One-Week Maternal Separation Caused Sex-Specific Changes in Behavior and Hippocampal Metabolomics of Offspring Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Animals
2.3. Animal Experimental Procedures
2.4. Behavioral Tests
2.4.1. Open Field Test (OFT)
2.4.2. Elevated Plus Mazes (EPM)
2.4.3. Novelty Suppressed Feeding Test (NSFT)
2.4.4. Forced Swimming Test (FST)
2.5. Collection of Biological Samples
2.6. Determination of Serum Corticosterone and Hippocampal NAD+ and NADH
2.7. Determination of Hippocampal Inflammatory Cytokines by Real-Time Fluorescence Quantification (qRT-PCR)
2.8. Hippocampal Untargeted Metabolomics Using UPLC-Q-TOF/MS
2.8.1. Hippocampal Tissue Processing
2.8.2. UPLC-Q-TOF/MS Analysis
2.8.3. Data Processing
2.8.4. Multivariate Analysis of UPLC-Q-TOF/MS Data
2.9. Statistical Analysis
3. Results
3.1. Effect of One-Week MS on Body Weight of Offspring Rats
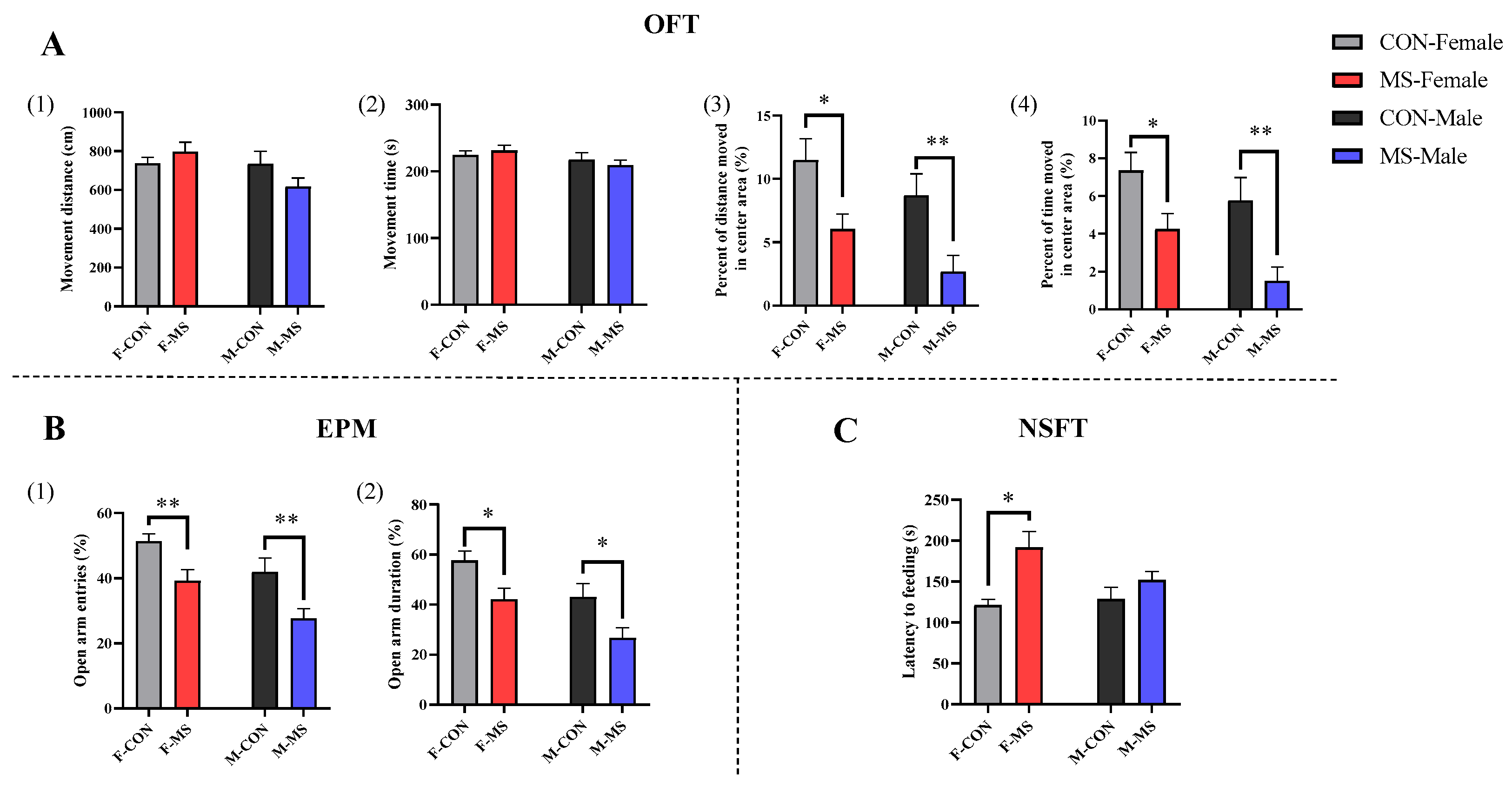
3.2. Effect of One-Week MS on Adolescent Behaviors of Offspring Rats
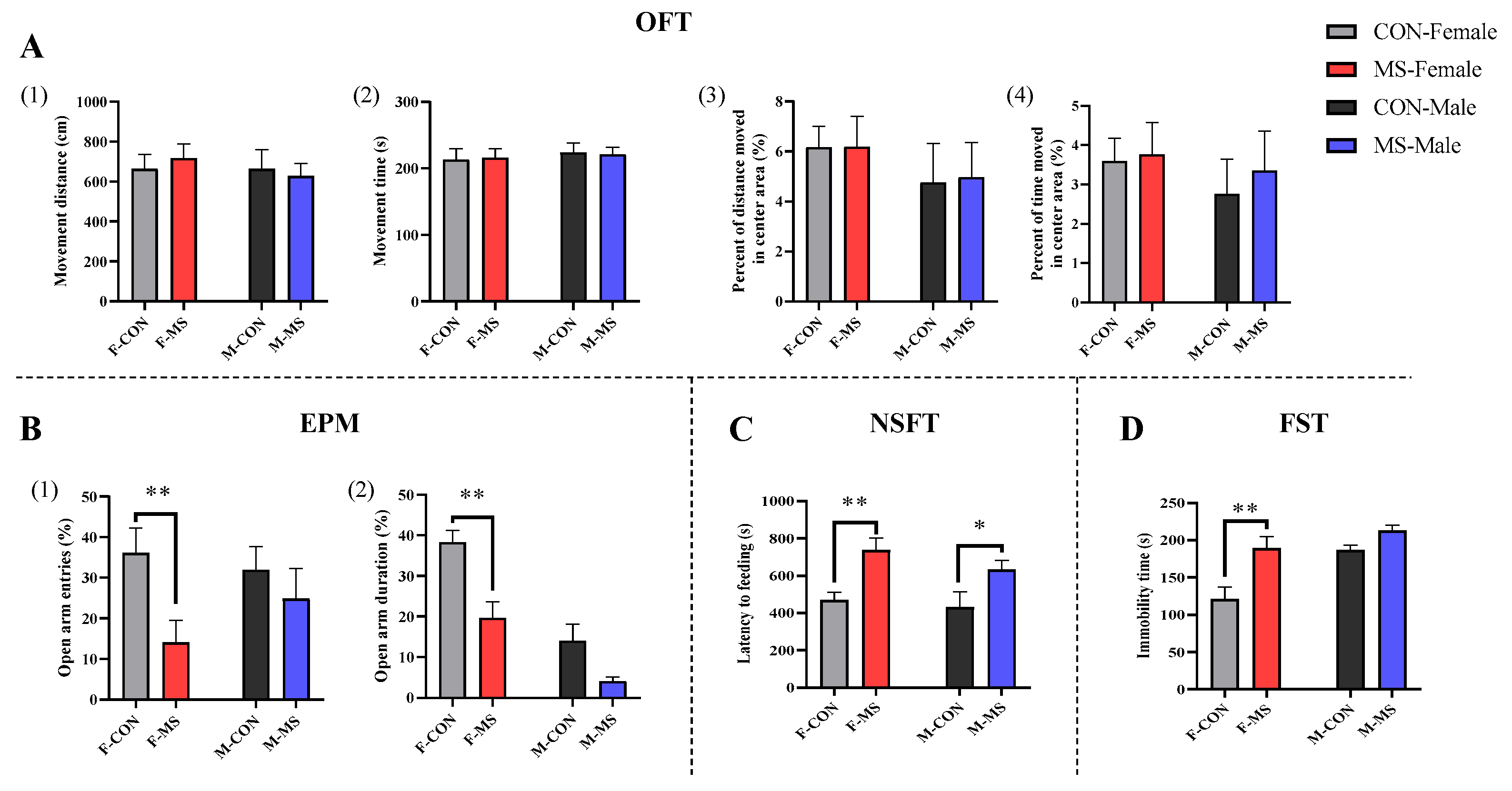
3.3. Effect of One-Week MS on Adulthood Behaviors of Offspring Rats
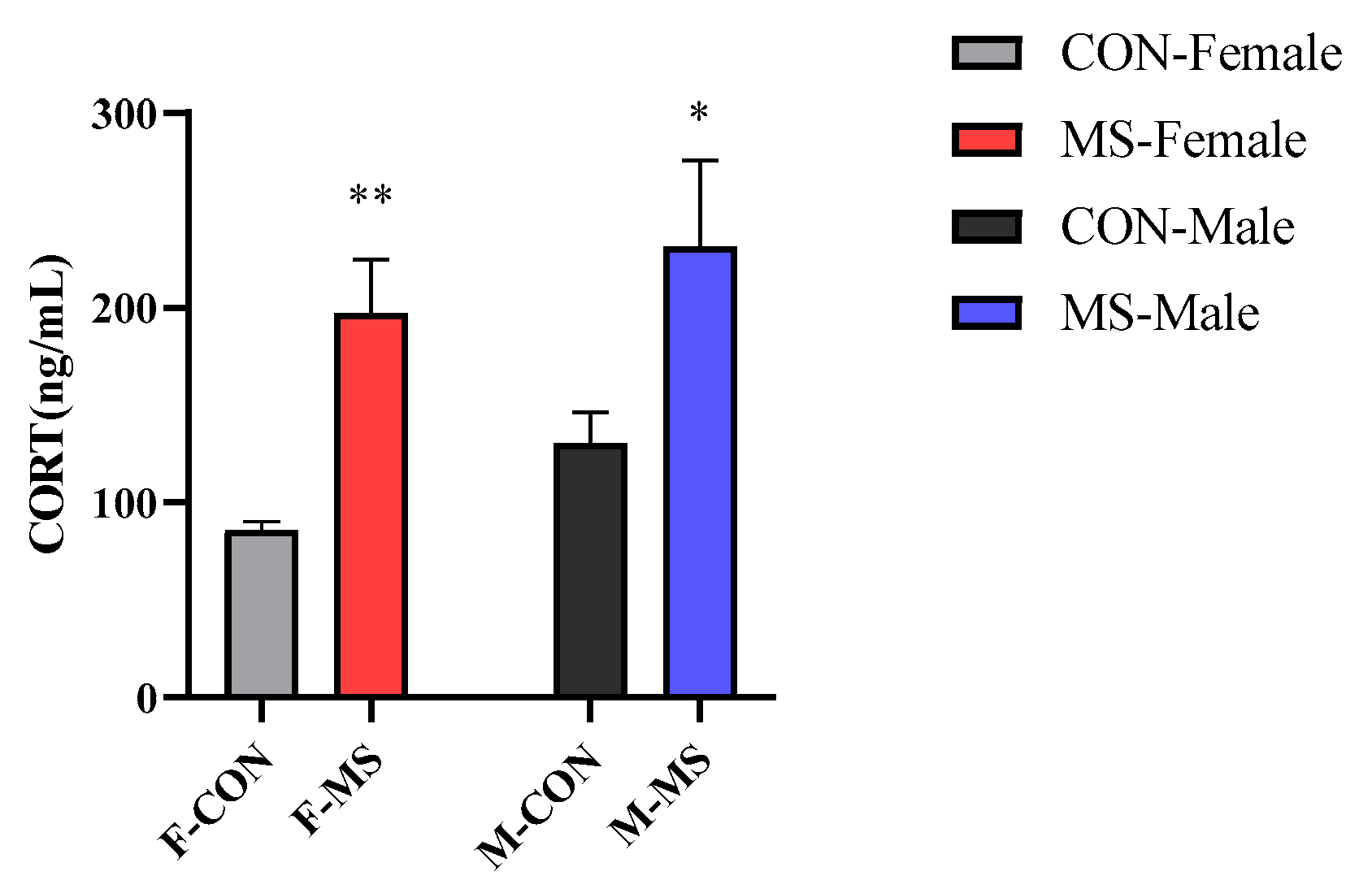
3.4. Effect of One-Week MS on Serum Corticosterone (CORT) of Offspring Rats
3.5. Effect of One-Week MS on Hippocampal Inflammatory Cytokines of Offspring Rats
3.6. Effect of One-Week MS on Hippocampal Untargeted Metabolomics of Offspring Rats
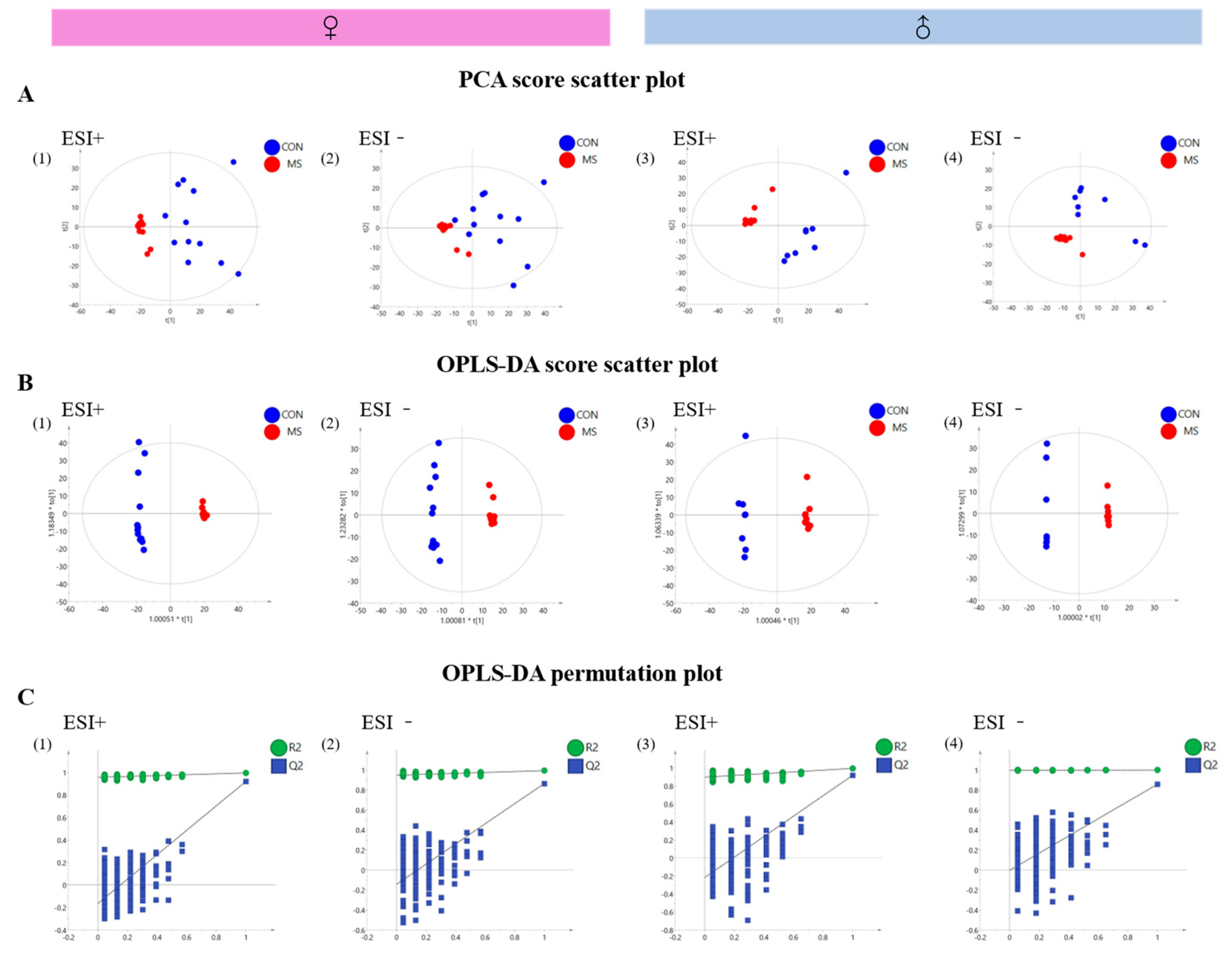
3.6.1. Validation of Analytical Methods
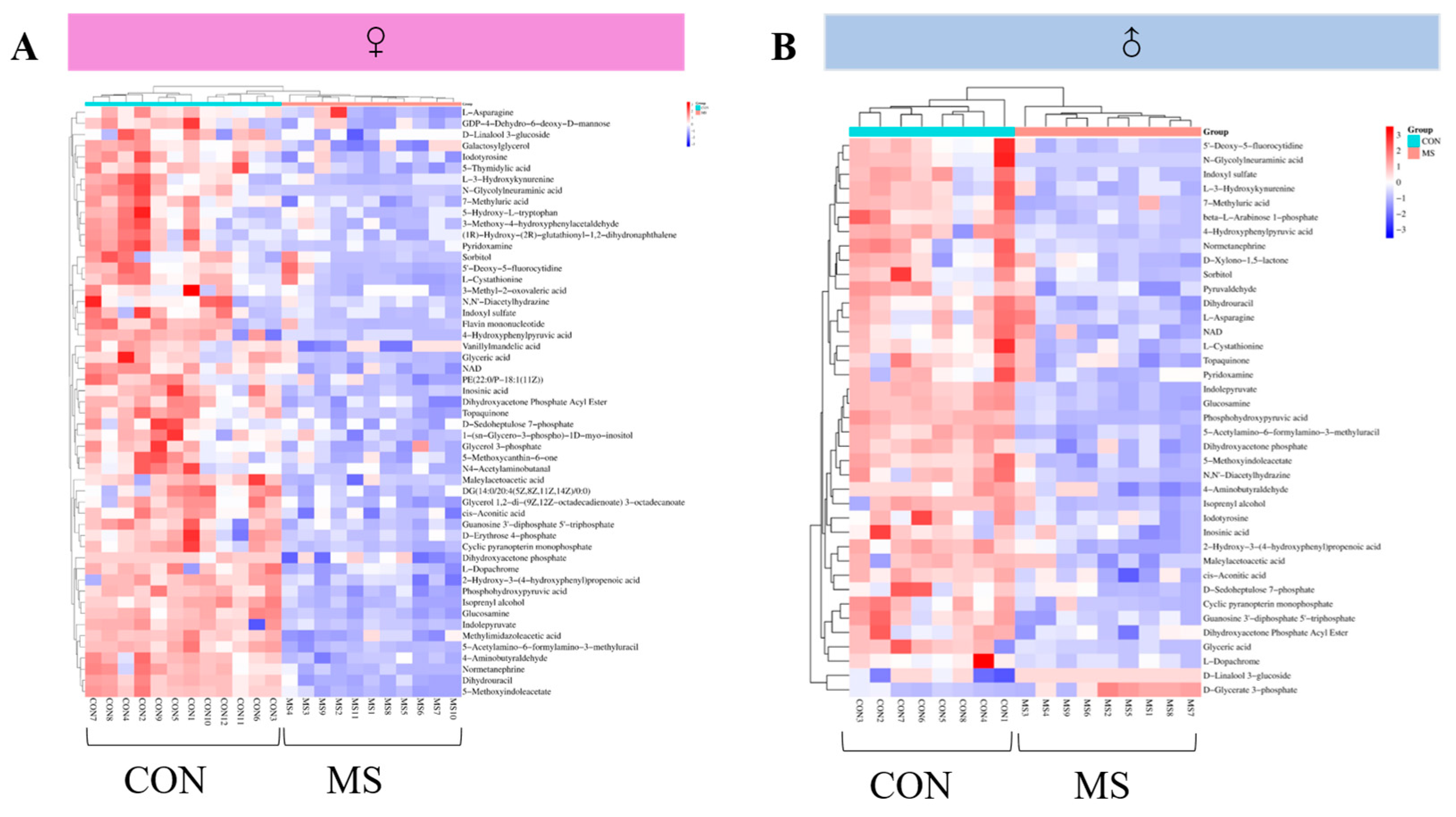
3.6.2. Analysis of Metabolic Profile in the Hippocampus
3.6.3. Identification of Differential Metabolites in the Hippocampus
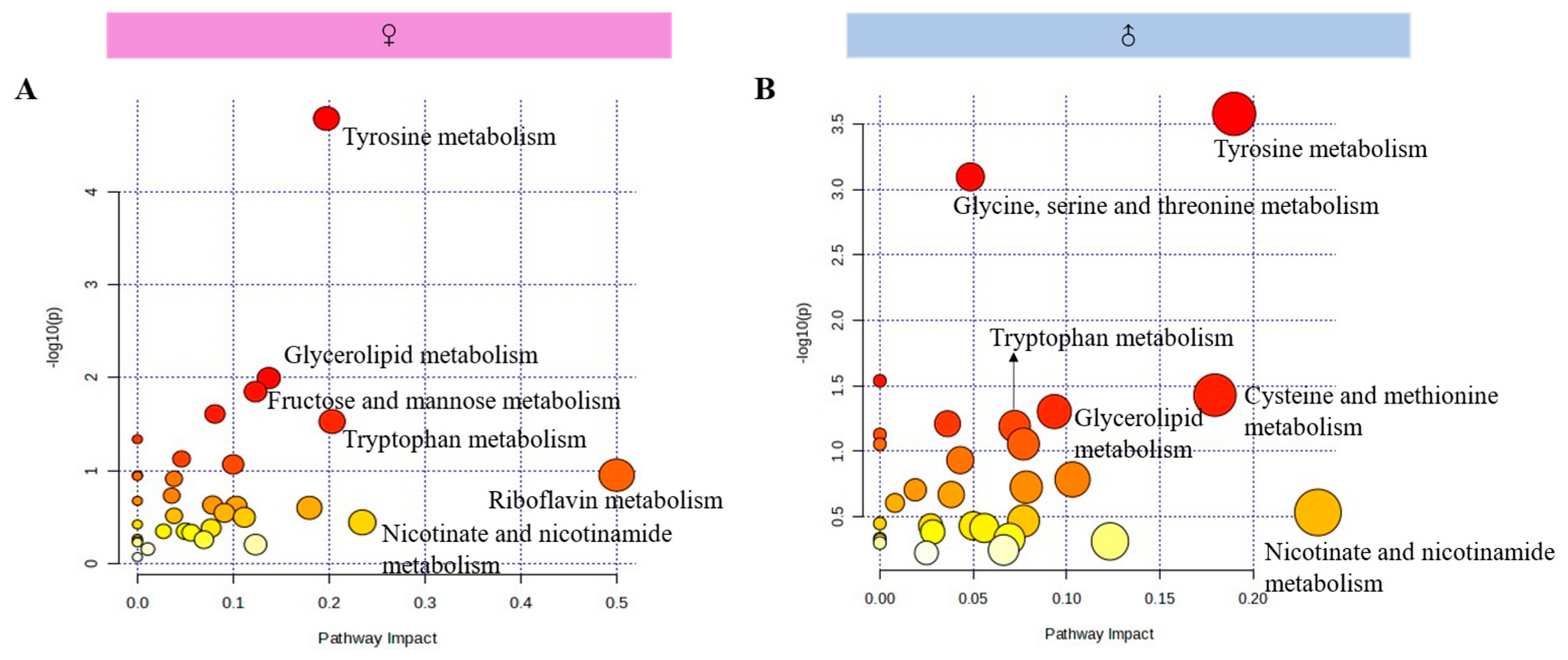
3.6.4. Metabolic Pathway Analysis on the Differential Metabolites in the Hippocampus
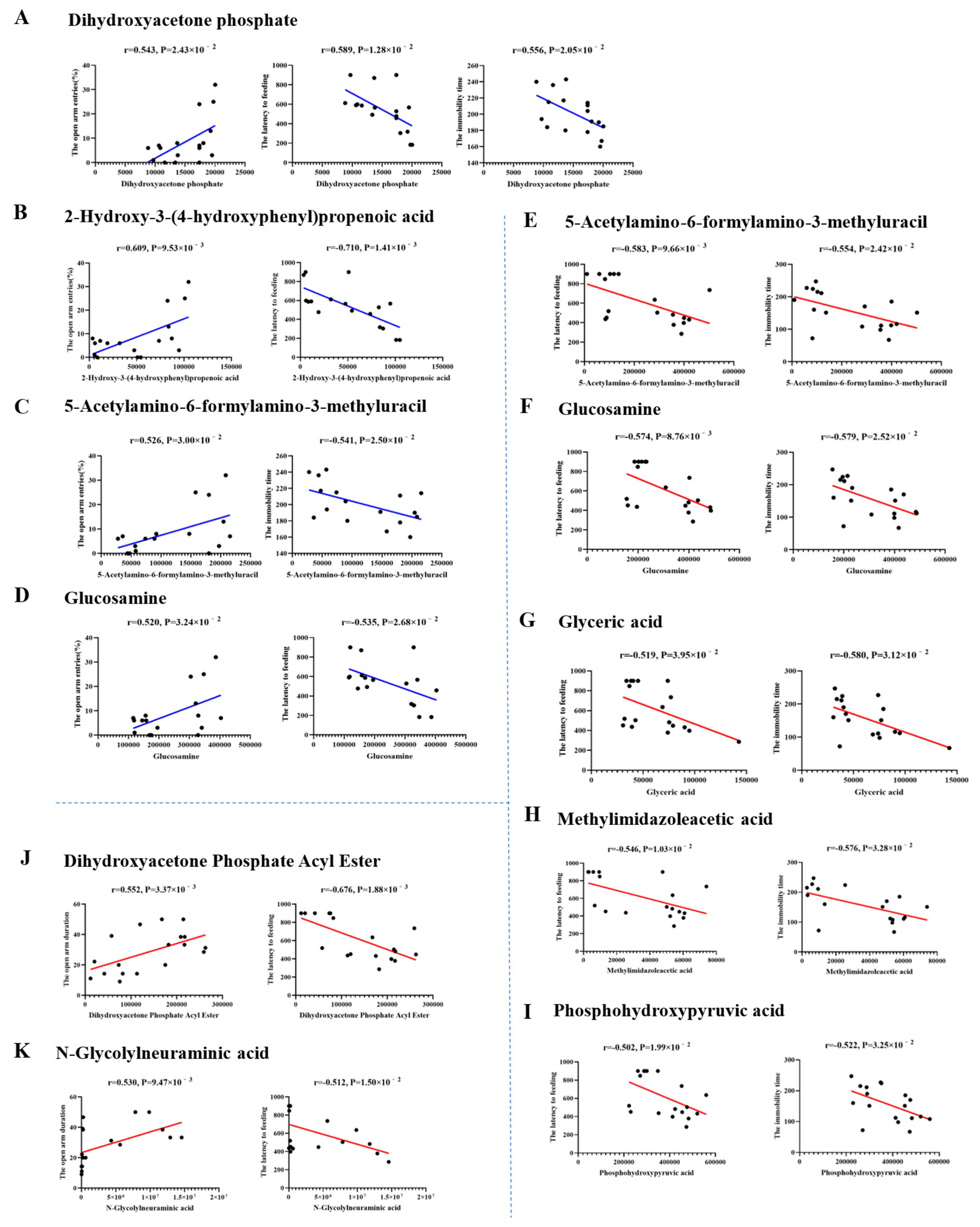
3.6.5. Correlation Analysis of Behavior Data and Hippocampal Metabolites
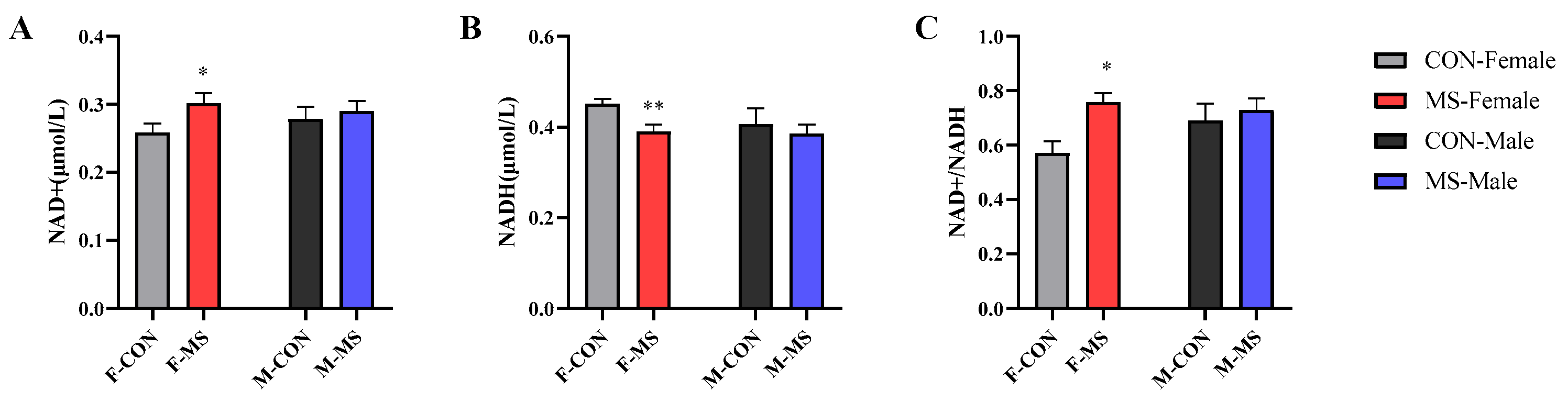
3.7. Effect of One-Week MS on Hippocampal NAD+ and NADH of Offspring Rats
4. Discussion
4.1. One-Week MS Induced Sex-Specific Anxiety- and Depression-like Behaviors in Offspring Rats During Adolescence and Adulthood
4.2. One-Week MS Induced Sex-Specific Effects of HPA Axis Overactivation and Hippocampal Inflammation in Offspring Rats
4.3. One-Week MS Showed Sex-Specific Effects on Hippocampal Metabolomics in Adulthood Offspring Rats
4.4. One-Week MS Induced Energy Metabolism Disorder in Hippocampal Cells of Offspring Rats
4.5. One-Week MS Induced Disfunction of Hippocampal Tyrosine and Tryptophan Metabolism in Offspring Rats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Zhang, A.; Liu, W.; Tao, F.; Sun, Y. Childhood separation from parents with cognitive and psychopathological outcomes in adolescence. Dev. Sci. 2022, 26, e13324. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.D.; Hübner, I.C.; Bennemann, B.; de Carvalho, C.R.; Brocardo, P.S. Effects of postnatal ethanol exposure and maternal separation on mood, cognition and hippocampal arborization in adolescent rats. Behav. Brain Res. 2021, 411, 113372. [Google Scholar] [CrossRef] [PubMed]
- Cevik, O.S.; Cevik, K.; Temel, G.O.; Sahin, L. Maternal separation increased memory function and anxiety without effects of environmental enrichment in male rats. Behav. Brain Res. 2023, 441, 114280. [Google Scholar] [CrossRef] [PubMed]
- Urb, M.; Anier, K.; Matsalu, T.; Aonurm-Helm, A.; Tasa, G.; Koppel, I.; Zharkovsky, A.; Timmusk, T.; Kalda, A. Glucocorticoid Receptor Stimulation Resulting from Early Life Stress Affects Expression of DNA Methyltransferases in Rat Prefrontal Cortex. J. Mol. Neurosci. 2019, 68, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, J.N.; Sierra-Fonseca, J.A.; Flores, R.J.; Saucedo, S.; Miranda-Arango, M.; O’Dell, L.E.; Gosselink, K.L. Early-life adversity increases anxiety-like behavior and modifies synaptic protein expression in a region-specific manner. Front. Behav. Neurosci. 2022, 16, 1008556. [Google Scholar] [CrossRef]
- Doreste-Mendez, R.; Ríos-Ruiz, E.J.; Rivera-López, L.L.; Gutierrez, A.; Torres-Reveron, A. Effects of Environmental Enrichment in Maternally Separated Rats: Age and Sex-Specific Outcomes. Front. Behav. Neurosci. 2019, 13, 198. [Google Scholar] [CrossRef]
- Jaimes-Hoy, L.; Pérez-Maldonado, A.; Narváez Bahena, E.; de la Cruz Guarneros, N.; Rodríguez-Rodríguez, A.; Charli, J.-L.; Soberón, X.; Joseph-Bravo, P. Sex Dimorphic Changes in Trh Gene Methylation and Thyroid-Axis Response to Energy Demands in Maternally Separated Rats. Endocrinology 2021, 162, bqab110. [Google Scholar] [CrossRef]
- Eskandari, F.; Salimi, M.; Hedayati, M.; Zardooz, H. Maternal separation induced resilience to depression and spatial memory deficit despite intensifying hippocampal inflammatory responses to chronic social defeat stress in young adult male rats. Behav. Brain Res. 2022, 425, 113810. [Google Scholar] [CrossRef]
- Nicolas, S.; McGovern, A.J.; Hueston, C.M.; O’Mahony, S.M.; Cryan, J.F.; O’Leary, O.F.; Nolan, Y.M. Prior maternal separation stress alters the dendritic complexity of new hippocampal neurons and neuroinflammation in response to an inflammatory stressor in juvenile female rats. Brain Behav. Immun. 2022, 99, 327–338. [Google Scholar] [CrossRef]
- Hill, J.; Inder, T.; Neil, J.; Dierker, D.; Harwell, J.; Van Essen, D. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. USA 2010, 107, 13135–13140. [Google Scholar] [CrossRef]
- Levitt, P. Structural and functional maturation of the developing primate brain. J. Pediatr. 2003, 143, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.S. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J. Comp. Psychol. 1934, 18, 385–403. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Britton, D.R.; Britton, K.T. A sensitive open field measure of anxiolytic drug activity. Pharmacol. Biochem. Behav. 1981, 15, 577–582. [Google Scholar] [CrossRef]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef]
- Li, C.-C.; Ye, F.; Xu, C.-X.; Jiang, N.; Chang, Q.; Liu, X.-M.; Pan, R.-L. Tryptophan-kynurenine metabolic characterization in the gut and brain of depressive-like rats induced by chronic restraint stress. J. Affect. Disord. 2023, 328, 273–286. [Google Scholar] [CrossRef]
- Yu, M.; Jia, H.-M.; Zhang, T.; Shang, H.; Zhang, H.-W.; Ma, L.-Y.; Zou, Z.-M. Gut Microbiota Is the Key to the Antidepressant Effect of Chaihu-Shu-Gan-San. Metabolites 2020, 10, 63. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef]
- Kim, E.-G.; Chang, W.; Shin, S.; Adhikari, A.S.; Seol, G.H.; Song, D.-Y.; Min, S.S. Maternal separation in mice leads to anxiety-like/aggressive behavior and increases immunoreactivity for glutamic acid decarboxylase and parvalbumin in the adolescence ventral hippocampus. Korean J. Physiol. Pharmacol. 2023, 27, 113–125. [Google Scholar] [CrossRef]
- Kolar, D.; Kleteckova, L.; Brozka, H.; Vales, K. Mini-review: Brain energy metabolism and its role in animal models of depression, bipolar disorder, schizophrenia and autism. Neurosci. Lett. 2021, 760, 136003. [Google Scholar] [CrossRef]
- Wei, R.-M.; Zhang, Y.-M.; Feng, Y.-Z.; Zhang, K.-X.; Zhang, J.-Y.; Chen, J.; Luo, B.-L.; Li, X.-Y.; Chen, G.-H. Resveratrol ameliorates maternal separation-induced anxiety- and depression-like behaviors and reduces Sirt1-NF-kB signaling-mediated neuroinflammation. Front. Behav. Neurosci. 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-S.; Kim, T.-W.; Park, H.-S.; Seo, T.-B.; Kim, Y.-P. Effects of treadmill exercise on activity, short-term memory, vascular dysfunction in maternal separation rats. J. Exerc. Rehabil. 2020, 16, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, S.; Esmaeilpour, K.; Shabani, M.; Sepehri, G.; Rajizadeh, M.A.; Maneshian, M.; Joushi, S.; Sheibani, V. Choline chloride modulates learning, memory, and synaptic plasticity impairments in maternally separated adolescent male rats. Int. J. Dev. Neurosci. 2021, 82, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, S.; Khodagholi, F.; Moghtadaei, M.; Behvarmanesh, A.; Kheradmand, A.; Ghazvini, H. Effects of Maternal Deprivation on Anxiety, Depression, and Empathy in Male and Female Offspring of Wistar Rats in the Face of Novel Objects. Galen Med. J. 2019, 8, e1093. [Google Scholar] [CrossRef]
- Oh, S.J.; Lee, N.; Nam, K.R.; Kang, K.J.; Lee, K.C.; Lee, Y.J.; Seok, J.-H.; Choi, J.Y. Effect of developmental stress on the in vivo neuronal circuits related to excitation–inhibition balance and mood in adulthood. Front. Psychiatry 2023, 14, 1086370. [Google Scholar] [CrossRef]
- Sapolsky, R.; Meaney, M. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986, 396, 65–76. [Google Scholar] [CrossRef]
- McCormick, C.M.; Vargas, J.; Junco, M.; Gomez, C.; Lajud, N. Early Life Stress Increases Metabolic Risk, HPA Axis Reactivity, and Depressive-Like Behavior When Combined with Postweaning Social Isolation in Rats. PLoS ONE 2016, 11, e0162665. [Google Scholar] [CrossRef]
- Eskandari, F.; Salimi, M.; Binayi, F.; Abdollahifar, M.-A.; Eftekhary, M.; Hedayati, M.; Ghanbarian, H.; Zardooz, H. Investigating the Effects of Maternal Separation on Hypothalamic-Pituitary-Adrenal Axis and Glucose Homeostasis under Chronic Social Defeat Stress in Young Adult Male Rat Offspring. Neuroendocrinology 2023, 113, 361–380. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.C.; Kuehn, A.; Muller, C.P.; Turner, J.D. The effects of early life adversity on the immune system. Psychoneuroendocrinology 2017, 82, 140–154. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid regulation of inflammation and its functional correlates: From HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Cao, K.; Lin, H.; Cui, S.; Shen, C.; Wen, W.; Mo, H.; Dong, Z.; Bai, S.; Yang, L.; et al. Early-Life Stress Induces Depression-Like Behavior and Synaptic-Plasticity Changes in a Maternal Separation Rat Model: Gender Difference and Metabolomics Study. Front. Pharmacol. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Liu, L.; Mo, X.; He, D.; Chen, X.; Xiao, R.; Cheng, Q.; Fatima, M.; Du, Y.; et al. Maternal separation regulates sensitivity of stress-induced depression in mice by affecting hippocampal metabolism. Physiol. Behav. 2024, 279, 114530. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, L.; Sheng, H. Mitochondria in depression: The dysfunction of mitochondrial energy metabolism and quality control systems. CNS Neurosci. Ther. 2024, 30, e14576. [Google Scholar] [CrossRef]
- Kestering-Ferreira, E.; Tractenberg, S.G.; Lumertz, F.S.; Orso, R.; Creutzberg, K.C.; Wearick-Silva, L.E.; Viola, T.W.; Grassi-Oliveira, R. Long-term Effects of Maternal Separation on Anxiety-Like Behavior and Neuroendocrine Parameters in Adult Balb/c Mice. Chronic Stress 2021, 5, 24705470211067181. [Google Scholar] [CrossRef]
- Rouhani, P.; Amoushahi, M.; Keshteli, A.H.; Saneei, P.; Afshar, H.; Esmaillzadeh, A.; Adibi, P. Dietary riboflavin intake in relation to psychological disorders in Iranian adults: An observational study. Sci. Rep. 2023, 13, 5152. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Pu, J.; Yang, L.; Zhou, X.; Liu, L.; Jiang, X.; Zhang, H.; Teng, T.; Tian, L.; et al. Integrated Metabolomics and Proteomics Analysis of Hippocampus in a Rat Model of Depression. Neuroscience 2018, 371, 207–220. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; Li, Y.; Yuan, Z.; Liu, Z.; Guo, J.; Li, X.; Zhang, W.; Tao, Y.; Mei, J. Fructus gardeniae ameliorates anxiety-like behaviors induced by sleep deprivation via regulating hippocampal metabolomics and gut microbiota. Front. Cell. Infect. Microbiol. 2023, 13, 1167312. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef]
- Vilela, F.C.; Vieira, J.S.; Vitor-Vieira, F.; Kalil-Cutti, B.; da Silva, J.R.T.; Giusti-Paiva, A.; da Silva, M.L. Maternal separation increases pain sensitivity by reducing the activity of serotonergic neurons in the dorsal raphe nucleus and noradrenergic neurons in locus coeruleus. Neurosci. Lett. 2021, 748, 135734. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, E.; de Kloet, E.R.; Schaaf, M.; Datson, N.A. Genetic dissection of corticosterone receptor function in the rat hippocampus. Eur. Neuropsychopharmacol. 2001, 11, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Greenough, W.T.; Carter, C.S.; Steerman, C.; DeVoogd, T.J. Sex differences in dendritic patterns in hamster preoptic area. Brain Res. 1977, 126, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ciofi, P.; Leroy, D.; Tramu, G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience 2006, 141, 1731–1745. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.; Ashburner, J.; Henson, R.N.A.; Friston, K.J.; Frackowiak, R.S.J. Cerebral Asymmetry and the Effects of Sex and Handedness on Brain Structure: A Voxel-Based Morphometric Analysis of 465 Normal Adult Human Brains. NeuroImage 2001, 14, 685–700. [Google Scholar] [CrossRef]
- Matsumoto, A.; Arai, Y. Male-Female Difference in Synaptic Organization of the Ventromedial Nucleus of the Hypothalamus in the Rat. Neuroendocrinology 1986, 42, 232–236. [Google Scholar] [CrossRef]
- Witelson, S.F.; Glezer, I.I.; Kigar, D.L. Women have greater density of neurons in posterior temporal cortex. J. Neurosci. 1995, 15, 3418–3428. [Google Scholar] [CrossRef]



| ESI+ | ESI− | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | RT (min) | m/z | Precision RSD (×100%) | Stability RSD (×100%) | No. | RT (min) | m/z | Precision RSD (×100%) | Stability RSD (×100%) |
| 1 | 1.63 | 204.6211 | 0.0479 | 0.0802 | 1 | 0.49 | 130.9669 | 0.0575 | 0.2138 |
| 2 | 1.75 | 306.0644 | 0.0215 | 0.1830 | 2 | 0.49 | 174.9561 | 0.0339 | 0.2112 |
| 3 | 1.95 | 663.3329 | 0.0347 | 0.1778 | 3 | 1.36 | 303.0507 | 0.0528 | 0.2080 |
| 4 | 2.13 | 998.5831 | 0.0364 | 0.1853 | 4 | 1.45 | 467.0912 | 0.0321 | 0.1786 |
| 5 | 2.24 | 646.0117 | 0.0198 | 0.0687 | 5 | 1.76 | 358.5540 | 0.0535 | 0.1677 |
| 6 | 2.24 | 861.7346 | 0.0305 | 0.0735 | 6 | 1.88 | 548.1155 | 0.0348 | 0.1774 |
| 7 | 2.26 | 647.0518 | 0.0401 | 0.0888 | 7 | 2.61 | 165.0556 | 0.0432 | 0.1127 |
| 8 | 2.72 | 705.2183 | 0.0396 | 0.2051 | 8 | 3.09 | 125.0979 | 0.0484 | 0.1951 |
| 9 | 4.55 | 250.1779 | 0.0186 | 0.1901 | 9 | 6.25 | 566.3448 | 0.0583 | 0.2466 |
| 10 | 8.01 | 517.3692 | 0.0369 | 0.1313 | 10 | 6.45 | 506.3250 | 0.0565 | 0.2150 |
| Metabolites | RT (min) | m/z | Adduction | Female CON vs. MS | Male CON vs. MS |
|---|---|---|---|---|---|
| d-Glycerate 3-phosphate | 3.40 | 204.0872 | [M + NH4]+ | ns | ↑ ** |
| d-Xylono-1,5-lactone | 17.95 | 171.1002 | [M + Na]+ | ns | ↓ * |
| Pyruvaldehyde | 18.01 | 95.05016 | [M + Na]+ | ns | ↓ ** |
| beta-l-Arabinose 1-phosphate | 0.97 | 231.1141 | [M + H]+ | ns | ↓ *** |
| 5-Hydroxy-l-tryptophan | 9.50 | 243.211 | [M + Na]+ | ↓ * | ns |
| d-Erythrose 4-phosphate | 1.98 | 201.0878 | [M + H]+ | ↓ ** | ns |
| 1-(sn-Glycero-3-phospho)-1d-myo-inositol | 4.10 | 333.206 | [M − H]− | ↓ ** | ns |
| GDP-4-Dehydro-6-deoxy-d-mannose | 5.69 | 588.3307 | [M + H]+ | ↓ ** | ns |
| Vanillylmandelic acid | 6.35 | 221.1562 | [M + Na]+ | ↓ ** | ns |
| 5-Methoxycanthin-6-one | 6.42 | 271.2286 | [M + Na − 2H]− | ↓ ** | ns |
| 3-Methoxy-4-hydroxyphenylacetaldehyde | 9.04 | 189.1632 | [M + Na]+ | ↓ ** | ns |
| N4-Acetylaminobutanal | 11.18 | 152.1497 | [M + Na]+ | ↓ ** | ns |
| (1R)-Hydroxy-(2R)-glutathionyl-1,2-dihydronaphthalene | 12.32 | 490.459 | [M + K]+ | ↓ ** | ns |
| PE(22:0/P-18:1(11Z)) | 2.04 | 787.1695 | [M + H]+ | ↓ *** | ns |
| DG(14:0/20:4(5Z,8Z,11Z,14Z)/0:0) | 2.26 | 627.8584 | [M + K]+ | ↓ *** | ns |
| Glycerol 1,2-di-(9Z,12Z-octadecadienoate) 3-octadecanoate | 2.31 | 884.4276 | [M + H]+ | ↓ *** | ns |
| Glycerol 3-phosphate | 2.87 | 171.0679 | [M − H]− | ↓ *** | ns |
| Methylimidazoleacetic acid | 5.19 | 179.1077 | [M + K]+ | ↓ *** | ns |
| Galactosylglycerol | 6.55 | 255.2431 | [M + H]+ | ↓ *** | ns |
| 3-Methyl-2-oxovaleric acid | 8.84 | 169.1011 | [M + K]+ | ↓ *** | ns |
| 5-Thymidylic acid | 17.95 | 361.172 | [M + K]+ | ↓ *** | ns |
| Flavin mononucleotide | 17.95 | 479.3335 | [M + Na]+ | ↓ *** | ns |
| d-Linalool 3-glucoside | 3.90 | 339.3827 | [M + Na]+ | ↓ * | ↑ * |
| l-Asparagine | 4.56 | 155.1065 | [M + Na]+ | ↓ * | ↓ * |
| d-Sedoheptulose 7-phosphate | 5.94 | 291.1621 | [M + H]+ | ↓ ** | ↓ * |
| Pyridoxamine | 9.06 | 191.1793 | [M + Na]+ | ↓ ** | ↓ * |
| l-Dopachrome | 0.90 | 232.118 | [M + K]+ | ↓ *** | ↓ * |
| Inosinic acid | 2.06 | 371.1956 | [M + Na]+ | ↓ *** | ↓ * |
| NADH | 8.61 | 664.432 | [M + H]+ | ↓ *** | ↓ * |
| Sorbitol | 3.25 | 200.2009 | [M + NH4]+ | ↓ * | ↓ ** |
| 5′-Deoxy-5-fluorocytidine | 8.18 | 263.237 | [M + Na]+ | ↓ * | ↓ ** |
| Iodotyrosine | 1.25 | 330.0728 | [M + Na]+ | ↓ ** | ↓ ** |
| l-3-Hydroxykynurenine | 8.08 | 242.2477 | [M + NH4]+ | ↓ ** | ↓ ** |
| Maleylacetoacetic acid | 0.57 | 239.1142 | [M + K]+ | ↓ *** | ↓ ** |
| Dihydroxyacetone Phosphate Acyl Ester | 1.40 | 199.072 | [M + H]+ | ↓ *** | ↓ ** |
| Glyceric acid | 4.05 | 107.0858 | [M + H]+ | ↓ *** | ↓ ** |
| Isoprenyl alcohol | 4.05 | 125.0963 | [M + K]+ | ↓ *** | ↓ ** |
| 4-Aminobutyraldehyde | 4.56 | 110.105 | [M + Na]+ | ↓ *** | ↓ ** |
| Dihydrouracil | 4.56 | 137.0963 | [M + Na]+ | ↓ *** | ↓ ** |
| Normetanephrine | 4.69 | 206.1903 | [M + Na]+ | ↓ *** | ↓ ** |
| Topaquinone | 6.42 | 234.1598 | [M + Na − 2H]− | ↓ *** | ↓ ** |
| l-Cystathionine | 7.43 | 261.2212 | [M + K]+ | ↓ *** | ↓ ** |
| N,N′-Diacetylhydrazine | 4.61 | 139.1118 | [M + Na]+ | ↓ * | ↓ *** |
| 4-Hydroxyphenylpyruvic acid | 4.05 | 198.1855 | [M + NH4]+ | ↓ ** | ↓ *** |
| Indoxyl sulfate | 7.12 | 214.2167 | [M + H]+ | ↓ ** | ↓ *** |
| cis-Aconitic acid | 0.64 | 175.1196 | [M + H]+ | ↓ *** | ↓ *** |
| Cyclic pyranopterin monophosphate | 1.40 | 386.214 | [M + Na]+ | ↓ *** | ↓ *** |
| Guanosine 3′-diphosphate 5′-triphosphate | 1.63 | 684.1546 | [M + H]+ | ↓ *** | ↓ *** |
| Dihydroxyacetone phosphate | 2.89 | 193.0497 | [M + Na]+ | ↓ *** | ↓ *** |
| Indolepyruvate | 4.03 | 226.1775 | [M + Na]+ | ↓ *** | ↓ *** |
| 2-Hydroxy-3-(4-hydroxyphenyl)propenoic acid | 4.05 | 181.1592 | [M + H]+ | ↓ *** | ↓ *** |
| Glucosamine | 4.05 | 180.1748 | [M + H]+ | ↓ *** | ↓ *** |
| 5-Methoxyindoleacetate | 4.56 | 228.1958 | [M + Na]+ | ↓ *** | ↓ *** |
| Phosphohydroxypyruvic acid | 4.71 | 207.0327 | [M + Na]+ | ↓ *** | ↓ *** |
| 5-Acetylamino-6-formylamino-3-methyluracil | 6.70 | 249.1845 | [M + Na]+ | ↓ *** | ↓ *** |
| 7-Methyluric acid | 7.35 | 205.1231 | [M + Na]+ | ↓ *** | ↓ *** |
| N-Glycolylneuraminic acid | 8.56 | 326.2705 | [M + H]+ | ↓ *** | ↓ *** |
| Pathway | NDMF | NDMM | Pathway | NDMF | NDMM |
|---|---|---|---|---|---|
| Tyrosine metabolism | 8 | 6 | Alanine, aspartate and glutamate metabolism | 1 | 1 |
| Glycine, serine and threonine metabolism | 3 | 5 | beta-Alanine metabolism | 1 | 1 |
| Tryptophan metabolism | 4 | 3 | Citrate cycle (TCA cycle) | 1 | 1 |
| Cysteine and methionine metabolism | 2 | 3 | Folate biosynthesis | 1 | 1 |
| Amino sugar and nucleotide sugar metabolism | 3 | 2 | Inositol phosphate metabolism | 1 | 1 |
| Fructose and mannose metabolism | 3 | 2 | Nicotinate and nicotinamide metabolism | 1 | 1 |
| Glycerolipid metabolism | 3 | 2 | Pantothenate and CoA biosynthesis | 1 | 1 |
| Glycerophospholipid metabolism | 3 | 2 | Phenylalanine, tyrosine and tryptophan biosynthesis | 1 | 1 |
| Pentose phosphate pathway | 3 | 2 | Ubiquinone and other terpenoid-quinone biosynthesis | 1 | 1 |
| Caffeine metabolism | 2 | 2 | Vitamin B6 metabolism | 1 | 1 |
| Drug metabolism–other enzymes | 2 | 2 | Pentose and glucuronate interconversions | 0 | 1 |
| Ether lipid metabolism | 2 | 2 | Pyruvate metabolism | 0 | 1 |
| Glyoxylate and dicarboxylate metabolism | 2 | 2 | Histidine metabolism | 1 | 0 |
| Purine metabolism | 2 | 2 | Metabolism of xenobiotics by cytochrome P450 | 1 | 0 |
| Glycolysis/Gluconeogenesis | 1 | 2 | Riboflavin metabolism | 1 | 0 |
| Arginine and proline metabolism | 2 | 1 | Valine, leucine and isoleucine biosynthesis | 1 | 0 |
| Galactose metabolism | 2 | 1 | Valine, leucine and isoleucine degradation | 1 | 0 |
| Pyrimidine metabolism | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, M.-C.; Chen, Y.-X.; Sun, X.-R.; Jiang, N.; Chang, Q.; Liu, X.-M.; Pan, R.-L. One-Week Maternal Separation Caused Sex-Specific Changes in Behavior and Hippocampal Metabolomics of Offspring Rats. Brain Sci. 2024, 14, 1275. https://doi.org/10.3390/brainsci14121275
Dong M-C, Chen Y-X, Sun X-R, Jiang N, Chang Q, Liu X-M, Pan R-L. One-Week Maternal Separation Caused Sex-Specific Changes in Behavior and Hippocampal Metabolomics of Offspring Rats. Brain Sciences. 2024; 14(12):1275. https://doi.org/10.3390/brainsci14121275
Chicago/Turabian StyleDong, Meng-Chen, Yu-Xin Chen, Xin-Ran Sun, Ning Jiang, Qi Chang, Xin-Min Liu, and Rui-Le Pan. 2024. "One-Week Maternal Separation Caused Sex-Specific Changes in Behavior and Hippocampal Metabolomics of Offspring Rats" Brain Sciences 14, no. 12: 1275. https://doi.org/10.3390/brainsci14121275
APA StyleDong, M.-C., Chen, Y.-X., Sun, X.-R., Jiang, N., Chang, Q., Liu, X.-M., & Pan, R.-L. (2024). One-Week Maternal Separation Caused Sex-Specific Changes in Behavior and Hippocampal Metabolomics of Offspring Rats. Brain Sciences, 14(12), 1275. https://doi.org/10.3390/brainsci14121275






