomioka, H. Impact of sarcopenia defined by carina-level skeletal muscle mass on the long-term prognosis of patients with idiopathic pulmonary fibrosis. Respir. Med. Res. 2022, 82, 100965. [Google Scholar] [CrossRef] [PubMed]
- Rutten, I.J.; Ubachs, J.; Kruitwagen, R.F.; van Dijk, D.P.; Beets-Tan, R.G.; Massuger, L.F.; Olde Damink, S.W.; Van Gorp, T. The influence of sarcopenia on survival and surgical complications in ovarian cancer patients undergoing primary debulking surgery. Eur. J. Surg. Oncol. 2017, 43, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, K.W.; Kang, J.; Ki, Y.-J.; Chang, M.; Han, J.-K.; Yang, H.-M.; Kang, H.-J.; Koo, B.-K.; Kim, H.-S. Sarcopenia index as a predictor of clinical outcomes in older patients with coronary artery disease. J. Clin. Med. 2020, 9, 3121. [Google Scholar] [CrossRef] [PubMed]
- Irshad, K.; Ashraf, I.; Azam, F.; Shaheen, A. Burnout prevalence and associated factors in medical students in integrated modular curriculum: A cross-sectional study. Pak. J. Med. Sci. 2022, 38, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.J.; Scott, K.M.; De Jonge, P.; Kessler, R.C. Epidemiology of anxiety disorders: From surveys to nosology and back. Dialogues Clin. Neurosci. 2022, 19, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin. Neurosci. 2022, 17, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Javaid, S.F.; Hashim, I.J.; Hashim, M.J.; Stip, E.; Samad, M.A.; Ahbabi, A.A. Epidemiology of anxiety disorders: Global burden and sociodemographic associations. Middle East Curr. Psychiatry 2023, 30, 44. [Google Scholar] [CrossRef]
- Kavelaars, R.; Ward, H.; Modi, K.M.; Mohandas, A. The burden of anxiety among a nationally representative US adult population. J. Affect. Disord. 2023, 336, 81–91. [Google Scholar] [CrossRef]
- Teesson, M.; Mitchell, P.B.; Deady, M.; Memedovic, S.; Slade, T.; Baillie, A. Affective and anxiety disorders and their relationship with chronic physical conditions in Australia: Findings of the 2007 National Survey of Mental Health and Wellbeing. Aust. N. Z. J. Psychiatry 2011, 45, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, J.; Honkanen, R.; Williams, L.; Leung, J.; Rauma, P.; Quirk, S.; Koivumaa-Honkanen, H. Depressive disorders, anxiety disorders and subjective mental health in common musculoskeletal diseases: A review. Maturitas 2019, 127, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Jacka, F.; Pasco, J.; Henry, M.; Dodd, S.; Nicholson, G.; Kotowicz, M.; Berk, M. The prevalence of mood and anxiety disorders in Australian women. Australas. Psychiatry 2010, 18, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Sareen, J.; Cox, B.J.; Clara, I.; Asmundson, G.J. The relationship between anxiety disorders and physical disorders in the US National Comorbidity Survey. Depress. Anxiety 2005, 21, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Pasco, J.A.; Williams, L.J.; Jacka, F.N.; Stupka, N.; Brennan-Olsen, S.L.; Holloway, K.L.; Berk, M. Sarcopenia and the Common Mental Disorders: A Potential Regulatory Role of Skeletal Muscle on Brain Function? Curr. Osteoporos. Rep. 2015, 13, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.V.C.; Schneider, L.P.; Fonseca, J.; Belo, L.F.; Bonomo, C.; Morita, A.A.; Furlanetto, K.C.; Felcar, J.M.; Rodrigues, A.; Franssen, F.M.E.; et al. Clinical impact of body composition phenotypes in patients with COPD: A retrospective analysis. Eur. J. Clin. Nutr. 2019, 73, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Staples, W.H.; Kays, A.; Richman, R. Examination of the correlation between physical and psychological measures in community-dwelling older adults. Clin. Interv. Aging 2020, 15, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Maes, M.; Solmi, M.; Brunoni, A.R.; Lange, S.; Husain, M.I.; Kurdyak, P.; Rehm, J.; Koyanagi, A. Is dynapenia associated with the onset and persistence of depressive and anxiety symptoms among older adults? Findings from the Irish longitudinal study on ageing. Aging Ment. Health 2021, 25, 468–475. [Google Scholar] [CrossRef]
- Gordon, B.R.; McDowell, C.P.; Lyons, M.; Herring, M.P. Associations between grip strength and generalized anxiety disorder in older adults: Results from the Irish longitudinal study on ageing. J. Affect. Disord. 2019, 255, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.L.; Nguyen, U.S.; Au, E.; Tan, K.C.; Kung, A.W. Association of handgrip strength with chronic diseases and multimorbidity: A cross-sectional study. Age 2013, 35, 929–941. [Google Scholar] [CrossRef]
- Alston, H.; Burns, A.; Davenport, A. Loss of appendicular muscle mass in haemodialysis patients is associated with increased self-reported depression, anxiety and lower general health scores. Nephrology 2018, 23, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Tyrovolas, S.; Koyanagi, A.; Olaya, B.; Ayuso-Mateos, J.L.; Miret, M.; Chatterji, S.; Tobiasz-Adamczyk, B.; Koskinen, S.; Leonardi, M.; Haro, J.M. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: A multi-continent study. J. Cachexia Sarcopenia Muscle 2016, 7, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Sheffield-Moore, M.; Yeckel, C.W.; Gilkison, C.; Jiang, J.; Achacosa, A.; Lieberman, S.A.; Tipton, K.; Wolfe, R.R.; Urban, R.J. Testosterone administration to older men improves muscle function: Molecular and physiological mechanisms. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E601–E607. [Google Scholar] [CrossRef]
- Anderson, L.J.; Liu, H.; Garcia, J.M. Sex Differences in Muscle Wasting. Adv. Exp. Med. Biol. 2017, 1043, 153–197. [Google Scholar] [PubMed]
- Zimmerman, M.; Chelminski, I.; Young, D.; Dalrymple, K. A clinically useful anxiety outcome scale. J. Clin. Psychiatry 2010, 71, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Han, C.; Ko, Y.H.; Yoon, S.; Pae, C.U.; Choi, J.; Kim, J.M.; Yoon, H.K.; Lee, H.; Patkar, A.A.; et al. A Korean validation study of the Clinically Useful Anxiety Outcome Scale: Comorbidity and differentiation of anxiety and depressive disorders. PLoS ONE 2017, 12, e0179247. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Devaux, M.; Sassi, F. Alcohol Consumption and Harmful Drinking: Trends and Social Disparities Across OECD Countries; OECD Health Working Papers, No. 79; OECD Publishing: Paris, France, 2015. [Google Scholar]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Di Leo, G.; Sardanelli, F. Statistical significance: p value, 0.05 threshold, and applications to radiomics—Reasons for a conservative approach. Eur. Radiol. Exp. 2020, 4, 18. [Google Scholar] [CrossRef]
- Li, Z.; Tong, X.; Ma, Y.; Bao, T.; Yue, J. Relationship between Low skeletal muscle mass and arteriosclerosis in western China: A cross-sectional study. Front. Cardiovasc. Med. 2021, 8, 735262. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, C.S.; Thang, L.A.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef] [PubMed]
- Favier, F.B.; Benoit, H.; Freyssenet, D. Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflug. Arch. 2008, 456, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Haynie, D.A.; Berg, S.; Johansson, B.; Gatz, M.; Zarit, S.H. Symptoms of depression in the oldest old: A longitudinal study. J. Gerontol. B Psychol. Sci. Soc. Sci. 2001, 56, P111–P118. [Google Scholar] [CrossRef] [PubMed]
- Vest, M.T.; Murphy, T.E.; Araujo, K.L.; Pisani, M.A. Disability in activities of daily living, depression, and quality of life among older medical ICU survivors: A prospective cohort study. Health Qual. Life Outcomes 2011, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral cytokine and chemokine alterations in depression: A meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The Role of Inflammation in Age-Related Sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef]
- Steptoe, A.; Demakakos, P.; de Oliveira, C.; Wardle, J. Distinctive biological correlates of positive psychological well-being in older men and women. Psychosom. Med. 2012, 74, 501–508. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Liu, X.; Yue, J.; Hou, L.; Xia, X.; Zuo, Z.; Liu, Y.; Jia, S.; Dong, B. Comorbid depressive and anxiety symptoms and frailty among older adults: Findings from the West China health and aging trend study. J. Affect. Disord. 2020, 277, 970–976. [Google Scholar] [CrossRef]
- Maggio, M.; Guralnik, J.M.; Longo, D.L.; Ferrucci, L. Interleukin-6 in aging and chronic disease: A magnificent pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 575–584. [Google Scholar] [CrossRef]
- Lebron-Milad, K.; Graham, B.M.; Milad, M.R. Low estradiol levels: A vulnerability factor for the development of posttraumatic stress disorder. Biol. Psychiatry 2012, 72, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Gracia, C.R.; Kapoor, S.; Ferdousi, T. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause 2005, 12, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Graham, B.M. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry 2017, 4, 73–82. [Google Scholar] [CrossRef]
- Giltay, E.J.; Enter, D.; Zitman, F.G.; Penninx, B.W.; van Pelt, J.; Spinhoven, P.; Roelofs, K. Salivary testosterone: Associations with depression, anxiety disorders, and antidepressant use in a large cohort study. J. Psychosom. Res. 2012, 72, 205–213. [Google Scholar] [CrossRef]
- Park, C.H.; Do, J.G.; Lee, Y.T.; Yoon, K.J. Sarcopenic obesity associated with high-sensitivity C-reactive protein in age and sex comparison: A two-center study in South Korea. BMJ Open 2018, 8, e021232. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Wong, S.K.; Wan Hasan, W.N.; Jolly, J.J.; Nur-Farhana, M.F.; Ima-Nirwana, S.; Chin, K.Y. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef]
- Burns, A.; Strawbridge, J.D.; Clancy, L.; Doyle, F. Exploring smoking, mental health and smoking-related disease in a nationally representative sample of older adults in Ireland—A retrospective secondary analysis. J. Psychosom. Res. 2017, 98, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Li, S.; Pan, L.; Zhang, N.; Jia, C. Association of anxiety disorders with the risk of smoking behaviors: A meta-analysis of prospective observational studies. Drug Alcohol. Depend. 2014, 145, 69–76. [Google Scholar] [CrossRef]
- Moylan, S.; Jacka, F.N.; Pasco, J.A.; Berk, M. Cigarette smoking, nicotine dependence and anxiety disorders: A systematic review of population-based, epidemiological studies. BMC Med. 2012, 10, 123. [Google Scholar] [CrossRef]
- Schuckit, M.; Hesselbrock, V. Alcohol dependence and anxiety disorders. Focus-Am. Psychiatr. Publ. Inc. 2004, 2, 440–453. [Google Scholar] [CrossRef]
- Urbano-Marquez, A.; Fernandez-Sola, J. Effects of alcohol on skeletal and cardiac muscle. Muscle Nerve 2004, 30, 689–707. [Google Scholar] [CrossRef] [PubMed]
- McDowell, C.P.; Gordon, B.R.; Andrews, K.L.; MacDonncha, C.; Herring, M.P. Associations of physical activity with anxiety symptoms and status: Results from The Irish longitudinal study on ageing. Epidemiol. Psychiatr. Sci. 2019, 28, 436–445. [Google Scholar] [CrossRef] [PubMed]
- McDowell, C.P.; Dishman, R.K.; Vancampfort, D.; Hallgren, M.; Stubbs, B.; MacDonncha, C.; Herring, M.P. Physical activity and generalized anxiety disorder: Results from The Irish Longitudinal Study on Ageing (TILDA). Int. J. Epidemiol. 2018, 47, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Latham, N.K.; Bennett, D.A.; Stretton, C.M.; Anderson, C.S. Systematic review of progressive resistance strength training in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Herring, M.P.; Johnson, K.E.; O’Connor, P.J. Exercise training and health-related quality of life in generalized anxiety disorder. Psychol. Sport Exerc. 2016, 27, 138–141. [Google Scholar] [CrossRef]
- Ortega, F.B.; Silventoinen, K.; Tynelius, P.; Rasmussen, F. Muscular strength in male adolescents and premature death: Cohort study of one million participants. BMJ 2012, 345, e7279. [Google Scholar] [CrossRef] [PubMed]
- Salmon, P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clin. Psychol. Rev. 2001, 21, 33–61. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.M.; Currie, K.C. Depression, anxiety and their relationship with chronic diseases: A review of the epidemiology, risk and treatment evidence. Med. J. Aust. 2009, 190, S54–S60. [Google Scholar] [CrossRef]
- Cabanas-Sánchez, V.; Esteban-Cornejo, I.; Parra-Soto, S.; Petermann-Rocha, F.; Gray, S.R.; Rodríguez-Artalejo, F.; Ho, F.K.; Pell, J.P.; Martínez-Gómez, D.; Celis-Morales, C. Muscle strength and incidence of depression and anxiety: Findings from the UK Biobank prospective cohort study. J. Cachexia Sarcopenia Muscle 2022, 13, 1983–1994. [Google Scholar] [CrossRef]
| Characteristics | Overall | Female | Male | p-Value |
|---|---|---|---|---|
| Number | 174,262 | 73,833 (42.37) | 100,429 (57.63) | |
| Age (year) | 42.25 ± 8.48 | 41.57 ± 8.42 | 42.75 ± 8.5 | <0.001 *** |
| Screening center (Seoul, %) | 59,997 (34.43) | 23,732 (32.14) | 36,265 (36.11) | <0.001 *** |
| Height (cm) | 168.11 ± 8.41 | 160.73 ± 5.22 | 173.54 ± 5.77 | <0.001 *** |
| Weight (kg) | 67.73 ± 13.52 | 57.17 ± 8.96 | 75.5 ± 10.77 | <0.001 *** |
| BMI (kg/m2) | 23.81 ± 3.49 | 22.13 ± 3.29 | 25.04 ± 3.1 | <0.001 *** |
| Appendicular Skeletal Muscle Mass (kg) | 20.9 ± 4.92 | 16.05 ± 2.11 | 24.47 ± 2.94 | <0.001 *** |
| SMI (kg/m2) * | 7.29 ± 1.14 | 6.2 ± 0.61 | 8.1 ± 0.65 | <0.001 *** |
| Smoker status | <0.001 *** | |||
| Never smoker | 89,666 (51.45) | 65,624 (88.88) | 24,042 (23.94) | |
| Former smoker | 57,437 (32.96) | 6257 (8.47) | 51,180 (50.96) | |
| Current smoker | 25,993 (14.92) | 1046 (1.42) | 24,947 (24.84) | |
| Heavy alcohol | 50,798 (29.15) | 7552 (10.23) | 43,246 (43.06) | <0.001 *** |
| Regular physical exercise | 25,764 (14.78) | 9310 (12.61) | 16,454 (16.38) | <0.001 *** |
| Comorbidities | ||||
| Diabetes (%) | 5813 (3.34) | 1186 (1.61) | 4627 (4.61) | <0.001 *** |
| Hypertension (%) | 18,007 (10.33) | 3220 (4.36) | 14,787 (14.72) | <0.001 *** |
| Dyslipidemia (%) | 30,063 (17.25) | 6628 (8.98) | 23,435 (23.33) | <0.001 *** |
| Laboratory Finding | ||||
| Fasting plasma glucose (mg/dL) | 98.13 ± 15.41 | 94.37 ± 12.71 | 100.9 ± 16.59 | <0.001 *** |
| HbA1c (%) | 5.51 ± 0.55 | 5.41 ± 0.46 | 5.59 ± 0.61 | <0.001 *** |
| Fasting insulin (µU/mL) | 7.72 ± 5.01 | 7.07 ± 4.7 | 8.19 ± 5.18 | <0.001 *** |
| Total Cholesterol (mg/dL) | 190.03 ± 33.97 | 185.64 ± 32.49 | 193.26 ± 34.67 | <0.001 *** |
| LDL-C (mg/dL) | 128.39 ± 33.08 | 120.69 ± 31.56 | 134.04 ± 33.04 | <0.001 *** |
| HDL-C (mg/dL) | 60.31 ± 16.3 | 68.48 ± 16.17 | 54.3 ± 13.55 | <0.001 *** |
| Triglycerides (mg/dL) | 120.77 ± 83.87 | 90.48 ± 53.82 | 143.03 ± 94.37 | <0.001 *** |
| HOMA-Ir † | 1.92 ± 1.47 | 1.7 ± 1.32 | 2.09 ± 1.55 | <0.001 *** |
| Systolic blood pressure (mmHg) | 110.68 ± 12.63 | 104.81 ± 11.73 | 115 ± 11.47 | <0.001 *** |
| Diastolic blood pressure(mmHg) | 71.25 ± 9.76 | 66.77 ± 8.74 | 74.54 ± 9.15 | <0.001 *** |
| Characteristics | Female | Effect Size (95% CI) | p-Value | Male | Effect Size (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Control | LMM | Control | LMM | |||||
| Number | 58,942 (79.83) | 14,891 (20.17) | 96,551 (96.14) | 3878 (3.86) | ||||
| Age (year) | 41.91 ± 8.3 | 40.25 ± 8.75 | 0.2 (0.18–0.22) | <0.001 | 42.67 ± 8.37 | 44.75 ± 11 | −0.24 (−0.28–−0.21) | <0.001 *** |
| Screening center (Seoul, %) | 19,307 (32.76) | 4425 (29.72) | 0.07 (0.05–0.08) | <0.001 | 34,966 (36.22) | 1299 (33.5) | 0.06 (0.02–0.09) | 0.001 |
| Height (cm) | 161.29 ± 5.19 | 158.48 ± 4.72 | 0.55 (0.53–0.57) | <0.001 | 173.72 ± 5.71 | 169 ± 5.43 | 0.83 (0.8–0.86) | <0.001 *** |
| Weight (kg) | 59.28 ± 8.61 | 48.81 ± 4.16 | 1.32 (1.3–1.34) | <0.001 | 76.18 ± 10.37 | 58.58 ± 5.32 | 0.83 (0.8–0.86) | <0.001 *** |
| BMI (kg/m2) | 22.8 ± 3.25 | 19.46 ± 1.72 | 1.11 (1.1–1.13) | <0.001 | 25.22 ± 3 | 20.54 ± 1.91 | 1.58 (1.54–1.61) | <0.001 *** |
| Appendicular Skeletal Muscle Mass (kg) | 16.66 ± 1.84 | 13.63 ± 1.09 | 1.76 (1.74–1.78) | <0.001 | 24.68 ± 2.78 | 19.28 ± 1.52 | 1.97 (1.93–2) | <0.001 *** |
| SMI (kg/m2) * | 6.39 ± 0.51 | 5.42 ± 0.24 | 2.08 (2.06–2.1) | <0.001 | 8.16 ± 0.6 | 6.74 ± 0.25 | 2.39 (2.36–2.42) | <0.001 *** |
| Smoker status | <0.001 | <0.001 *** | ||||||
| Never smoker | 52,181 (88.53) | 13,443 (90.28) | 22,948 (23.77) | 1094 (28.21) | ||||
| Former smoker | 5161 (8.76) | 1096 (7.36) | 49,374 (51.14) | 1806 (46.57) | ||||
| Current smoker | 855 (1.45) | 191 (1.28) | 0.01 (0–0.03) | 23,993 (24.85) | 954 (24.6) | 0.01 (−0.03–0.04) | ||
| Heavy alcohol | 6130 (10.4) | 1422 (9.55) | 0.03 (0.01–0.05) | <0.001 | 41,862 (43.36) | 1384 (35.69) | 0.15 (0.12–0.19) | <0.001 *** |
| Regular Physical exercise | 8043 (13.65) | 1267 (8.51) | 0.16 (0.14–0.17) | <0.001 | 16,025 (16.6) | 429 (11.06) | 0.15 (0.12–0.18) | <0.001 *** |
| Comorbidities | ||||||||
| Diabetes (%) | 991 (1.68) | 195 (1.31) | 0.03 (0.01–0.05) | 0.001 | 4411 (4.57) | 216 (5.57) | −0.05 (−0.08–−0.02) | 0.004 |
| Hypertension (%) | 2771 (4.7) | 449 (3.02) | 0.08 (0.06–0.1) | <0.001 | 14,353 (14.87) | 434 (11.19) | 0.1 (0.07–0.14) | <0.001 *** |
| Dyslipidemia (%) | 5423 (9.2) | 1205 (8.09) | 0.04 (0.02–0.06) | <0.001 | 22,693 (23.5) | 742 (19.13) | 0.1 (0.07–0.14) | <0.001 *** |
| Laboratory Finding | ||||||||
| Fasting glucose (mg/dL) | 94.88 ± 13.14 | 92.36 ± 10.63 | 0.2 (0.18–0.22) | <0.001 | 100.98 ± 16.46 | 98.95 ± 19.48 | 0.12 (0.09–0.15) | <0.001 *** |
| HbA1c (%) | 5.43 ± 0.47 | 5.33 ± 0.38 | 0.21 (0.19–0.23) | <0.001 | 5.59 ± 0.6 | 5.55 ± 0.72 | 0.07 (0.03–0.1) | <0.001 *** |
| Fasting insulin (µU/mL) | 7.37 ± 5.01 | 5.89 ± 2.92 | 0.32 (0.3–0.34) | <0.001 | 8.29 ± 5.22 | 5.66 ± 3.14 | 0.51 (0.48–0.54) | <0.001 *** |
| Total Cholesterol (mg/dL) | 185.62 ± 32.51 | 185.7 ± 32.39 | 0 (−0.02–0.02) | 0.79 | 193.39 ± 34.67 | 189.91 ± 34.57 | 0.1 (0.07–0.13) | <0.001 *** |
| LDL-C (mg/dL) | 121.23 ± 31.68 | 118.53 ± 31.01 | 0.09 (0.07–0.1) | <0.001 | 134.25 ± 32.98 | 128.79 ± 34 | 0.17 (0.13–0.2) | <0.001 *** |
| HDL-C (mg/dL) | 67.45 ± 16.13 | 72.56 ± 15.67 | −0.32 (−0.34–−0.3) | <0.001 | 54.05 ± 13.41 | 60.74 ± 15.25 | −0.5 (−0.53–−0.46) | <0.001 *** |
| Triglycerides (mg/dL) | 92.94 ± 56.61 | 80.75 ± 39.51 | 0.23 (0.21–0.25) | <0.001 | 144.08 ± 94.88 | 116.93 ± 75.98 | 0.29 (0.26–0.32) | <0.001 *** |
| HOMA-Ir † | 1.78 ± 1.41 | 1.37 ± 0.76 | 0.32 (0.3–0.33) | <0.001 | 2.12 ± 1.56 | 1.41 ± 0.92 | 0.46 (0.43–0.49) | <0.001 *** |
| Systolic blood pressure (mmHg) | 105.76 ± 11.86 | 101.08 ± 10.41 | 0.4 (0.39–0.42) | <0.001 | 115.22 ± 11.42 | 109.46 ± 11.14 | 0.5 (0.47–0.54) | <0.001 *** |
| Diastolic blood pressure (mmHg) | 67.2 ± 8.85 | 65.08 ± 8.07 | 0.24 (0.23–0.26) | <0.001 | 74.63 ± 9.15 | 72.21 ± 8.83 | 0.27 (0.23–0.3) | <0.001 *** |
| Anxiety Level (CUXOS Score) | |||
|---|---|---|---|
| ≤20 | >20 | ||
| Female | |||
| Unadjusted Model | 1 (reference) | 1.13 (1.08–1.17) | |
| Model 1 | 1 (reference) | 1.12 (1.08–1.16) | |
| Model 2 | 1 (reference) | 1.13 (1.09–1.17) | |
| Model 3 | 1 (reference) | 1.13 (1.08–1.17) | |
| Male | |||
| Unadjusted Model | 1 (reference) | 1.16 (1.07–1.25) | |
| Model 1 | 1 (reference) | 1.15 (1.06–1.25) | |
| Model 2 | 1 (reference) | 1.16 (1.07–1.25) | |
| Model 3 | 1 (reference) | 1.17 (1.08–1.27) | |
| Anxiety Level (CUXOS Score) | |||
| ≤20 | 21–30 | >30 | |
| Female | |||
| Unadjusted Model | 1 (reference) | 1.09 (1.04–1.15) | 1.18 (1.12–1.24) |
| Model 1 | 1 (reference) | 1.1 (1.05–1.15) | 1.15 (1.09–1.22) |
| Model 2 | 1 (reference) | 1.1 (1.05–1.15) | 1.17 (1.1–1.23) |
| Model 3 | 1 (reference) | 1.1 (1.05–1.15) | 1.17 (1.11–1.24) |
| Male | |||
| Unadjusted Model | 1 (reference) | 1.1 (1–1.2) | 1.29 (1.14–1.46) |
| Model 1 | 1 (reference) | 1.08 (0.98–1.18) | 1.31 (1.16–1.49) |
| Model 2 | 1 (reference) | 1.08 (0.98–1.19) | 1.34 (1.18–1.51) |
| Model 3 | 1 (reference) | 1.09 (0.99–1.2) | 1.35 (1.19–1.53) |
| Anxiety Level (CUXOS Score) | |||
| ≤20 | 21–40 | >40 | |
| Female | |||
| Unadjusted Model | 1 (reference) | 1.12 (1.07–1.16) | 1.2 (1.09–1.32) |
| Model 1 | 1 (reference) | 1.12 (1.07–1.16) | 1.14 (1.04–1.26) |
| Model 2 | 1 (reference) | 1.12 (1.08–1.17) | 1.17 (1.06–1.28) |
| Model 3 | 1 (reference) | 1.12 (1.08–1.17) | 1.18 (1.07–1.3) |
| Male | |||
| Unadjusted Model | 1 (reference) | 1.15 (1.06–1.24) | 1.27 (0.99–1.63) |
| Model 1 | 1 (reference) | 1.14 (1.05–1.23) | 1.32 (1.03–1.69) |
| Model 2 | 1 (reference) | 1.14 (1.05–1.24) | 1.34 (1.05–1.72) |
| Model 3 | 1 (reference) | 1.15 (1.06–1.25) | 1.36 (1.06–1.74) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Jung, S.; Lee, M.Y.; Park, C.-H.; Cho, S.J. Exploring the Association between Elevated Anxiety Symptoms and Low Skeletal Muscle Mass among Asymptomatic Adults: A Population-Based Study in Republic of Korea. Brain Sci. 2024, 14, 438. https://doi.org/10.3390/brainsci14050438
Kim E, Jung S, Lee MY, Park C-H, Cho SJ. Exploring the Association between Elevated Anxiety Symptoms and Low Skeletal Muscle Mass among Asymptomatic Adults: A Population-Based Study in Republic of Korea. Brain Sciences. 2024; 14(5):438. https://doi.org/10.3390/brainsci14050438
Chicago/Turabian StyleKim, Eunsoo, Sra Jung, Mi Yeon Lee, Chul-Hyun Park, and Sung Joon Cho. 2024. "Exploring the Association between Elevated Anxiety Symptoms and Low Skeletal Muscle Mass among Asymptomatic Adults: A Population-Based Study in Republic of Korea" Brain Sciences 14, no. 5: 438. https://doi.org/10.3390/brainsci14050438
APA StyleKim, E., Jung, S., Lee, M. Y., Park, C.-H., & Cho, S. J. (2024). Exploring the Association between Elevated Anxiety Symptoms and Low Skeletal Muscle Mass among Asymptomatic Adults: A Population-Based Study in Republic of Korea. Brain Sciences, 14(5), 438. https://doi.org/10.3390/brainsci14050438







