Abstract
Chemical synapses are essential for neuronal information storage and relay. The synaptic signal received or sent from spatially distinct subcellular compartments often generates different outcomes due to the distance or physical property difference. Therefore, the final output of postsynaptic neurons is determined not only by the type and intensity of synaptic inputs but also by the synaptic subcellular location. How synaptic subcellular specificity is determined has long been the focus of study in the neurodevelopment field. Genetic studies from invertebrates such as Caenorhabditis elegans (C. elegans) have uncovered important molecular and cellular mechanisms required for subcellular specificity. Interestingly, similar molecular mechanisms were found in the mammalian cerebellum, hippocampus, and cerebral cortex. This review summarizes the comprehensive advances in the cellular and molecular mechanisms underlying synaptic subcellular specificity, focusing on studies from C. elegans and rodents.
1. Introduction
The human brain contains roughly 100 billion morphologically diverse neurons connected by over 100 trillion synapses. They are required for neural information storage and relays that are essential for locomotion, sensation, learning, memory, cognition, and other activities. Chemical synapses are one primary type of synaptic junction with asymmetric presynaptic and postsynaptic specialization [1]. At the presynaptic terminus, synaptic vesicles fuse with the presynaptic membrane and release neurotransmitters upon action potential, which is usually calcium dependent. The released neurotransmitters then bind to the corresponding receptors and activate or suppress the postsynaptic neurons [2,3,4,5]. As neurons have extremely complex morphologies, and each neuron forms thousands of synapses on average, the final output of any given neuron is determined by the sum of total synaptic inputs, which are affected not only by synaptic properties but also the physical location [6]. Therefore, to ensure proper neuronal information transmission and processing, specific presynaptic termini must precisely target specific postsynaptic subcellular domains of particular target cells [7,8,9,10,11]. Synaptic subcellular specificity is based on either presynaptic or postsynaptic locations depending on the model of study. For example, subcellular specificity was based on presynaptic localization in the C. elegans model and postsynaptic subcellular compartments in mammalian brain systems [9,11].
The synaptic specificity can be regulated at different steps during neurodevelopment, including neurogenesis, neuronal migration, axon guidance, synaptic targeting, synaptic formation, and synaptic pruning. How neurons select synaptic targets during development has been a fundamental question in neuroscience. Based on Peter’s rule, proposed by Peter and Feldman in 1976, synaptic connections are stochastically determined by the degree of anatomically overlapping of axons and dendrites [12]. Peter’s rule was supported by some studies [13,14]. However, as the spatial and temporal resolution increases, more and more evidence suggests that synapses are formed by nonrandom mechanisms [15]. In this review, we summarize comprehensive advances in regulating subcellular specificity in the synaptic targeting process, covering the roles of secreted/extracellular molecules, adhesion/transmembrane molecules, and cytoplasmic/intracellular molecules in the synaptic subcellular specificity. We also review the recent findings on the roles of neuronal activity and non-neuronal tissues in synaptic subcellular specificity.
2. Secreted Molecules
Many secreted signaling molecules, originally identified as guidance cues, are found to play multiple roles in neuronal development, including neurogenesis, cell migration, and synaptogenesis [16,17,18,19]. More recently, genetic studies in invertebrates demonstrate that many secreted molecules are required for synaptic subcellular specificity in both invertebrates [20,21,22,23] and mammals [24,25]. In this review, we focus on netrin/DCC, semaphorin/plexin, Wnt, and Slit/Robo, as they are involved in synaptic subcellular specificity.
2.1. Netrin
Netrin and netrin receptors were first identified in C. elegans (UNC-6/netrin and its receptors UNC-40/DCC and UNC-5) for their roles in behavioral coordination, cell migration, and axon guidance [26]. A few years later, their roles in Drosophila and vertebrate axon guidance were reported [27,28,29,30,31,32,33,34], suggesting that the functions of netrin and its receptors are highly conserved. Recent studies have demonstrated that netrin also regulates synaptic subcellular specificity [20,23,35,36,37].
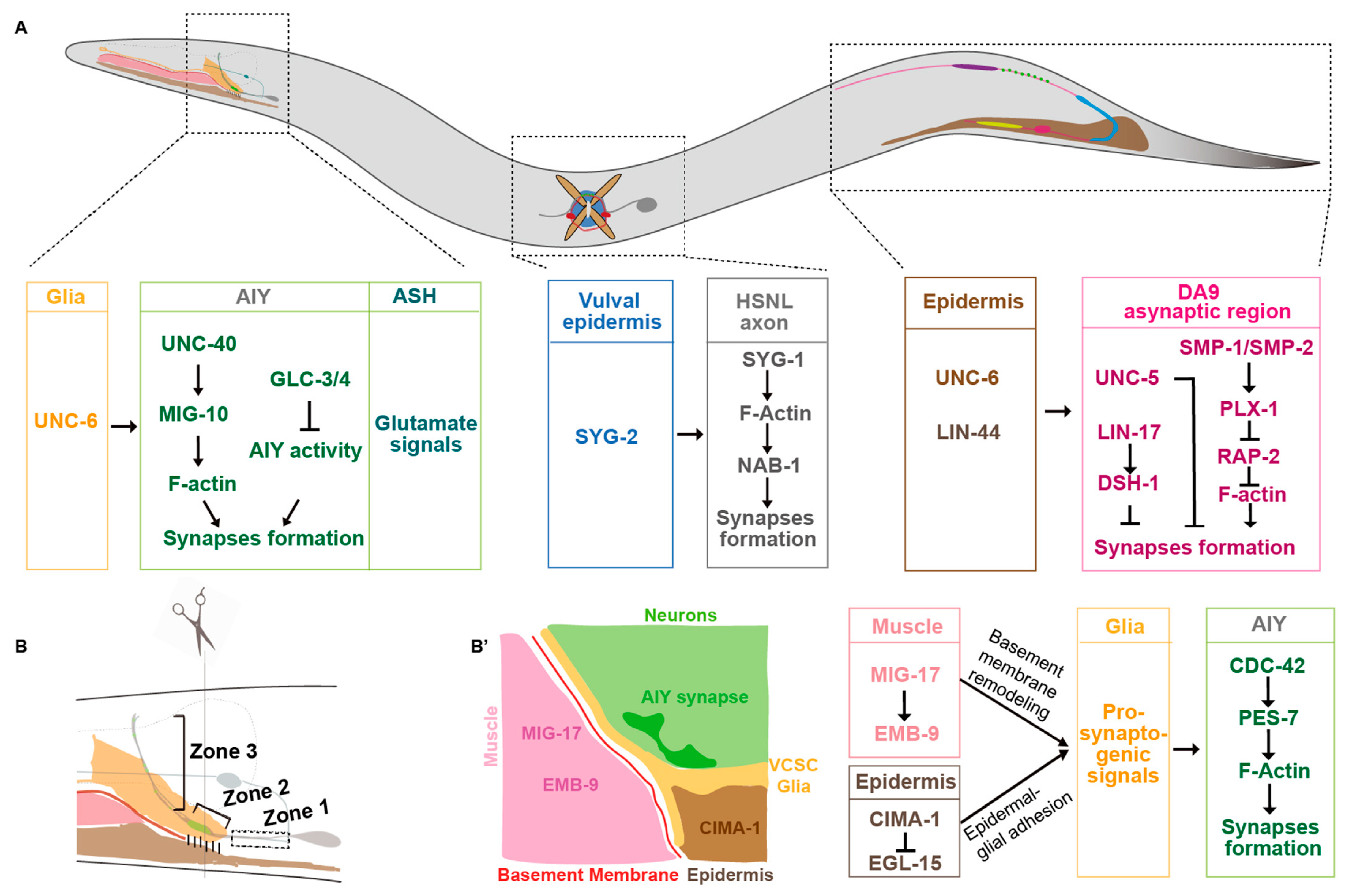
In C. elegans heads, Amphid Interneuron Y (AIY) neurites anatomically can be divided into three zones. The ventral proximal Zone 1, the distal Zone 3 in the nerve ring, and the link Zone 2 are asynaptic, synaptic sparse, and synaptic enriched regions, respectively (Figure 1A,B) [20,38]. The synaptic specificity at the Zone 2 region is mainly determined by the UNC-6/netrin secreted from the ventral cephalic sheath glial cells (VCSCs), which contact AIY Zone 2 [20]. During embryogenesis, the VCSCs regulate the AIY synaptic subcellular specificity by expressing UNC-6/netrin [20]. Extending the VCSC glia to Zone 1 by genetic manipulation promotes ectopic synaptic formation in this region. This suggests that netrin acts as a short-range signaling molecule to regulate the synaptic assembly locally [20]. This specificity requires the AIY-expressed UNC-40/DCC receptor, which is specifically localized to the presynaptic sites [20]. In addition, UNC-6 and UNC-40 signaling also regulate the subcellular localization of serotonin neurosecretory–motor (NSM) neurons neurosecretory presynaptic terminals [36]. Furthermore, the dorsal axonic presynaptic localization of the ventral cord “dorsal A 9” (DA9) motor neuron in the tail is also regulated by the UNC-6/netrin signaling (Figure 1A) [37]. The DA9 dendrite and ventral axon accumulate more UNC-5 receptors than the dorsal axon, which receives the UNC-6 signals expressed in the ventral posterior side and locally inhibits the presynaptic assembly [37]. In unc-6 or unc-5 loss-of-function mutants, DA9 presynaptic proteins mislocalize to the ventral dendrite. Consistently, ectopic expressing UNC-6 in the presynaptic region is sufficient to exclude the endogenous synaptic localization. These data support that UNC-6 and UNC-5 localize the synaptic position by inhibiting the presynaptic assembly (Figure 1A) [37].
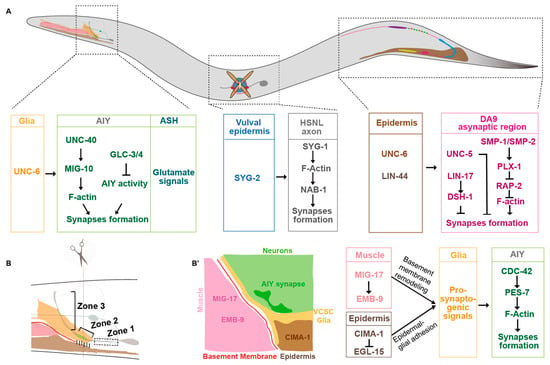
Figure 1.
Presynaptic subcellular specificity in C. elegans. (A) A diagram of C. elegans. Some molecular mechanisms underlying the synaptic subcellular specificity in the head, middle body, and tail neurons are described. (B) A cartoon describes the anatomy of different tissues in the head (brown, epidermis; pink, muscle; red, basement membrane; yellow, VCSC glia; gray, AIY and ASH neurons; light green, nerve ring; green, AIY presynaptic sites. The AIY presynaptic pattern is stereotypic: the ventral asynaptic Zone 1 region (dashed box), the synaptic enriched Zone 2 region (skewed bracket), and the distal synaptic sparse Zone 3 region (vertical bracket). (B’) A cartoon describes the cross section at the AIY Zone 2 region. The molecular mechanisms underlying AIY synaptic subcellular specificity during embryogenesis are described in (A), whereas those during postembryonic development are described in (B’).
The role of UNC-6/netrins signaling in synaptic subcellular specificity is also reported in Drosophila [23]. The dendrites of A08a interneurons can be anatomically divided into medial and lateral regions, which receive inputs from dbd and A021 neurons, respectively. When overexpressing netrin receptor UNC-5 in the dbd neurons, it forms ectopic synapses on A08a lateral dendrites to avoid the netrins in the middle line region [23], suggesting the repulsive role of UNC-5 in synaptic subcellular targeting. Studies from both C. elegans and Drosophila suggest that netrin regulates synaptic subcellular specificity by promoting or inhibiting synaptogenesis depending on the receptors. The UNC-40 receptor is pro-synaptogenic, whereas the UNC-5 is anti-synaptogenic. Similarly, netrin-1 and DCC are involved in synaptic formation during development in vertebrate brains [35,39]. In frog embryos, injecting exogenous netrin-1 into the optical tectum promotes presynaptic formation rapidly in retinal ganglion cells [35]. In rodents, netrin-1 promotes the number and strength of excitatory synapses between cortical neurons in embryonic neuronal culture [39]. In the ventral tegmental area (VTA) of adult mice, netrin-1 loss-of-function in GABAergic or dopaminergic neurons leads to excitatory synapse reduction, suggesting the important roles of netrin-1 in synaptic maintenance [40]. Although their functions in synaptic subcellular specificity have not been reported, the conserved roles in pro-synaptogenesis in vertebrates suggest that netrin/DCC signaling may also regulate synaptic subcellular specificity in mammals.
2.2. Semaphorins
Semaphorins are a class of proteins with a roughly 500 amino acid semaphorin domain, which are subdivided into eight subclasses based on their origins. The subclasses 1 and 2 are from invertebrates, 3–7 are from vertebrates, and the V is from virus [41]. The subclasses 2–3 and V are secreted, whereas the rest are membrane bound [41]. Semaphorins have a broad spectrum function in neurodevelopment, including neurogenesis, cell migration, and axon guidance [42]. Recent studies indicate that semaphorins are involved in synaptic subcellular specificity.
In C. elegans, axons of DA8 and DA9 motor neurons overlap in the dorsal nerve cord. However, each of them innervates a unique, non-overlapping muscle region [43]. This “synaptic tiling” phenotype is partially regulated by two transmembrane semaphorins SMP-1 and SMP-2 [22]. In SMP-1 mutants, presynaptic sites of DA8 and DA9 fail to tile, and they overlap. Interestingly, transmembrane SMP-1/SMP-2 and the receptor PLX-1 both act in the DA9 neuron to prevent the presynaptic sites overlapping between DA8 and DA9 through a cis interaction (Figure 1A). SMP-1/SMP-2 and PLX-1 are enriched at the asynaptic region tightly adjacent to the synaptic region to inhibit the synapse formation most likely through RAP-2/Rap2A GTPase, which regulates synaptic assembly through the effector MIG-15/TNIK (Figure 1A) [22,44].
Mammalian semaphorins also regulate synaptic subcellular specificity. Semaphorin3F (Sema3F), a secreted form of semaphorins, is required for the synaptic subcellular specificity in mouse dentate gyrus granule cells and neocortical pyramidal cells [25]. Specifically, mice without Sema3F display increased spine number and size in the approximal but not distal dendrites. Sema3F inhibits spine formation in the specific subcellular region by interacting with the holoreceptor Neuropilin2 and plexin A3 [25]. Transmembrane semaphorins also regulate synaptic formation and specificity. Sema5A, 5B, and 6A regulate the laminal-specific innervation in the inner plexiform layer [45]. Semaphorin4A and 4D promote, while semaphorin5A inhibits, synapse formation in hippocampal neurons [46,47,48,49]. These studies clearly demonstrate the conserved roles of semaphorins in regulating synaptic subcellular specificity.
2.3. Wnts
Wnts are highly conserved glycoproteins involved in many aspects of neurodevelopment and plasticity, including neurogenesis, axon guidance, and synaptogenesis [50,51]. The defects of the Wnt signaling are associated with many neurological disorders, including autism spectrum disorder (ASD), Alzheimer’s disease (AD), and Parkinson’s disease (PD) [52,53,54]. Wnts are also involved in synaptic subcellular specificity.
Studies in C. elegans demonstrate that Wnt signaling plays a complex role in presynaptic formation. Klassen and his colleagues found that the Wnt ligand LIN-44 secreted from the tail prevents synaptic formation at the DA9 posterior asynaptic region, which is mediated by localizing the frizzled receptor LIN-17 in that region. The frizzled receptor prevents local synaptic assembly by regulating the intracellular component disheveled DSH-1 (Figure 1A) [21]. The presynaptic subcellular specificity of DA8 motor neurons, whose synaptic sites are localized anteriorly next to DA9′s, is regulated by another Wnt, EGL-20 [55]. EGL-20, secreted from more anterior sites, locates a different frizzled receptor MIG-1 to the DA8 posterior asynaptic region, by which it inhibits the presynaptic assembly in the DA8 posterior region (Figure 1A) [55]. Therefore, two Wnt signaling pathways collaborate to regulate DA8/DA9 synaptic subcellular specificity by inhibiting synaptic formation at their posterior regions [21,55]. These data collectively demonstrate that Wnt signaling regulates synaptic subcellular specificity by inhibiting synaptic assembly. Interestingly, Wnts also play positive roles in synaptogenesis [56,57,58]. The Wnt ligand CWN-2 promotes synaptogenesis in AIY through a canonical Wnt signaling pathway [58].
In mammals, Wnt-7a expressed in granule cells promotes presynaptic assembly in cerebellum mossy fibers [59,60]. In the hippocampus, Wnt7a positively regulates dendritic spine development [61]. In contrast, Wnt5a has been showed to have both pro- and anti-synaptogenic functions. In mouse hippocampal tissue culture, Wnt5a activates a noncanonical signaling pathway and decreases active β-catenin levels, resulting in a reduction in the number of presynaptic puncta in hippocampal cultures [56]. In rat primary culture systems, Wnt5a promotes the recruitment of PSD-95 to the dendritic spine and regulates the dendritic spine morphogenesis [62,63]. The pro- and anti-synaptogenic roles of Wnts are probably due to the diversity and complexity of Wnt signaling and neurons.
Wnt signaling can also regulate synaptic subcellular specificity in combination with other factors. In C. elegans, loss of EGL-20/Wnt leads to axonal tiling defects in the DD5 and DD6 neurons, whereas presynaptic tiling is unaffected. However, when both EGL-20/Wnt and the gap junction protein UNC-9 are ablated, the synaptic tiling between these two neurons is lost [64].
2.4. Slit/Robo
Robo and the ligand Slits, first identified as a cue for preventing axons from recrossing midline in both Drosophila and mammals [65,66,67], are now found involved in developmental and pathogenic processes, including neurogenesis, angiogenesis, and cancer progression [68,69,70]. Recent studies find that Slits/Robo signaling is also involved in the synaptic subcellular specificity [23]. In Drosophila, Slit is highly expressed in the A08a midline domain, where low-Robo dbd neurons form synapses. When overexpressing robo-2 in dbd neurons, they will avoid the normal medial region and form synapses ectopically in the lateral region [23]. In mice, Robo2 is involved in establishing synaptic specificity in hippocampal CA1. Postsynaptically, Robo2 is present and necessary for the formation of excitatory (E) synapses, particularly in proximal dendritic compartments, with no impact on inhibitory (I) synapses and limited relevance to distal dendritic compartments [24].
Interestingly, these secreted synaptic subcellular specificity regulators also regulate axon guidance. Axon or dendrite guidance and synaptogenesis are sequential steps in neurodevelopment. Factors perturbating guidance will also affect synaptogenesis and specificity later. Therefore, it is challenging to determine whether the synaptic subcellular defects in the above-mentioned mutants happen during or after the axon guidance process. Future work should address this question.
3. Cell Adhesion/Transmembrane Molecules
Cell adhesion molecules (CAMs) are a group of cell membrane proteins regulating cell–cell and cell–extracellular matrix (ECM) interactions [71]. CAMs are recognized as key regulators in synaptic formation and organization [72,73,74]. Many of them are also involved in synaptic target recognition and subcellular specificity [75,76,77,78,79,80,81]. Therefore, they are often closely associated with various neurodevelopment and psychiatric disorders [82,83]. Here, we will focus on the role of immunoglobulin superfamily cell adhesion molecules (IgSF CAMs). Some other CAMs are discussed either with secreted (transmembrane semaphorins) or intracellular (latrophilin, teneurin, FLRT, and Nrg) regulators.
IgSF CAMs are a superfamily of adhesion molecules with various numbers of immunoglobulin domains. This family of CAMs plays an important role in synaptic development. For example, SynCAMs promote synaptogenesis in vitro through homophilic Ig domain interactions [84]; Sidekicks regulate laminar targeting in the inner plexiform layer during vertebrate retina development [85]. Recent studies have indicated that IgSF CAMs are involved in synaptic subcellular specificity in both invertebrates and vertebrates.
C. elegans hermaphrodite-specific neurons (HSNs) are located in the middle of the body close to the vulva, with a long process extending to the nerve ring in the head. The left HSN (HSNL) forms synapses specifically onto the vulval muscle interfaces. The presynaptic location specificity of HSNL is mediated by the heterophilic interactions between two IgSF CAMs SYG-1 and SYG-2 [86,87]. The ligand SYG-2 is expressed in vulval epithelial cells. It binds to and recruits SYG-1 in the HSNL axon at the specific subcellular sites and promotes local F-actin assembly. Then, F-actin promotes presynaptic assembly by interacting with its binding partner NAB-1 (Figure 1A) [88].
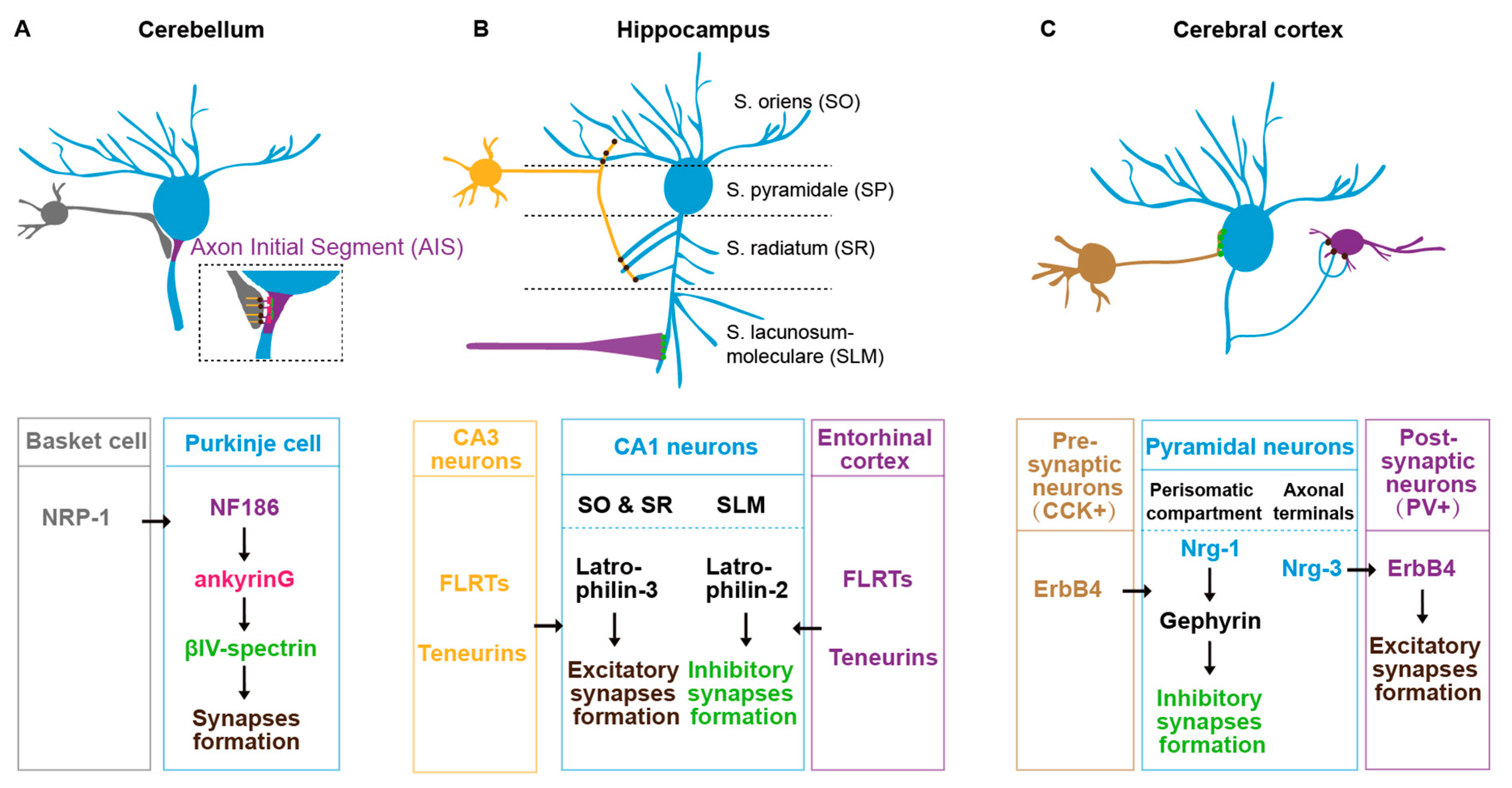
Several IgSF family L1CAMs have been found to regulate synaptic subcellular specificity in mice. In the cerebellum, basket cells form synaptic connections specifically onto the axon initial segment (AIS) of Purkinje cells [89]). This specificity is mediated by L1CAM neurofascin186 (NF186). NF186 forms an ankyrinG-dependent gradient along the AIS-soma axis of the Purkinje cell [89]. The NF186 expressed in AIS trans-synaptic binds to the Neuropilin-1 (NRP1) in basket cells to promote the synaptic assembly at the specific subcellular sites (Figure 2A) [90]. In the neocortex, L1CAM regulates the chandelier cells to form synaptic structure specifically onto the pyramidal AIS, which also requires ankyrinG in the pyramidal neurons [91]. In the cingulate cortex, L1CAM and ankyrin regulate basket cells to target onto the soma of pyramidal cells [92]. Disruption of L1CAM-ankyrin binding causes a significant decrease in the synaptic number between basket cells and the soma of pyramidal cells [92]. In addition, heterophilic interactions of different Ig family members between presynaptic and postsynaptic membranes are required for the synaptic assembly at specific subcellular locations [93]. For example, in the spinal cord, the presynaptic inhibitory axon–axon synapses are specified by two L1 family immunoglobulin NrCAM/CHL1 expressed in GABAergic interneurons and another IgSF CAM NB2 in the sensory neurons [93,94]. Therefore, the role of IgSF family CAMs in synaptic subcellular specificity seems very common in different species and cross CNS regions.
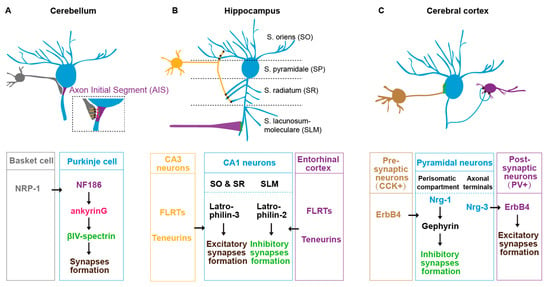
Figure 2.
Postsynaptic subcellular specificity in mouse brain. (A) The synaptic subcellular specificity between basket cells (grey) and Purkinje cells (blue) axon initial segment (AIS) in mouse cerebellum. (B) The synaptic subcellular specificity of the CA1 pyramidal neurons (blue) in mouse hippocampus. The dendrites in the S. oriens (SO) and S. radiatum (SR) regions receive synaptic inputs from CA3 pyramidal neurons (yellow), whereas the dendrites in S. lacunosum-moleculare (SLM) receive synaptic inputs from entorhinal cortex (purple). (C) The synaptic subcellular specificity of pyramidal neurons (blue) in the cerebral cortex. Neuregulin (Nrg)1 and Nrg3 in pyramidal neurons are sorted into perisomatic and axonic domains, which are required for synaptic targeting from CCK neurons (brown) and to PV neurons (purple).
4. Intracellular Molecules
The extracellular signaling functions through transmembrane proteins and intracellular factors to regulate synaptic assembly. Therefore, the final executors are intracellular factors.
In C. elegans, CED-10/Rac1, MIG-10/Lamellipodin, CDC-42/CDC42, and PES-7/IQGAP are specifically localized to the AIY Zone 2 regions in response to extracellular signals mainly from VCSC glia, where they promote local presynaptic assembly [95,96]. Ectopic CDC-42 or PES-7 localization in the zone 1 region results in the ectopic presynaptic assembly (Figure 1B’) [95].
In the mouse cerebral cortex, presynaptic somatostatin-expressing (SST+) interneurons, parvalbumin-expressing (PV+) basket cells, and chandelier cells form synapses specifically on the distal dendrites, somata/proximal dendrites, and axon initial segments of pyramidal cells through specifically expressing Cbln4, Lgi2, and Fgf13, respectively [97]. Interestingly, Cbln4 only promotes the synaptic formation when expressed in SST+, but not in basket cells or chandelier cells ([97], suggesting that the synaptic subcellular specificity regulated by Cbln4 is cell-type specific.
Synaptic factor sorting and trafficking are required for the synaptic subcellular specificity. Local synaptic regulators have to be sorted and transported to the synaptic sites. Therefore, the sorting and trafficking of those synaptic regulators determine the synaptic subcellular specificity. In the mouse hippocampal CA1 region, the pyramidal neurons form regional-specific synaptic connections regulated by subcellular sorting synaptic adhesion molecules [98,99,100]. For example, the stratum oriens (SO) and stratum radiatum (SR) layers receive synaptic inputs from CA3 pyramidal neurons and the stratum lacunosum-moleculare (SLM) from the entorhinal cortex. This subcellular specificity is determined by subcellular specific sorting of the latrophilin-2/latrophilin-3 GPCR in the postsynaptic CA1 pyramidal neurons. The latrophilin-2 is localized specifically to SLM, whereas the latrophilin-3 is localized to SO and SR, where they promote synaptic assembly through binding to two presynaptic ligands teneurin and fibronectin leucine-rich repeat transmembrane proteins (FLRTs) (Figure 2B) [99]. Similarly, in the cerebral cortex, the pyramidal neurons sort neuregulin (Nrg)1 and Nrg3 in distinct subcellular domains, which mediates the inhibitory input and excitatory output synapses in the perisomatic and axonic regions (Figure 2C) [101].
While intracellular sorting and trafficking of many proteins is regulated by extracellular signaling, some are sorted intrinsically. In neuromuscular junctions, the synaptic subcellular specificity of the muscle cells is determined by the muscle-expressed MuSK, which is independent of the presynaptic motor neurons [102,103,104]. The MuSK is specifically localized to the central region of the muscle, which dictates the location of motor axons and synapses [105].
In addition to proteins, RNA sorting may also be involved in synaptic specificity. Although mRNA sorting has not been found to regulate synaptic subcellular specificity, its synaptic localization and the local translation are well-recognized [106,107,108]. Future work should focus on understanding the molecular mechanisms underlying the sorting and trafficking of local synaptic regulators.
5. Neuronal Activity-Dependent Mechanism
In addition to the intrinsic factors, environment-dependent neuronal activity can also regulate neurodevelopment, including proliferation, differentiation, migration, axon guidance [109,110,111,112], and synaptic plasticity [113,114,115,116,117,118,119,120,121]. Recently, neuronal activity has also been shown to regulate synaptic subcellular specificity [122,123].
Environmental temperature can affect neuronal activity and synaptic subcellular specificity. A recent study found that C. elegans AIY presynaptic specificity is regulated by the temperature-sensitive glutamatergic ASH neurons (Figure 1A) [123]. High cultivated temperature or overexpressing vesicle glutamate transporter EAT-4 induces ectopic synaptic formation in the AIY asynaptic Zone 1, which is mediated by a pair of presynaptic glutamatergic ASH neurons. Interestingly, these glutamate signals are sensed by a pair of inhibitory glutamate-gated chloride channels, GLC-3 and GLC-4, in the AIY interneurons [123]. The AIY synaptic defect induced by eat-4(OE) appears at the newly hatched larval L1 stage, suggesting that the glutamatergic neuronal activity regulates AIY synaptic subcellular specificity during embryogenesis, and it most likely regulates the synaptic formation, not the plasticity. Consistently, high-temperature treatment, specifically during the embryonic development stage, is sufficient to induce the synaptic subcellular specificity defects [123]. In mice, enriched environments have been shown to promote the expression of transcription factor NPAS4 in hippocampal pyramidal neurons, which specifically increases somatic inhibitory synapses through upregulating BDNF [122]. The above studies demonstrate that environmental conditions play an important role in synaptic subcellular specificity.
6. Glia
Glia are closely associated with neurons and synapses, with profound effects on synaptogenesis, synaptic pruning and maturation, and synaptic subcellular specificity [89,124,125]. In C. elegans, as discussed in previous sections, the VCSC glia promote or maintain presynaptic structure at specific subcellular regions [20,126].
Glial cells also regulate synaptic subcellular specificity in vertebrates. In the mouse cerebral cortex, Bergmann glial (BG) fibers guide the GABAergic stellate axons towards Purkinje cell dendrites, which are mediated by the Close Homologue of L1(CHL1). CHL1 localized along BG fibers promotes stellate innervation of Purkinje dendrites [127]. Microglia also play important roles in synaptogenesis during adulthood. Reshef and his colleagues examined the effects of microglial depletion on the density and size of spines on the distal apical dendrites of adult-born granule cells (abGCs) within the external plexiform layer (EPL), where GCs form reciprocal synapses with mitral cells (MCs). They found that spine density was 25% lower in microglial depletion mice when compared with control mice [128]. The critical role of glia in specific synaptic pruning or specific neuronal compartments suggests their contribution to synaptic subcellular specificity [129,130]. Together, these findings indicate that glia have a general role in the modulation of synaptogenesis throughout the entire life span of animals.
7. Non-Neural Cells
During development, neural and non-neural cells are precisely coordinated. Therefore, communication between neural and non-neural tissues is critical for cell–cell and synaptic connection. Studies have demonstrated that non-neural tissues, including the epidermis, muscle, and intestine, are involved in synaptic development and specificity [37,55,58,87,126,131].
In the C. elegans nerve system, the presynaptic subcellular distribution in AIY interneurons is determined before hatching. Although animals increase up to 100-fold in volume during postembryonic development, the presynaptic pattern is maintained. The maintenance of AIY presynaptic pattern requires epidermal expressed the sialin homolog CIMA-1 specifically during the postembryonic developmental stage [126]. In cima-1 loss-of-function mutants, presynaptic sites are normal in newly hatched animals. However, synaptic subcellular defects appear since the adult stage, which requires normal growth. The size of C. elegans is determined mainly by the epidermal- or muscle-expressed genes. Animals with dumpy morphologies, however, do not form the ectopic synapses in cima-1 mutants [126]. Therefore, synaptic subcellular specificity is also affected by genes or molecules expressed in non-neuronal tissues, particularly those involved in body size growth. Muscle activity contributes to body elongation [132,133,134]. Interestingly, MIG-17, a secreted ADAMTS family metalloprotease, which is mainly expressed in the muscle tissue, regulates the AIY synaptic subcellular specificity [131]. Intestinal Wnt and neuropeptide signaling can also regulate synaptogenesis in the nervous system [58,135]. The above studies indicate that non-neuronal tissues also play a critical role in synaptic development and subcellular specificity.
8. Conclusions and the Remaining Questions
Synaptic specificity is a prerequisite for normal neuronal functions and animal behaviors. With the increasing levels of resolution, the concept of synaptic subcellular specificity begins to be appreciated. Studies have shown that synaptic subcellular specificity is a conserved phenomenon present in both invertebrates and vertebrates. Through advanced imaging technology, we have gained some cellular and molecular insights. However, this is only the tip of the iceberg. Many questions remain to be addressed. First, the human brain has a myriad of types of neurons, and different types of neurons most likely use different sets of molecules to determine the synaptic subcellular specificity [8,11,136]. We only know very few of them so far. Secondly, although we have identified some molecules required for synaptic subcellular specificity, it is largely unknown how the synaptic subcellular border is determined. Thirdly, intracellular sorting of synaptic regulators is fundamental for synaptic subcellular specificity. However, it is largely unknown how subcellular sorting is regulated. Fourthly, we know very little about the role of synaptic remodeling at the subcellular level [137,138]. Finally, we have a lot to learn about the roles of non-neural tissues and neuronal activity in regulating synaptic subcellular specificity. Future studies should address those questions.
Funding
Work in the Shao lab is supported by the Ministry of Science and Technology, China (2021YFA0909300), National Natural Science Foundation of China (32170828).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank members of the Shao lab for their insightful comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hendi, A.; Kurashina, M.; Mizumoto, K. Intrinsic and extrinsic mechanisms of synapse formation and specificity in C. elegans. Cell. Mol. Life Sci. 2019, 76, 2719–2738. [Google Scholar] [CrossRef] [PubMed]
- Calahorro, F.; Izquierdo, P.G. The presynaptic machinery at the synapse of C. elegans. Invertebr. Neurosci. 2018, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.; Haucke, V. Clathrin-mediated endocytosis at synapses. Traffic 2007, 8, 1129–1136. [Google Scholar] [CrossRef]
- Sudhof, T.C. The synaptic vesicle cycle: A cascade of protein-protein interactions. Nature 1995, 375, 645–653. [Google Scholar] [CrossRef]
- Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef] [PubMed]
- Holtmaat, A.; Svoboda, K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009, 10, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.; Shao, Z.; Colón-Ramos, D.A. The cell biology of synaptic specificity during development. Curr. Opin. Neurobiol. 2013, 23, 1018–1026. [Google Scholar] [CrossRef]
- Huang, Z.J. Subcellular organization of GABAergic synapses: Role of ankyrins and L1 cell adhesion molecules. Nat. Neurosci. 2006, 9, 163–166. [Google Scholar] [CrossRef]
- Sanes, J.R.; Yamagata, M. Many Paths to Synaptic Specificity. Annu. Rev. Cell Dev. Biol. 2009, 25, 161–195. [Google Scholar] [CrossRef]
- Shen, K.; Scheiffele, P. Genetics and Cell Biology of Building Specific Synaptic Connectivity. Annu. Rev. Neurosci. 2010, 33, 473–507. [Google Scholar] [CrossRef]
- Yogev, S.; Shen, K. Cellular and molecular mechanisms of synaptic specificity. Annu. Rev. Cell Dev. Biol. 2014, 30, 417–437. [Google Scholar] [CrossRef]
- Peters, A.; Feldman, M.L. The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. I. General description. J. Neurocytol. 1976, 5, 63–84. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.J.; Kalinski, C.A.; Hobert, O. Neuronal contact predicts connectivity in the C. elegans brain. Curr. Biol. 2023, 33, 2315–2320.e2. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.L.; Moradi, K.; Ascoli, G.A. Weighing the Evidence in Peters’ Rule: Does Neuronal Morphology Predict Connectivity? Trends Neurosci. 2017, 40, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J. Synaptic configuration and reconfiguration in the neocortex are spatiotemporally selective. Anat. Sci. Int. 2024, 99, 17–33. [Google Scholar] [CrossRef]
- Dickson, B.J. Molecular mechanisms of axon guidance. Science 2002, 298, 1959–1964. [Google Scholar] [CrossRef]
- Kolodkin, A.L.; Tessier-Lavigne, M. Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harb. Perspect. Biol. 2011, 3, a001727. [Google Scholar] [CrossRef]
- Silhankova, M.; Korswagen, H.C. Migration of neuronal cells along the anterior-posterior body axis of C. elegans: Wnts are in control. Curr. Opin. Genet. Dev. 2007, 17, 320–325. [Google Scholar] [CrossRef]
- Stoeckli, E.T. Understanding axon guidance: Are we nearly there yet? Development 2018, 145, dev151415. [Google Scholar] [CrossRef]
- Colón-Ramos, D.A.; Margeta, M.A.; Kang, S. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 2007, 318, 103–106. [Google Scholar] [CrossRef]
- Klassen, M.P.; Shen, K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell 2007, 130, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, K.; Shen, K. Interaxonal interaction defines tiled presynaptic innervation in C. elegans. Neuron 2013, 77, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Sales, E.C.; Heckman, E.L.; Warren, T.L.; Doe, C.Q. Regulation of subcellular dendritic synapse specificity by axon guidance cues. eLife 2019, 8, e43478. [Google Scholar] [CrossRef]
- Blockus, H.; Rolotti, S.V.; Szoboszlay, M.; Peze-Heidsieck, E.; Ming, T.; Schroeder, A.; Apostolo, N.; Vennekens, K.M.; Katsamba, P.S.; Bahna, F.; et al. Synaptogenic activity of the axon guidance molecule Robo2 underlies hippocampal circuit function. Cell Rep. 2021, 37, 109828. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.S.; Rubio, M.E.; Clem, R.L.; Johnson, D.; Case, L.; Tessier-Lavigne, M.; Huganir, R.L.; Ginty, D.D.; Kolodkin, A.L. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature 2009, 462, 1065–1069. [Google Scholar] [CrossRef]
- Hedgecock, E.M.; Culotti, J.G.; Hall, D.H. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 1990, 4, 61–85. [Google Scholar] [CrossRef]
- Keino-Masu, K.; Masu, M.; Hinck, L.; Leonardo, E.D.; Chan, S.S.; Culotti, J.G.; Tessier-Lavigne, M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 1996, 87, 175–185. [Google Scholar] [CrossRef]
- Kennedy, T.E.; Serafini, T.; de la Torre, J.R.; Tessier-Lavigne, M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 1994, 78, 425–435. [Google Scholar] [CrossRef]
- Leonardo, E.D.; Hinck, L.; Masu, M.; Keino-Masu, K.; Ackerman, S.L.; Tessier-Lavigne, M. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 1997, 386, 833–838. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Doyle, J.L.; Serafini, T.; Kennedy, T.E.; Tessier-Lavigne, M.; Goodman, C.S.; Dickson, B.J. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron 1996, 17, 203–215. [Google Scholar] [CrossRef]
- Serafini, T.; Colamarino, S.A.; Leonardo, E.D.; Wang, H.; Beddington, R.; Skarnes, W.C.; Tessier-Lavigne, M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 1996, 87, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Serafini, T.; Kennedy, T.E.; Galko, M.J.; Mirzayan, C.; Jessell, T.M.; Tessier-Lavigne, M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 1994, 78, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Vosberg, D.E.; Leyton, M.; Flores, C. The Netrin-1/DCC guidance system: Dopamine pathway maturation and psychiatric disorders emerging in adolescence. Mol. Psychiatry 2020, 25, 297–307. [Google Scholar] [CrossRef]
- Wadsworth, W.G.; Bhatt, H.; Hedgecock, E.M. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 1996, 16, 35–46. [Google Scholar] [CrossRef]
- Manitt, C.; Nikolakopoulou, A.M.; Almario, D.R.; Nguyen, S.A.; Cohen-Cory, S. Netrin participates in the development of retinotectal synaptic connectivity by modulating axon arborization and synapse formation in the developing brain. J. Neurosci. 2009, 29, 11065–11077. [Google Scholar] [CrossRef][Green Version]
- Nelson, J.C.; Colon-Ramos, D.A. Serotonergic neurosecretory synapse targeting is controlled by netrin-releasing guidepost neurons in Caenorhabditis elegans. J. Neurosci. 2013, 33, 1366–1376. [Google Scholar] [CrossRef]
- Poon, V.Y.; Klassen, M.P.; Shen, K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature 2008, 455, 669–673. [Google Scholar] [CrossRef] [PubMed]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. 1986, 314, 1–340. [Google Scholar]
- Goldman, J.S.; Ashour, M.A.; Magdesian, M.H.; Tritsch, N.X.; Harris, S.N.; Christofi, N.; Chemali, R.; Stern, Y.E.; Thompson-Steckel, G.; Gris, P.; et al. Netrin-1 promotes excitatory synaptogenesis between cortical neurons by initiating synapse assembly. J. Neurosci. 2013, 33, 17278–17289. [Google Scholar] [CrossRef]
- Cline, M.M.; Juarez, B.; Hunker, A.; Regiarto, E.G.; Hariadi, B.; Soden, M.E.; Zweifel, L.S. Netrin-1 regulates the balance of synaptic glutamate signaling in the adult ventral tegmental area. eLife 2023, 12, e83760. [Google Scholar] [CrossRef]
- Goodman, C.S.; Kolodkin, A.L.; Luo, Y.; Püschel, A.W.; Raper, J.A. Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell 1999, 97, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Limoni, G.; Niquille, M. Semaphorins and Plexins in central nervous system patterning: The key to it all? Curr. Opin. Neurobiol. 2021, 66, 224–232. [Google Scholar] [CrossRef]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1976, 275, 327–348. [Google Scholar] [CrossRef]
- Chen, X.; Shibata, A.C.; Hendi, A.; Kurashina, M.; Fortes, E.; Weilinger, N.L.; MacVicar, B.A.; Murakoshi, H.; Mizumoto, K. Rap2 and TNIK control Plexin-dependent tiled synaptic innervation in C. elegans. eLife 2018, 7, e38801. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, R.L.; Nguyen-Ba-Charvet, K.T.; Parray, A.; Badea, T.C.; Chedotal, A.; Kolodkin, A.L. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature 2011, 470, 259–263. [Google Scholar] [CrossRef]
- Adel, S.S.; Pranske, Z.J.; Kowalski, T.F.; Kanzler, N.; Ray, R.; Carmona, C.; Paradis, S. Plexin-B1 and Plexin-B2 play non-redundant roles in GABAergic synapse formation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, S.H.; Song, J.; Mironova, Y.; Ming, G.L.; Kolodkin, A.L.; Giger, R.J. Semaphorin 5A inhibits synaptogenesis in early postnatal- and adult-born hippocampal dentate granule cells. eLife 2014, 3, e04390. [Google Scholar] [CrossRef]
- Kuzirian, M.S.; Moore, A.R.; Staudenmaier, E.K.; Friedel, R.H.; Paradis, S. The class 4 semaphorin Sema4D promotes the rapid assembly of GABAergic synapses in rodent hippocampus. J. Neurosci. 2013, 33, 8961–8973. [Google Scholar] [CrossRef]
- McDermott, J.E.; Goldblatt, D.; Paradis, S. Class 4 Semaphorins and Plexin-B receptors regulate GABAergic and glutamatergic synapse development in the mammalian hippocampus. Mol. Cell. Neurosci. 2018, 92, 50–66. [Google Scholar] [CrossRef]
- Ciani, L.; Salinas, P.C. WNTs in the vertebrate nervous system: From patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005, 6, 351–362. [Google Scholar] [CrossRef]
- Zwamborn, R.A.J.; Snijders, C.; An, N.; Thomson, A.; Rutten, B.P.F.; de Nijs, L. Wnt Signaling in the Hippocampus in Relation to Neurogenesis, Neuroplasticity, Stress and Epigenetics. Prog. Mol. Biol. Transl. Sci. 2018, 158, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.A.; Khan, M.; V, M.; Kar, D. The Molecular Basis of Wnt/β-Catenin Signaling Pathways in Neurodegenerative Diseases. Int. J. Cell Biol. 2023, 2023, 9296092. [Google Scholar] [CrossRef]
- Dickins, E.M.; Salinas, P.C. Wnts in action: From synapse formation to synaptic maintenance. Front. Cell. Neurosci. 2013, 7, 162. [Google Scholar] [CrossRef]
- Fazel Darbandi, S.; Robinson Schwartz, S.E.; Pai, E.L.; Everitt, A.; Turner, M.L.; Cheyette, B.N.R.; Willsey, A.J.; State, M.W.; Sohal, V.S.; Rubenstein, J.L.R. Enhancing WNT Signaling Restores Cortical Neuronal Spine Maturation and Synaptogenesis in Tbr1 Mutants. Cell Rep. 2020, 31, 107495. [Google Scholar] [CrossRef]
- Mizumoto, K.; Shen, K. Two Wnts instruct topographic synaptic innervation in C. elegans. Cell Rep. 2013, 5, 389–396. [Google Scholar] [CrossRef]
- Davis, E.K.; Zou, Y.; Ghosh, A. Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Dev. 2008, 3, 32. [Google Scholar] [CrossRef]
- Park, M.; Shen, K. WNTs in synapse formation and neuronal circuitry. EMBO J. 2012, 31, 2697–2704. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Q.; Shao, Z. Wnts Promote Synaptic Assembly Through T-Cell Specific Transcription Factors in Caenorhabditis elegans. Front. Mol. Neurosci. 2018, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.C.; Lucas, F.R.; Salinas, P.C. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell 2000, 100, 525–535. [Google Scholar] [CrossRef]
- Lucas, F.R.; Salinas, P.C. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev. Biol. 1997, 192, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Ciani, L.; Boyle, K.A.; Dickins, E.; Sahores, M.; Anane, D.; Lopes, D.M.; Gibb, A.J.; Salinas, P.C. Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca2+/Calmodulin-dependent protein kinase II. Proc. Natl. Acad. Sci. USA 2011, 108, 10732–10737. [Google Scholar] [CrossRef] [PubMed]
- Farias, G.G.; Alfaro, I.E.; Cerpa, W.; Grabowski, C.P.; Godoy, J.A.; Bonansco, C.; Inestrosa, N.C. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J. Biol. Chem. 2009, 284, 15857–15866. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, D.; Lindsay, C.B.; Gonzalez-Billault, C.; Inestrosa, N.C. Wnt5a modulates dendritic spine dynamics through the regulation of Cofilin via small Rho GTPase activity in hippocampal neurons. J. Neurochem. 2021, 158, 673–693. [Google Scholar] [CrossRef] [PubMed]
- Hendi, A.; Niu, L.G.; Snow, A.W.; Ikegami, R.; Wang, Z.W.; Mizumoto, K. Channel-independent function of UNC-9/Innexin in spatial arrangement of GABAergic synapses in C. elegans. eLife 2022, 11, e80555. [Google Scholar] [CrossRef] [PubMed]
- Brose, K.; Bland, K.S.; Wang, K.H.; Arnott, D.; Henzel, W.; Goodman, C.S.; Tessier-Lavigne, M.; Kidd, T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 1999, 96, 795–806. [Google Scholar] [CrossRef]
- Kidd, T.; Bland, K.S.; Goodman, C.S. Slit Is the Midline Repellent for the Robo Receptor in Drosophila. Cell 1999, 96, 785–794. [Google Scholar] [CrossRef]
- Kidd, T.; Brose, K.; Mitchell, K.J.; Fetter, R.D.; Tessier-Lavigne, M.; Goodman, C.S.; Tear, G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 1998, 92, 205–215. [Google Scholar] [CrossRef]
- Blockus, H.; Chédotal, A. Slit-Robo signaling. Development 2016, 143, 3037–3044. [Google Scholar] [CrossRef]
- Kim, M.; Lee, C.H.; Barnum, S.J.; Watson, R.C.; Li, J.; Mastick, G.S. Slit/Robo signals prevent spinal motor neuron emigration by organizing the spinal cord basement membrane. Dev. Biol. 2019, 455, 449–457. [Google Scholar] [CrossRef]
- Mastick, G.S.; Farmer, W.T.; Altick, A.L.; Nural, H.F.; Dugan, J.P.; Kidd, T.; Charron, F. Longitudinal axons are guided by Slit/Robo signals from the floor plate. Cell Adhes. Migr. 2010, 4, 337–341. [Google Scholar] [CrossRef][Green Version]
- Kim, H.N.; Ruan, Y.; Ogana, H.; Kim, Y.M. Cadherins, Selectins, and Integrins in CAM-DR in Leukemia. Front. Oncol. 2020, 10, 592733. [Google Scholar] [CrossRef]
- De Arce, K.P.; Ribic, A.; Chowdhury, D.; Watters, K.; Thompson, G.J.; Sanganahalli, B.G.; Lippard, E.T.C.; Rohlmann, A.; Strittmatter, S.M.; Missler, M.; et al. Concerted roles of LRRTM1 and SynCAM 1 in organizing prefrontal cortex synapses and cognitive functions. Nat. Commun. 2023, 14, 459. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, X.J.; Yang, J.; Li, S.; Li, L.; Lv, X.; Ma, J.; Shi, S.H. Developmental neuronal origin regulates neocortical map formation. Cell Rep. 2023, 42, 112170. [Google Scholar] [CrossRef]
- Sudhof, T.C. The cell biology of synapse formation. J. Cell Biol. 2021, 220, e202103052. [Google Scholar] [CrossRef]
- Dalva, M.B.; McClelland, A.C.; Kayser, M.S. Cell adhesion molecules: Signalling functions at the synapse. Nat. Rev. Neurosci. 2007, 8, 206–220. [Google Scholar] [CrossRef]
- Galindo, S.E.; Shin, G.J.; Millard, S.S.; Grueber, W.B. Regulated alternative splicing of Dscam2 is required for somatosensory circuit wiring. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gerrow, K.; El-Husseini, A. Cell adhesion molecules at the synapse. Front. Biosci. 2006, 11, 2400–2419. [Google Scholar] [CrossRef][Green Version]
- Obst-Pernberg, K.; Redies, C. Cadherins and synaptic specificity. J. Neurosci. Res. 1999, 58, 130–138. [Google Scholar] [CrossRef]
- Prigge, C.L.; Dembla, M.; Sharma, A.; El-Quessny, M.; Kozlowski, C.; Paisley, C.E.; Miltner, A.M.; Johnson, T.M.; Della Santina, L.; Feller, M.B.; et al. Rejection of inappropriate synaptic partners in mouse retina mediated by transcellular FLRT2-UNC5 signaling. Dev. Cell 2023, 58, 2080–2096.e7. [Google Scholar] [CrossRef] [PubMed]
- Sytnyk, V.; Leshchyns’ka, I.; Schachner, M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci. 2017, 40, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Dombrovski, M.; Mirshahidi, P.; Nern, A.; LoCascio, S.A.; Zipursky, S.L.; Kurmangaliyev, Y.Z. Brain wiring determinants uncovered by integrating connectomes and transcriptomes. Curr. Biol. 2023, 33, 3998–4005.e6. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Walker, D.I.; Lin, Y.; Smith, M.R.; Mandl, K.D.; Jones, D.P.; Kong, S.W. Association between Neuroligin-1 polymorphism and plasma glutamine levels in individuals with autism spectrum disorder. eBioMedicine 2023, 95, 104746. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, X. SynCAMs in Normal Vertebrate Neural Development and Neuropsychiatric Disorders: From the Perspective of the OCAs. Mol. Neurobiol. 2023. [Google Scholar] [CrossRef]
- Biederer, T.; Sara, Y.; Mozhayeva, M.; Atasoy, D.; Liu, X.; Kavalali, E.T.; Südhof, T.C. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 2002, 297, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, M.; Weiner, J.A.; Sanes, J.R. Sidekicks: Synaptic Adhesion Molecules that Promote Lamina-Specific Connectivity in the Retina. Cell 2002, 110, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Bargmann, C.I. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell 2003, 112, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Fetter, R.D.; Bargmann, C.I. Synaptic Specificity Is Generated by the Synaptic Guidepost Protein SYG-2 and Its Receptor, SYG-1. Cell 2004, 116, 869–881. [Google Scholar] [CrossRef]
- Chia, P.H.; Patel, M.R.; Shen, K. NAB-1 instructs synapse assembly by linking adhesion molecules and F-actin to active zone proteins. Nat. Neurosci. 2012, 15, 234–242. [Google Scholar] [CrossRef]
- Ango, F.; Cristo, G.d.; Higashiyama, H.; Bennett, V.; Wu, P.; Huang, Z.J. Ankyrin-Based Subcellular Gradient of Neurofascin, an Immunoglobulin Family Protein, Directs GABAergic Innervation at Purkinje Axon Initial Segment. Cell 2004, 119, 257–272. [Google Scholar] [CrossRef]
- Telley, L.; Cadilhac, C.; Cioni, J.M.; Saywell, V.; Jahannault-Talignani, C.; Huettl, R.E.; Sarrailh-Faivre, C.; Dayer, A.; Huber, A.B.; Ango, F. Dual Function of NRP1 in Axon Guidance and Subcellular Target Recognition in Cerebellum. Neuron 2016, 91, 1276–1291. [Google Scholar] [CrossRef]
- Tai, Y.; Gallo, N.B.; Wang, M.; Yu, J.R.; Van Aelst, L. Axo-axonic Innervation of Neocortical Pyramidal Neurons by GABAergic Chandelier Cells Requires AnkyrinG-Associated L1CAM. Neuron 2019, 102, 358–372.e9. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Maness, P.F. Perisomatic GABAergic innervation in prefrontal cortex is regulated by ankyrin interaction with the L1 cell adhesion molecule. Cereb. Cortex 2010, 20, 2684–2693. [Google Scholar] [CrossRef]
- Ashrafi, S.; Betley, J.N.; Comer, J.D.; Brenner-Morton, S.; Bar, V.; Shimoda, Y.; Watanabe, K.; Peles, E.; Jessell, T.M.; Kaltschmidt, J.A. Neuronal Ig/Caspr Recognition Promotes the Formation of Axoaxonic Synapses in Mouse Spinal Cord. Neuron 2014, 81, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Betley, J.N.; Wright, C.V.; Kawaguchi, Y.; Erdelyi, F.; Szabo, G.; Jessell, T.M.; Kaltschmidt, J.A. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 2009, 139, 161–174. [Google Scholar] [CrossRef]
- Dong, X.; Jin, S.; Shao, Z. Glia Promote Synaptogenesis through an IQGAP PES-7 in C. elegans. Cell Rep. 2020, 30, 2614–2626.e2. [Google Scholar] [CrossRef]
- Stavoe, A.K.H.; Colon-Ramos, D.A. Netrin instructs synaptic vesicle clustering through Rac GTPase, MIG-10, and the actin cytoskeleton. J. Cell Biol. 2012, 197, 75–88. [Google Scholar] [CrossRef]
- Favuzzi, E.; Deogracias, R.; Marques-Smith, A.; Maeso, P.; Jezequel, J.; Exposito-Alonso, D.; Balia, M.; Kroon, T.; Hinojosa, A.J.; Maraver, E.F.; et al. Distinct molecular programs regulate synapse specificity in cortical inhibitory circuits. Science 2019, 363, 413–417. [Google Scholar] [CrossRef]
- Anderson, G.R.; Maxeiner, S.; Sando, R.; Tsetsenis, T.; Malenka, R.C.; Südhof, T.C. Postsynaptic adhesion GPCR latrophilin-2 mediates target recognition in entorhinal-hippocampal synapse assembly. J. Cell Biol. 2017, 216, 3831–3846. [Google Scholar] [CrossRef]
- Sando, R.; Jiang, X.; Südhof, T.C. Latrophilin GPCRs direct synapse specificity by coincident binding of FLRTs and teneurins. Science 2019, 363, eaav7969. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Vanderlinden, J.; Vints, K.; Ribeiro, L.F.; Vennekens, K.M.; Gounko, N.V.; Wierda, K.D.; de Wit, J. A Modular Organization of LRR Protein-Mediated Synaptic Adhesion Defines Synapse Identity. Neuron 2018, 99, 329–344.e7. [Google Scholar] [CrossRef]
- Exposito-Alonso, D.; Osório, C.; Bernard, C.; Pascual-García, S.; del Pino, I.; Marín, O.; Rico, B. Subcellular sorting of neuregulins controls the assembly of excitatory-inhibitory cortical circuits. eLife 2020, 9, e57000. [Google Scholar] [CrossRef]
- Lin, W.; Burgess, R.W.; Dominguez, B.; Pfaff, S.L.; Sanes, J.R.; Lee, K.F. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 2001, 410, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Arber, S.; William, C.; Li, L.; Tanabe, Y.; Jessell, T.M.; Birchmeier, C.; Burden, S.J. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 2001, 30, 399–410. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Prescott, E.D.; Burden, S.J.; Wang, J.C. DNA topoisomerase IIbeta and neural development. Science 2000, 287, 131–134. [Google Scholar] [CrossRef]
- Kim, N.; Burden, S.J. MuSK controls where motor axons grow and form synapses. Nat. Neurosci. 2008, 11, 19–27. [Google Scholar] [CrossRef]
- Glock, C.; Heumüller, M.; Schuman, E.M. mRNA transport & local translation in neurons. Curr. Opin. Neurobiol. 2017, 45, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Holt, C.E.; Martin, K.C.; Schuman, E.M. Local translation in neurons: Visualization and function. Nat. Struct. Mol. Biol. 2019, 26, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.C.; Zukin, R.S. RNA trafficking and local protein synthesis in dendrites: An overview. J. Neurosci. 2006, 26, 7131–7134. [Google Scholar] [CrossRef]
- Luhmann, H.J.; Khazipov, R. Neuronal activity patterns in the developing barrel cortex. Neuroscience 2018, 368, 256–267. [Google Scholar] [CrossRef]
- Penn, A.A. Early Brain Wiring: Activity-Dependent Processes. Schizophr. Bull. 2001, 27, 337–347. [Google Scholar] [CrossRef]
- Spitzer, N.C. Electrical activity in early neuronal development. Nature 2006, 444, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Wamsley, B.; Fishell, G. Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat. Rev. Neurosci. 2017, 18, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef]
- Golovin, R.M.; Broadie, K. Developmental experience-dependent plasticity in the first synapse of the Drosophila olfactory circuit. J. Neurophysiol. 2016, 116, 2730–2738. [Google Scholar] [CrossRef] [PubMed]
- Grunwald Kadow, I.C. State-dependent plasticity of innate behavior in fruit flies. Curr. Opin. Neurobiol. 2019, 54, 60–65. [Google Scholar] [CrossRef]
- Haas, K.F.; Broadie, K. Roles of ubiquitination at the synapse. Biochim. Biophys. Acta 2008, 1779, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.P.; Hobert, O. Neurexin controls plasticity of a mature, sexually dimorphic neuron. Nature 2018, 553, 165–170. [Google Scholar] [CrossRef]
- Mansvelder, H.D.; Verhoog, M.B.; Goriounova, N.A. Synaptic plasticity in human cortical circuits: Cellular mechanisms of learning and memory in the human brain? Curr. Opin. Neurobiol. 2019, 54, 186–193. [Google Scholar] [CrossRef]
- Neves, G.; Cooke, S.F.; Bliss, T.V. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat. Rev. Neurosci. 2008, 9, 65–75. [Google Scholar] [CrossRef]
- Sachse, S.; Rueckert, E.; Keller, A.; Okada, R.; Tanaka, N.K.; Ito, K.; Vosshall, L.B. Activity-dependent plasticity in an olfactory circuit. Neuron 2007, 56, 838–850. [Google Scholar] [CrossRef]
- Zhao, H.; Nonet, M.L. A retrograde signal is involved in activity-dependent remodeling at a C. elegans neuromuscular junction. Development 2000, 127, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Bloodgood, B.L.; Sharma, N.; Browne, H.A.; Trepman, A.Z.; Greenberg, M.E. The activity-dependent transcription factor NPAS4 regulates domain-specific inhibition. Nature 2013, 503, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Witvliet, D.; Wu, M.; Kang, L.; Shao, Z. Temperature regulates synaptic subcellular specificity mediated by inhibitory glutamate signaling. PLoS Genet. 2021, 17, e1009295. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, A.P.; Allen, N.J. The diverse actions of astrocytes during synaptic development. Curr. Opin. Neurobiol. 2017, 47, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Farizatto, K.L.G.; Baldwin, K.T. Astrocyte-synapse interactions during brain development. Curr. Opin. Neurobiol. 2023, 80, 102704. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Watanabe, S.; Christensen, R.; Jorgensen, E.M.; Colon-Ramos, D.A. Synapse location during growth depends on glia location. Cell 2013, 154, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Ango, F.; Wu, C.; Van der Want, J.J.; Wu, P.; Schachner, M.; Huang, Z.J.; Ghosh, A. Bergmann Glia and the Recognition Molecule CHL1 Organize GABAergic Axons and Direct Innervation of Purkinje Cell Dendrites. PLoS Biol. 2008, 6, e103. [Google Scholar] [CrossRef]
- Reshef, R.; Kudryavitskaya, E.; Shani-Narkiss, H.; Isaacson, B.; Rimmerman, N.; Mizrahi, A.; Yirmiya, R. The role of microglia and their CX3CR1 signaling in adult neurogenesis in the olfactory bulb. eLife 2017, 6, e30809. [Google Scholar] [CrossRef]
- Baalman, K.; Marin, M.A.; Ho, T.S.; Godoy, M.; Cherian, L.; Robertson, C.; Rasband, M.N. Axon initial segment-associated microglia. J. Neurosci. 2015, 35, 2283–2292. [Google Scholar] [CrossRef]
- Favuzzi, E.; Huang, S.; Saldi, G.A.; Binan, L.; Ibrahim, L.A.; Fernandez-Otero, M.; Cao, Y.; Zeine, A.; Sefah, A.; Zheng, K.; et al. GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell 2021, 184, 4048–4063.e32. [Google Scholar] [CrossRef]
- Fan, J.; Ji, T.; Wang, K.; Huang, J.; Wang, M.; Manning, L.; Dong, X.; Shi, Y.; Zhang, X.; Shao, Z.; et al. A muscle-epidermis-glia signaling axis sustains synaptic specificity during allometric growth in Caenorhabditis elegans. eLife 2020, 9, e55890. [Google Scholar] [CrossRef]
- Chisholm, A.D.; Hardin, J. Epidermal morphogenesis. WormBook; The C. elegans Research Community, Ed.; WormBook Research Community: New York, NY, USA, 2005; pp. 1–22. [Google Scholar] [CrossRef]
- Chisholm, A.D.; Hsiao, T.I. The Caenorhabditis elegans epidermis as a model skin. I: Development, patterning, and growth. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Vuong-Brender, T.T.; Yang, X.; Labouesse, M.C. elegans Embryonic Morphogenesis. Curr. Top. Dev. Biol. 2016, 116, 597–616. [Google Scholar] [CrossRef]
- Shi, Y.; Qin, L.; Wu, M.; Zheng, J.; Xie, T.; Shao, Z. Gut neuroendocrine signaling regulates synaptic assembly in C. elegans. EMBO Rep. 2022, 23, e53267. [Google Scholar] [CrossRef] [PubMed]
- Apóstolo, N.; de Wit, J. Compartmentalized distributions of neuronal and glial cell-surface proteins pattern the synaptic network. Curr. Opin. Neurobiol. 2019, 57, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qi, Y.B. Building stereotypic connectivity: Mechanistic insights into structural plasticity from C. elegans. Curr. Opin. Neurobiol. 2018, 48, 97–105. [Google Scholar] [CrossRef]
- Kurup, N.; Jin, Y. Neural circuit rewiring: Insights from DD synapse remodeling. Worm 2016, 5, e1129486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).