Awake Craniotomy in Conscious Sedation: The Role of A2 Agonists
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Study Sample
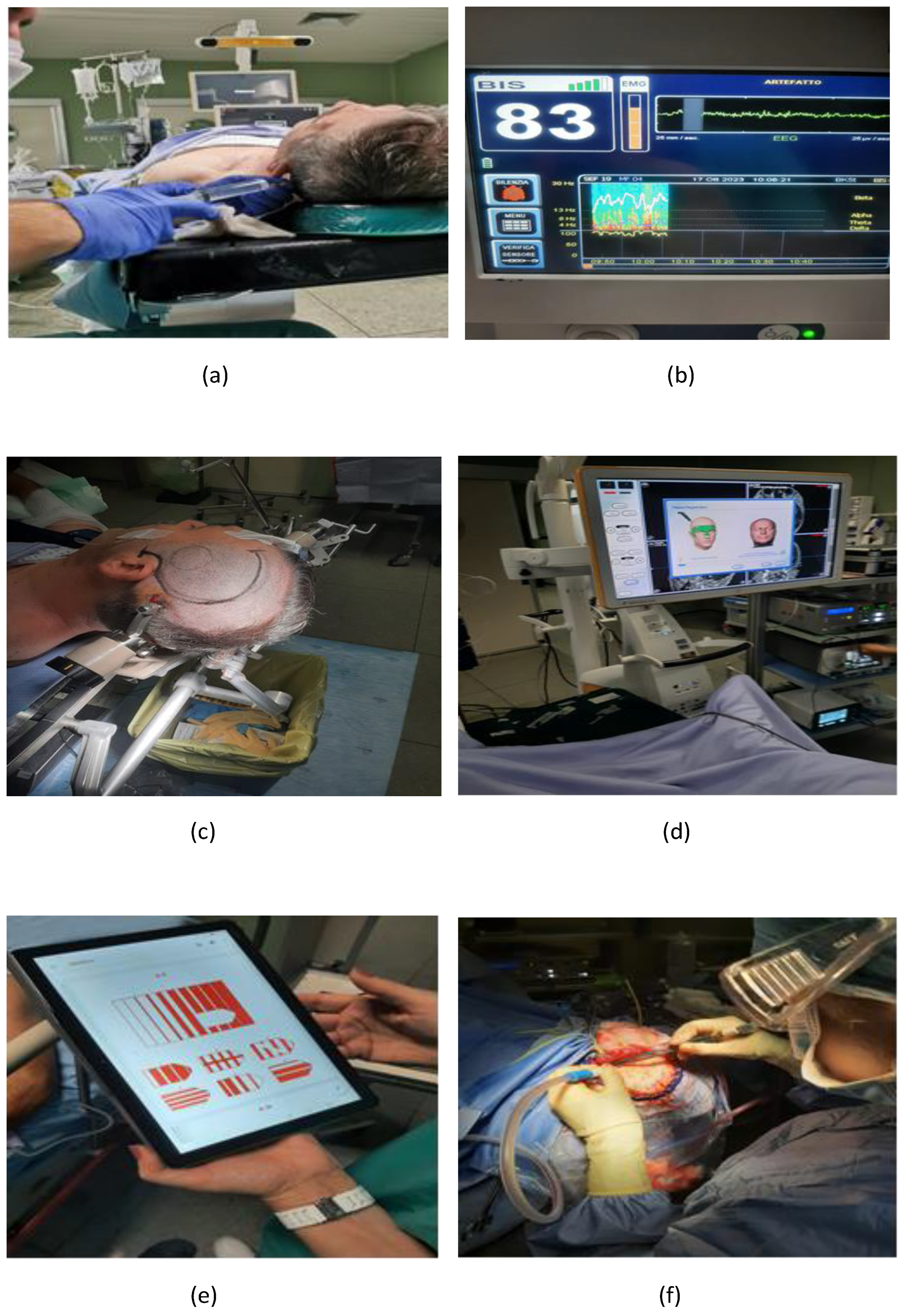
2.3. Anesthesiological Procedure and Comprehensive Approach
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
3.2. Hemodynamic Characteristics
3.3. Respiratory Characteristics
3.4. Side Effect Detected
3.5. Procedural Steps
3.6. Postoperative Evaluation
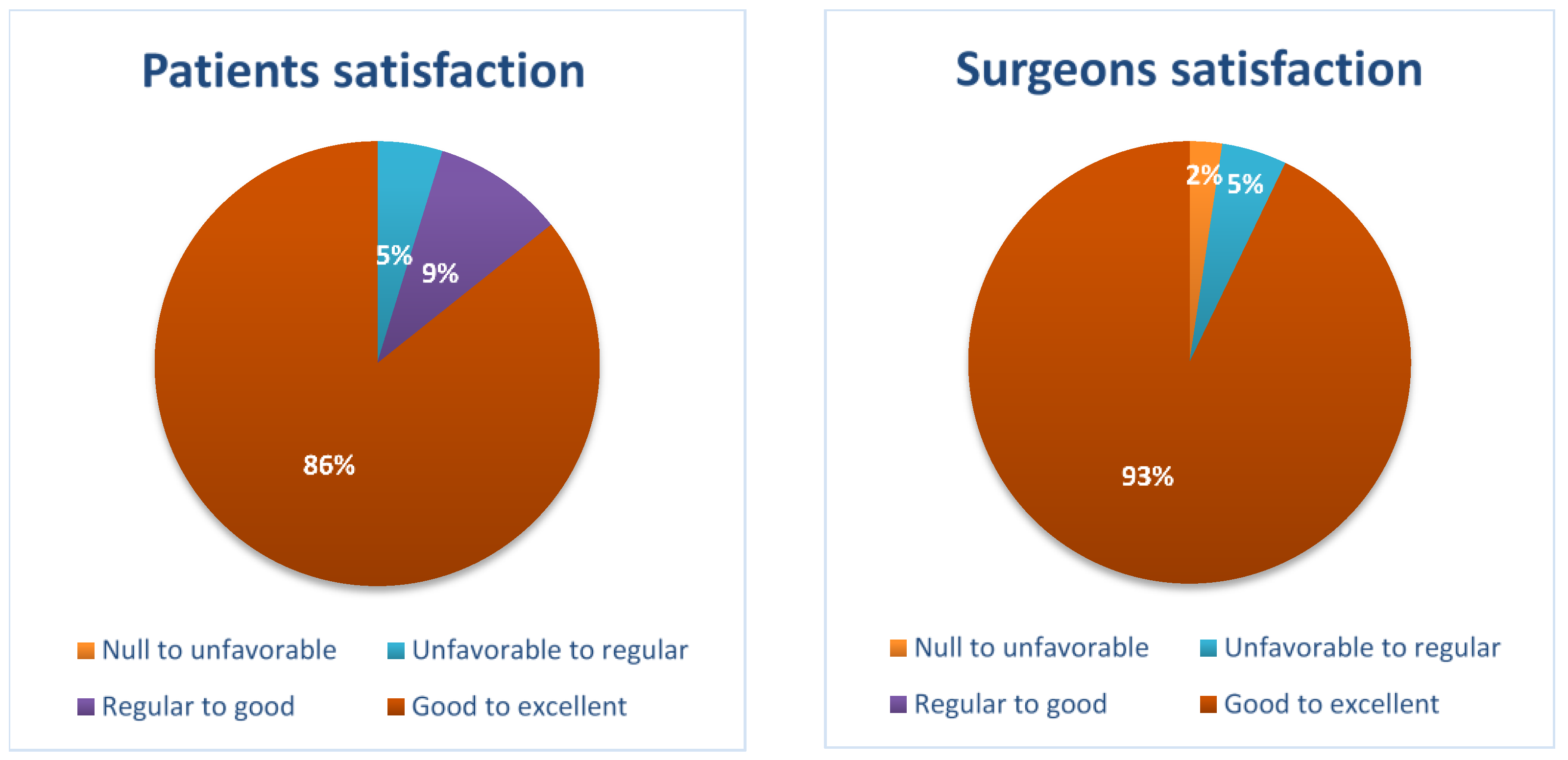
3.7. Patient and Surgeon Satisfaction Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulikov, A.; Lubnin, A. Anesthesia for awake craniotomy. Curr. Opin. Anaesthesiol. 2018, 31, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, F.; Guerra, C.; Rosa, G. Update on anesthesia for craniotomy. Curr. Opin. Anaesthesiol. 2013, 26, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R. Biased, Bitopic, Opioid-Adrenergic Tethered Compounds May Improve Specificity, Lower Dosage and Enhance Agonist or Antagonist Function with Reduced Risk of Tolerance and Addiction. Pharmaceuticals 2022, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Zetlaoui, P.J.; Gauthier, E.; Benhamou, D. Ultrasound-guided scalp nerve blocks for neurosurgery: A narrative review. Anaesth. Crit. Care Pain. Med. 2020, 39, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, J.S.; Gordin, V. Alpha 2 agonists in regional anesthesia and analgesia. Curr. Opin. Anaesthesiol. 2001, 14, 751–753. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, G.; Izzi, A.; Manuali, A.; Bisceglia, G.; Tancredi, A.; Marchello, V.; Recchia, A.; Tonti, M.P.; Icolaro, N.; Fazzari, E.; et al. Anesthetic Management for Awake Craniotomy Applied to Neurosurgery. Brain Sci. 2023, 13, 1031. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, N.; Nicholson, S.; Rigamonti, A.; Hare, G.M.T.; Cusimano, M.; Garavaglia, M.; Pshonyak, I.; Das, S. Awake craniotomy using dexmedetomidine and scalp blocks: A retrospective cohort study. Can. J. Anaesth. 2018, 65, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Natalini, D.; Ganau, M.; Rosenkranz, R.; Petrinic, T.; Fitzgibbon, K.; Antonelli, M.; Prisco, L. Comparison of the Asleep-Awake-Asleep Technique and Monitored Anesthesia Care During Awake Craniotomy: A Systematic Review and Meta-analysis. J. Neurosurg. Anesthesiol. 2022, 34, e1–e13. [Google Scholar] [CrossRef]
- Tonner, P.H.; Bein, B. Classic electroencephalographic parameters: Median frequency, spectral edge frequency etc. Best. Pract. Res. Clin. Anaesthesiol. 2006, 20, 147–159. [Google Scholar] [CrossRef]
- Renna, M.S.; Metcalfe, A.; Ellard, D.; Davies, D. A patient satisfaction survey investigating pre- and post-operative information provision in lower limb surgery. BMC Musculoskelet. Disord. 2020, 21, 754. [Google Scholar] [CrossRef]
- Murphy, M.; Sternschuss, G.; Haff, R.; van Raalte, H.; Saltz, S.; Lucente, V. Quality of life and surgical satisfaction after vaginal reconstructive vs obliterative surgery for the treatment of advanced pelvic organ prolapse. Am. J. Obstet. Gynecol. 2008, 198, 573.e1–573.e7. [Google Scholar] [CrossRef]
- Garavaglia, M.M.; Das, S.; Cusimano, M.D.; Crescini, C.; Mazer, C.D.; Hare, G.M.; Rigamonti, A. Anesthetic approach to high-risk patients and prolonged awake craniotomy using dexmedetomidine and scalp block. J. Neurosurg. Anesthesiol. 2014, 26, 226–233. [Google Scholar] [CrossRef]
- Chan, M.T.V.; Hedrick, T.L.; Egan, T.D.; García, P.S.; Koch, S.; Purdon, P.L.; Ramsay, M.A.; Miller, T.E.; McEvoy, M.D.; Gan, T.J. Perioperative Quality Initiative (POQI) 6 Workgroup. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on the Role of Neuromonitoring in Perioperative Outcomes: Electroencephalography. Anesth. Analg. 2020, 130, 1278–1291. [Google Scholar] [CrossRef]
- Kim, M.C.; Fricchione, G.L.; Brown, E.N.; Akeju, O. Role of electroencephalogram oscillations and the spectrogram in monitoring anaesthesia. BJA Educ. 2020, 20, 166–172. [Google Scholar] [CrossRef]
- Yuan, Y.; Peizhi, Z.; Xiang, W.; Yanhui, L.; Ruofei, L.; Shu, J.; Qing, M. Intraoperative seizures and seizures outcome in patients undergoing awake craniotomy. J. Neurosurg. Sci. 2019, 63, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Paquin-Lanthier, G.; Subramaniam, S.; Leong, K.W.; Daniels, A.; Singh, K.; Takami, H.; Chowdhury, T.; Bernstein, M.; Venkatraghavan, L. Risk Factors and Characteristics of Intraoperative Seizures During Awake Craniotomy: A Retrospective Cohort Study of 562 Consecutive Patients with a Space-occupying Brain Lesion. J. Neurosurg. Anesthesiol. 2003, 2, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Roca, E.; Pallud, J.; Guerrini, F.; Panciani, P.P.; Fontanella, M.; Spena, G. Stimulation-related intraoperative seizures during awake surgery: A review of available evidences. Neurosurg. Rev. 2020, 43, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Maruyama, T.; Komatsu, R.; Ozaki, M. Intraoperative panic attack in patients undergoing awake craniotomy: A retrospective analysis of risk factors. J. Anesth. 2021, 35, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Choi, S.H. Anesthetic considerations for awake craniotomy. Anesth. Pain Med. 2020, 15, 269–274. [Google Scholar] [CrossRef]
- Kwinta, B.M.; Myszka, A.M.; Bigaj, M.M.; Krzyżewski, R.M.; Starowicz-Filip, A. Intra- and postoperative adverse events in awake craniotomy for intrinsic supratentorial brain tumors. Neurol. Sci. 2021, 42, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.G.; Thomas, S.G.; Babu, K.S.; Daniel, R.T.; Chacko, G.; Prabhu, K.; Cherian, V.; Korula, G. Awake craniotomy and electrophysiological mapping for eloquent area tumours. Clin. Neurol. Neurosurg. 2013, 115, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Bernstein, M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: A prospective trial of 200 cases. J. Neurosurg. 1999, 90, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Tomasino, B.; Guarracino, I.; Ius, T.; Maieron, M.; Skrap, M. Real-Time Neuropsychological Testing Protocol for Left Temporal Brain Tumor Surgery: A Technical Note and Case Report. Front. Hum. Neurosci. 2021, 15, 760569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Nie, C.; Lu, H.; Zhang, L.; Chen, H.L.; Wang, S.Y.; Li, W.; Shen, S.; Wang, H. Monitored Anesthetic Care Combined with Scalp Nerve Block in Awake Craniotomy: An Effective Attempt at Enhanced Recovery After Neurosurgery. World Neurosurg. 2021, 154, e509–e519. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, T.; Bernstein, M. Awake craniotomy for brain tumor: Indications, technique and benefits. Expert. Rev. Neurother. 2014, 14, 1405–1415. [Google Scholar] [CrossRef]
- Bonhomme, V.; Franssen, C.; Hans, P. Awake craniotomy. Eur. J. Anaesthesiol. 2009, 26, 906–912. [Google Scholar] [CrossRef]
- Wang, A.T.; Pillai, P.; Guran, E.; Carter, H.; Minasian, T.; Lenart, J.; Vandse, R. Anesthetic Management of Awake Craniotomy for Resection of the Language and Motor Cortex Vascular Malformations. World Neurosurg. 2020, 143, e136–e148. [Google Scholar] [CrossRef]

| Patients (n = 42) | |
|---|---|
| Sex (Male/Female)—n(%) | 27(64.3%)/15(35.7%) |
| Age—(mean ± sd) | 58 ± 8 |
| Weight—(mean ± sd) | 78 ± 11 |
| Surgery area | |
| Temporal sn—n(%) | 21(50%) |
| Parietal sn—n(%) | 1(2%) |
| Frontal sn—n(%) | 2(5%) |
| Temporo-parietal sn—n(%) | 7(17%) |
| Fronto-parietal sn—n(%) | 4(9%) |
| Fronto-temporo-parietal sn—n(%) | 7(17%) |
| Type of lesion | |
| Oligodendroglioma—n(%) | 34(81%) |
| AVM—n(%) | 4(9%) |
| Astrocitoma—n(%) | 2(5%) |
| Metastasi—n(%) | 2(5%) |
| ASA | |
| 1—n(%) | 2(5%) |
| 2—n(%) | 35(83%) |
| 3—n(%) | 5(12%) |
| 4—n(%) | 0(0) |
| GCS preoperative | |
| 13—n(%) | 3(7%) |
| 14—n(%) | 5(12%) |
| 15—n(%) | 34(81%) |
| Suspension of the procedure—n(%) | 1(2%) |
| Phase | Parameter | Patients (n = 42) |
|---|---|---|
| Phase 1 | ABP (mmHg)—(mean ± sd) | 71 ± 5 |
| HR (bpm)—(mean ± sd) | 76 ± 10 | |
| SV (mL)—(mean ± sd) | 69 ± 13 | |
| Phase 2 | ABP (mmHg)—(mean ± sd) | 74 ± 5 |
| HR (bpm)—(mean ± sd) | 79 ± 6 | |
| SV (mL)—(mean ± sd) | 66 ± 14 | |
| Phase 3 | ABP (mmHg)—(mean ± sd) | 72 ± 5 |
| HR (bpm)—(mean ± sd) | 67 ± 9 | |
| SV (mL)—(mean ± sd) | 67 ± 12 |
| Phase. | Parameter | Patients (n = 42) |
|---|---|---|
| Phase 1 | SpO2 (%)—(mean ± sd) | 98 ± 1 |
| PH—(mean ± sd) | 7.41 ± 0.04 | |
| PaO2 (mmHg)—(mean ± sd) | 115 ± 22 | |
| P/F—(mean ± sd) | 326 ± 61 | |
| PaCO2 (mmHg)—(mean ± sd) | 40 ± 4 | |
| HCO3− (mmol/L)—(mean ± sd) | 24 ± 2 | |
| Lactate (mg/dl)—(mean ± sd) | 0.9 ± 0.3 | |
| Phase 2 | SpO2 (%)—(mean ± sd) | 99 ± 1 |
| PH—(mean ± sd) | 7.40 ± 0.04 | |
| PaO2 (mmHg)—(mean ± sd) | 122 ± 18 | |
| P/F—(mean ± sd) | 336 ± 51 | |
| PaCO2 (mmHg)—(mean ± sd) | 40 ± 2 | |
| HCO3− (mmol/L)—(mean ± sd) | 24 ± 2 | |
| Lactate (mg/dL)—(mean ± sd) | 1.0 ± 0.3 | |
| Phase 3 | SpO2 (%)—(mean ± sd) | 98 ± 1 |
| PH—(mean ± sd) | 7.40 ± 0.05 | |
| PaO2 (mmHg)—(mean ± sd) | 120 ± 20 | |
| P/F—(mean ± sd) | 325 ± 47 | |
| PaCO2 (mmHg)—(mean ± sd) | 35 ± 3 | |
| HCO3− (mmol/L)—(mean ± sd) | 23 ± 2 | |
| Lactate (mg/dL)—(mean ± sd) | 1.0 ± 0.7 |
| Effect | Patients (n = 42) |
|---|---|
| Epilepsy—n(%) | 1(2%) |
| Agitation—n(%) | 2(5%) |
| Aphasia—n(%) | 3(7%) |
| Feature | Patients (n = 42) |
|---|---|
| Duration of procedure (min)—(mean ± sd) | 240 ± 62 |
| Awake time (min)—(mean ± sd) | 120 ± 45 |
| Sleep time (min)—(mean ± sd) | 120 ± 32 |
| Ttpe (s)—(mean ± sd) | 142 ± 8 |
| tD (s)—(mean ± sd) | 94 ± 7 |
| Dose of Remifentanil (mg)—(mean ± sd) | 4.2 ± 1.3 |
| Dose of Dexmedetomidine (µg)—(mean ± sd) | 996 ± 316 |
| SEF (Hz)—(mean ± sd) | 18 ± 1 |
| Patiens (n = 42) | |
|---|---|
| Aldrete scale | |
| 10—n(%) | 38(91%) |
| 8—n(%) | 3(7%) |
| 7—n(%) | 1(2%) |
| Postoperative GCS | |
| 12—n(%) | 1(2%) |
| 13—n(%) | 2(5%) |
| 14—n(%) | 2(5%) |
| 15—n(%) | 37(88%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzi, A.; Mincolelli, G.; D’Onofrio, G.; Marchello, V.; Manuali, A.; Icolaro, N.; Mirabella, L.; Riefolo, A.; Mazzotta, B.; Barile, A.; et al. Awake Craniotomy in Conscious Sedation: The Role of A2 Agonists. Brain Sci. 2024, 14, 147. https://doi.org/10.3390/brainsci14020147
Izzi A, Mincolelli G, D’Onofrio G, Marchello V, Manuali A, Icolaro N, Mirabella L, Riefolo A, Mazzotta B, Barile A, et al. Awake Craniotomy in Conscious Sedation: The Role of A2 Agonists. Brain Sciences. 2024; 14(2):147. https://doi.org/10.3390/brainsci14020147
Chicago/Turabian StyleIzzi, Antonio, Giuseppe Mincolelli, Grazia D’Onofrio, Vincenzo Marchello, Aldo Manuali, Nadia Icolaro, Lucia Mirabella, Anna Riefolo, Barbara Mazzotta, Alessio Barile, and et al. 2024. "Awake Craniotomy in Conscious Sedation: The Role of A2 Agonists" Brain Sciences 14, no. 2: 147. https://doi.org/10.3390/brainsci14020147
APA StyleIzzi, A., Mincolelli, G., D’Onofrio, G., Marchello, V., Manuali, A., Icolaro, N., Mirabella, L., Riefolo, A., Mazzotta, B., Barile, A., Gorgoglione, L. P., & Del Gaudio, A. (2024). Awake Craniotomy in Conscious Sedation: The Role of A2 Agonists. Brain Sciences, 14(2), 147. https://doi.org/10.3390/brainsci14020147







