The Effect of Bilateral, Two-Level Cervical Sympathetic Chain Blocks on Specific Symptom Clusters for Traumatic Brain Injury, Independent of Concomitant PTSD Symptoms
Abstract
1. Introduction
2. Materials and Methods
Per published guidelines, all patients had a bilateral 2LCSB at the 4th cervical vertebra and 6th cervical vertebra, with the right side performed on day 1, and the left side performed on day 2 [50]. The blocks were performed on subsequent days to eliminate the risk of inadvertent bilateral blockade of the recurrent laryngeal nerve and subsequent potential airway compromise. A Doppler ultrasound scan was utilized prior to every procedure to clearly identify the vertebral artery and vein, as well as other vasculature. A 50-mm 25-gauge needle was utilized under ultrasound-guidance (General Electric Logic e with an 8–12 MHz broadband linear transducer) using a lateral, in-plane approach at both the 6th cervical vertebra level (using 6–8 mL of 0.5% ropivacaine) and the 4th cervical vertebra level (using 1.5–2 mL of 0.5% ropivacaine). All procedures were performed at an established musculoskeletal practice by pain and sports medicine fellowship-trained physicians who have collectively performed more than 5000 SGBs. Horner’s syndrome, a sign of a successful blockade of the stellate ganglion, is characterized by ptosis, miosis, and scleral injection and was scored by two independent observers at 5 min post-block per published guidelines [47]. All patients met the minimum clinical threshold for an acceptable Horner’s syndrome of 4 out of 6 points by both observers.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DOD TBI Worldwide Numbers [Internet]. Military Health System. 2018. Available online: https://www.health.mil/Military-Health-Topics/Centers-of-Excellence/Traumatic-Brain-Injury-Center-of-Excellence/DOD-TBI-Worldwide-Numbers (accessed on 4 September 2024).
- Cassidy, J.D.; Carroll, L.; Peloso, P.; Borg, J.; von Holst, H.; Holm, L.; Kraus, J.; Coronado, V. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the who collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 2004, 36, 28–60. [Google Scholar] [CrossRef] [PubMed]
- Haarbauer-Krupa, J.; Pugh, M.J.; Prager, E.M.; Harmon, N.; Wolfe, J.; Yaffe, K. Epidemiology of Chronic Effects of Traumatic Brain Injury. J. Neurotrauma 2021, 38, 3235–3247. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and National Burden of Traumatic Brain Injury and Spinal Cord injury, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Riggio, S. Traumatic Brain Injury and Its Neurobehavioral Sequelae. Psychiatr. Clin. N. Am. 2010, 33, 807–819. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Neurological Disorders and Stroke Traumatic Brain Injury (TBI). Available online: https://www.ninds.nih.gov/health-information/disorders/traumatic-brain-injury-tbi (accessed on 4 September 2024).
- McCrea, M.; Hammeke, T.; Olsen, G.; Leo, P.; Guskiewicz, K. Unreported Concussion in High School Football Players. Clin. J. Sport Med. 2004, 14, 13–17. [Google Scholar] [CrossRef]
- Reger, M.A.; Brenner, L.A.; du Pont, A. Traumatic Brain Injury and Veteran Mortality After the War in Afghanistan. JAMA Netw. Open 2022, 5, e2148158. [Google Scholar] [CrossRef]
- Stewart, I.J.; Howard, J.T.; Poltavskiy, E.; Dore, M.; Amuan, M.E.; Ocier, K.; Walker, L.E.; Alcover, K.C.; Jo Pugh, M. Traumatic Brain Injury and Subsequent Risk of Brain Cancer in US Veterans of the Iraq and Afghanistan Wars. JAMA Netw. Open 2024, 7, e2354588. [Google Scholar] [CrossRef]
- Brenner, L.A.; Forster, J.E.; Gradus, J.L.; Hostetter, T.A.; Hoffmire, C.A.; Walsh, C.G.; Mary Jo Larson Stearns-Yoder, K.A.; Adams, R.S. Associations of Military-Related Traumatic Brain Injury with New-Onset Mental Health Conditions and Suicide Risk. JAMA Netw. Open 2023, 6, e2326296. [Google Scholar] [CrossRef]
- Hoge, C.W.; McGurk, D.; Thomas, J.L.; Cox, A.L.; Engel, C.C.; Castro, C.A. Mild Traumatic Brain Injury in U.S. Soldiers Returning from Iraq. N. Engl. J. Med. 2008, 358, 453–463. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Laskowitz, D.; Grant, G. Translational Research in Traumatic Brain Injury; CRC Press: Boca Raton, FL, USA, 2016; Chapter 3; ISBN 9781466584914. [Google Scholar]
- Tang-Schomer, M.D.; Patel, A.R.; Baas, P.W.; Smith, D.H. Mechanical Breaking of Microtubules in Axons during Dynamic Stretch Injury Underlies Delayed Elasticity, Microtubule Disassembly, and Axon Degeneration. FASEB J. 2010, 24, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.H.; Stewart, W. Tackling Concussion, beyond Hollywood. Lancet Neurol. 2016, 15, 662–663. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Mudher, A.; Colin, M.; Dujardin, S.; Medina, M.; Dewachter, I.; Alavi Naini, S.M.; Mandelkow, E.-M.; Mandelkow, E.; Buée, L.; Goedert, M.; et al. What Is the Evidence That Tau Pathology Spreads through Prion-like Propagation? Acta Neuropathol. Commun. 2017, 5, 99. [Google Scholar] [CrossRef]
- Marklund, N.; Vedung, F.; Lubberink, M.; Tegner, Y.; Johansson, J.; Blennow, K.; Zetterberg, H.; Fahlström, M.; Haller, S.; Stenson, S.; et al. Tau Aggregation and Increased Neuroinflammation in Athletes after Sports-Related Concussions and in Traumatic Brain Injury Patients—A PET/MR Study. NeuroImage Clin. 2021, 30, 102665. [Google Scholar] [CrossRef]
- McKee, A.C.; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Dirk Keene, C.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.-P.; Stewart, W.; et al. The First NINDS/NIBIB Consensus Meeting to Define Neuropathological Criteria for the Diagnosis of Chronic Traumatic Encephalopathy. Acta Neuropathol. 2015, 131, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Black, K.L.; Hanks, R.A.; Wood, D.L.; Zafonte, R.D.; Cullen, N.; Cifu, D.X.; Englander, J.; Francisco, G.E. Blunt versus Penetrating Violent Traumatic Brain Injury. J. Head Trauma Rehabil. 2002, 17, 489–496. [Google Scholar] [CrossRef]
- Warden, D. Military TBI during the Iraq and Afghanistan Wars. J. Head Trauma Rehabil. 2006, 21, 398–402. [Google Scholar] [CrossRef]
- Risdall, J.E.; Menon, D.K. Traumatic Brain Injury. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 241–250. [Google Scholar] [CrossRef]
- Cernak, I.; Noble-Haeusslein, L.J. Traumatic Brain Injury: An Overview of Pathobiology with Emphasis on Military Populations. J. Cereb. Blood Flow Metab. 2009, 30, 255–266. [Google Scholar] [CrossRef]
- Ling, G.S.; Ecklund, J.M. Traumatic Brain Injury in Modern War. Curr. Opin. Anaesthesiol. 2011, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Faden, A.; Demediuk, P.; Panter, S.; Vink, R. The Role of Excitatory Amino Acids and NMDA Receptors in Traumatic Brain Injury. Science 1989, 244, 798–800. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, S.M.; Leclercq, P.D.; Moyes, L.; Graham, D.I.; Smith, C.; Griffin, W.S.T.; Nicoll, J.a.R. Long-Term Intracerebral Inflammatory Response after Traumatic Brain Injury. Forensic Sci. Int. 2004, 146, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and White Matter Degeneration Persist for Years after a Single Traumatic Brain Injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef]
- Xiong, Y.; Gu, Q.; Peterson, P.L.; Muizelaar, J.P.; Lee, C.P. Mitochondrial Dysfunction and Calcium Perturbation Induced by Traumatic Brain Injury. J. Neurotrauma 1997, 14, 23–34. [Google Scholar] [CrossRef]
- Bruns, J.; Hauser, W.A. The Epidemiology of Traumatic Brain Injury: A Review. Epilepsia 2003, 44, 2–10. [Google Scholar] [CrossRef]
- Andriessen, T.M.J.C.; Jacobs, B.; Vos, P.E. Clinical Characteristics and Pathophysiological Mechanisms of Focal and Diffuse Traumatic Brain Injury. J. Cell. Mol. Med. 2010, 14, 2381–2392. [Google Scholar] [CrossRef]
- Mulvaney, S.W.; Lynch, J.H.; Rae, K.L.; Mahadevan, S.; Dineen, K.J. The Successful Use of Bilateral 2-Level Ultrasound-Guided Stellate Ganglion Block to Improve Traumatic Brain Injury Symptoms: A Retrospective Analysis of 23 Patients. Mil. Med. 2024, 10, e2573–e2577. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5-TR) [Internet]. Psychiatry.org. American Psychiatric Association. 2013. Available online: https://www.psychiatry.org/psychiatrists/practice/dsm (accessed on 4 September 2024).
- Kaplan, G.B.; Leite-Morris, K.A.; Wang, L.; Rumbika, K.K.; Heinrichs, S.C.; Zeng, X.; Wu, L.; Arena, D.T.; Teng, Y.D. Pathophysiological Bases of Comorbidity: Traumatic Brain Injury and Post-Traumatic Stress Disorder. J. Neurotrauma 2018, 35, 210–225. [Google Scholar] [CrossRef]
- van Amelsvoort, T.; Hernaus, D. Effect of Pharmacological Interventions on the Fronto-Cingulo-Parietal Cognitive Control Network in Psychiatric Disorders: A Transdiagnostic Systematic Review of fMRI Studies. Front. Psychiatry 2016, 7, 82. [Google Scholar] [CrossRef]
- Monsour, M.; Ebedes, D.; Borlongan, C.V. A review of the pathology and treatment of TBI and PTSD. Exp. Neurol. 2022, 351, 114009. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, M.; Reddy, V.; Singh, P. Neuroanatomy, Stellate Ganglion [Internet]; StatPearls Publishing: Petersburg, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539807/ (accessed on 4 September 2024).
- Lebovits, A.H.; Yarmush, J.; Lefkowitz, M. Reflex Sympathetic Dystrophy and Posttraumatic Stress Disorder. Clin. J. Pain 1990, 6, 153–157. [Google Scholar] [CrossRef]
- Mulvaney, S.W.; Lynch, J.H.; de Leeuw, J.; Schroeder, M.; Kane, S. Neurocognitive Performance is Not Degraded After Stellate Ganglion Block Treatment for Post-Traumatic Stress Disorder: A Case Series. Mil. Med. 2015, 180, e601–e604. [Google Scholar] [CrossRef]
- Moore, D.C. Stellate Ganglion Block; Thomas: Springfield, IL, USA, 1954. [Google Scholar]
- Baek, J.H. Characteristics of the Middle Cervical Sympathetic Ganglion: A Systematic Review and Meta-Analysis. Pain Physician 2018, 1, 9–18. [Google Scholar] [CrossRef]
- Mulvaney, S.; Curtis, K.; Ibrahim, T. Comparison C6 Stellate Ganglion versus C6 and C4 Cervical Sympathetic Chain Blocks for Treatment of Posttraumatic Stress Disorder (PTSD): Analysis of 147 Patients. J. Neurol. Disord. Stroke 2020, 7, 1163. [Google Scholar]
- Lipov, E.G.; Jacobs, R.; Springer, S.; Candido, K.D.; Knezevic, N.N. Utility of Cervical Sympathetic Block in Treating Post-Traumatic Stress Disorder in Multiple Cohorts: A Retrospective Analysis. Pain Physician 2022, 25, 77–85. [Google Scholar] [PubMed]
- Mulvaney, S.W.; McLean, B.; De Leeuw, J. The Use of Stellate Ganglion Block in the Treatment of Panic/Anxiety Symptoms with Combat-Related Post-Traumatic Stress Disorder; Preliminary Results of Long-Term Follow-Up: A Case Series. Pain Pract. 2010, 10, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, S.W.; Lynch, J.H.; Hickey, M.J.; Rahman-Rawlins, T.; Schroeder, M.; Kane, S.; Lipov, E. Stellate Ganglion Block Used to Treat Symptoms Associated with Combat-Related Post-Traumatic Stress Disorder: A Case Series of 166 Patients. Mil. Med. 2014, 179, 1133–1140. [Google Scholar] [CrossRef]
- Schore, A.N. Dysregulation of the Right Brain: A Fundamental Mechanism of Traumatic Attachment and the Psychopathogenesis of Posttraumatic Stress Disorder. Aust. N. Z. J. Psychiatry 2002, 36, 9–30. [Google Scholar] [CrossRef]
- Wittling, W. The right hemisphere and the human stress response. Acta Physiol. Scand. 1997, 640, 55–59. [Google Scholar]
- Mulvaney, S.W.; Lynch, J.H.; Kotwal, R.S. Clinical Guidelines for Stellate Ganglion Block to Treat Anxiety Associated with Posttraumatic Stress Disorder. J. Spec. Oper. Med. 2015, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Rae Olmsted, K.L.; Bartoszek, M.; Mulvaney, S.; McLean, B.; Turabi, A.; Young, R.; Kim, E.; Vandermaas-Peeler, R.; Morgan, J.K.; Constantinescu, O.; et al. Effect of Stellate Ganglion Block Treatment on Posttraumatic Stress Disorder Symptoms. JAMA Psychiatry 2020, 77, 130. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.H.; Muench, P.D.; Okiishi, J.C.; Means, G.E.; Mulvaney, S.W. Behavioral health clinicians endorse stellate ganglion block as a valuable intervention in the treatment of trauma-related disorders. J. Investig. Med. 2021, 69, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, S.W.; Lynch, J.H.; Curtis, K.E.; Ibrahim, T.S. The Successful Use of Left-sided Stellate Ganglion Block in Patients That Fail to Respond to Right-sided Stellate Ganglion Block for the Treatment of Post-traumatic Stress Disorder Symptoms: A Retrospective Analysis of 205 Patients. Mil. Med. 2021, 187, e826–e829. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. Generalized Anxiety Disorder 7; PsycTESTS Dataset; APA PsycTests: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- Lynch, J.H.; Mulvaney, S.W.; Bryan, C.J.; Hernandez, D. Stellate Ganglion Block Reduces Anxiety Symptoms by Half: A Case Series of 285 Patients. J. Pers. Med. 2023, 13, 958. [Google Scholar] [CrossRef]
- Meyers, J.E.; English, J.; Miller, R.M.; Lee, A.J. Normative Data for the Neurobehavioral Symptom Inventory. Appl. Neuropsychol. Adult 2015, 22, 427–434. [Google Scholar] [CrossRef]
- Weathers, F.W.; Litz, B.T.; Keane, T.M.; Palmieri, P.A.; Marx, B.P.; Schnurr, P.P. The PTSD Checklist for DSM-5 (PCL-5)—Standard [Internet]. 2023. Available online: https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp (accessed on 4 September 2024).
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing generalized anxiety disorder. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Andrews, R.J.; Fonda, J.R.; Levin, L.K.; McGlinchey, R.E.; Milberg, W.P. Comprehensive analysis of the predictors of neurobehavioral symptom reporting in veterans. Neurology 2018, 91, e732–e745. [Google Scholar] [CrossRef]
- Silva, M.A. Review of the Neurobehavioral Symptom Inventory. Rehabil. Psychol. 2020, 66, 170–182. [Google Scholar] [CrossRef]
- Yang, X.; Shi, Z.; Li, X.; Li, J. Impacts of stellate ganglion block on plasma NF-κB and inflammatory factors of TBI patients. Int. J. Clin. Exp. Med. 2015, 8, 15630–15638. [Google Scholar]
- Kim, D.H. The Effect of Oxygen Administration on Regional Cerebral Oxygen Saturation after Stellate Ganglion Block on the Non-Blocked Side. Pain Physician 2013, 16, 117–124. [Google Scholar] [CrossRef] [PubMed]
- ter Laan, M.; van Dijk, J.M.C.; Elting, J.W.J.; Staal, M.J.; Absalom, A.R. Sympathetic regulation of cerebral blood flow in humans: A review. Br. J. Anaesth. 2013, 111, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Blevins, C.A.; Weathers, F.W.; Davis, M.T.; Witte, T.K.; Domino, J.L. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J. Trauma. Stress 2015, 28, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bovin, M.J.; Marx, B.P.; Weathers, F.W.; Gallagher, M.W.; Rodriguez, P.; Schnurr, P.P.; Keane, T.M. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition (PCL-5) in veterans. Psychol. Assess. 2016, 28, 1379–1391. [Google Scholar] [CrossRef]
- Marx, B.P.; Lee, D.J.; Norman, S.B.; Bovin, M.J.; Sloan, D.M.; Weathers, F.W.; Keane, T.M.; Schnurr, P.P. Reliable and clinically significant change in the clinician-administered PTSD Scale for DSM-5 and PTSD Checklist for DSM-5 among male veterans. Psychol. Assess. 2021, 34, 197–203. [Google Scholar] [CrossRef]
- Wortmann, J.H.; Jordan, A.H.; Weathers, F.W.; Resick, P.A.; Dondanville, K.A.; Hall-Clark, B.; Foa, E.B.; Young-McCaughan, S.; Yarvis, J.S.; Hembree, E.A.; et al. Psychometric analysis of the PTSD Checklist-5 (PCL-5) among treatment-seeking military service members. Psychol. Assess. 2016, 28, 1392–1403. [Google Scholar] [CrossRef]

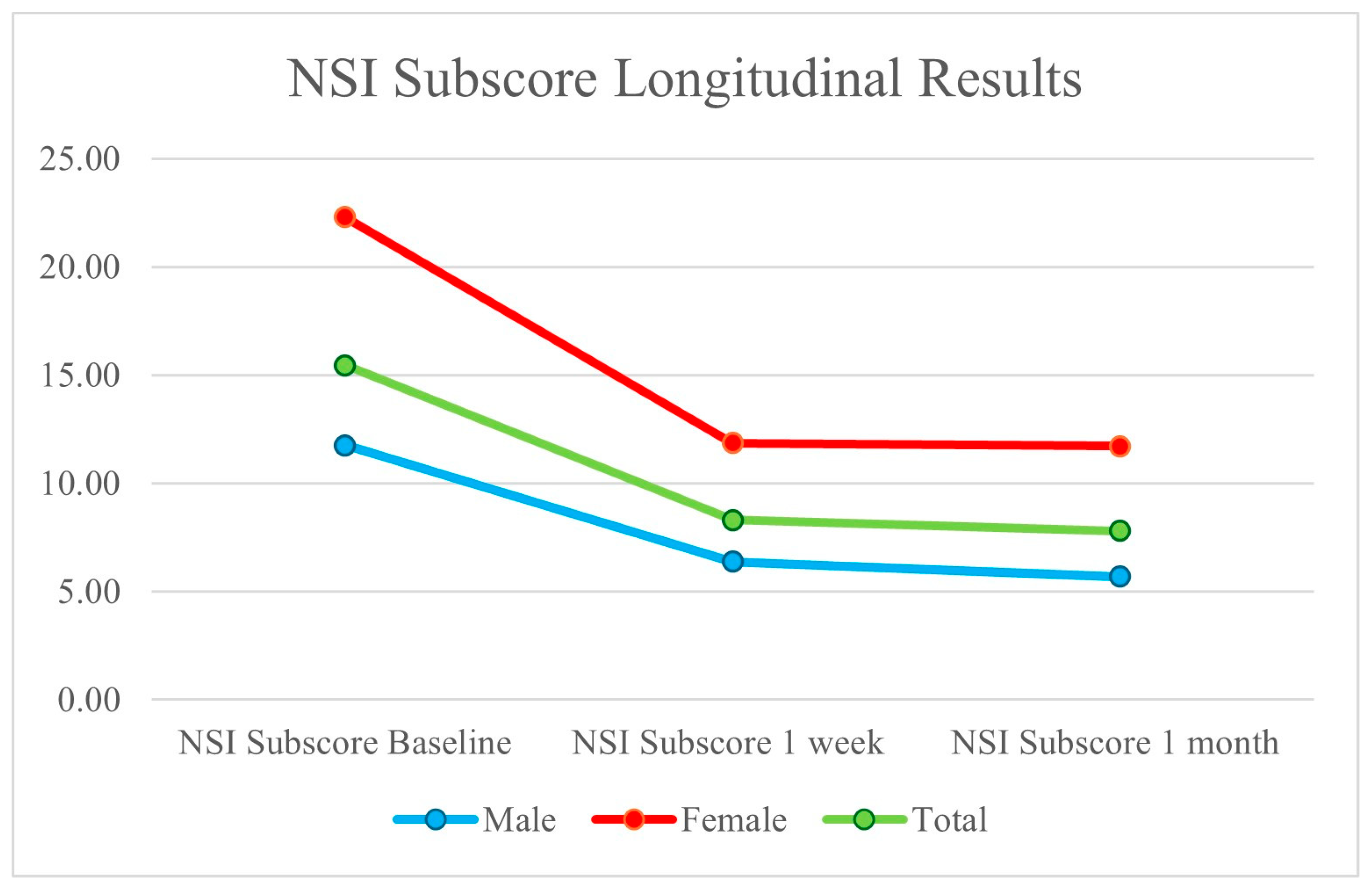
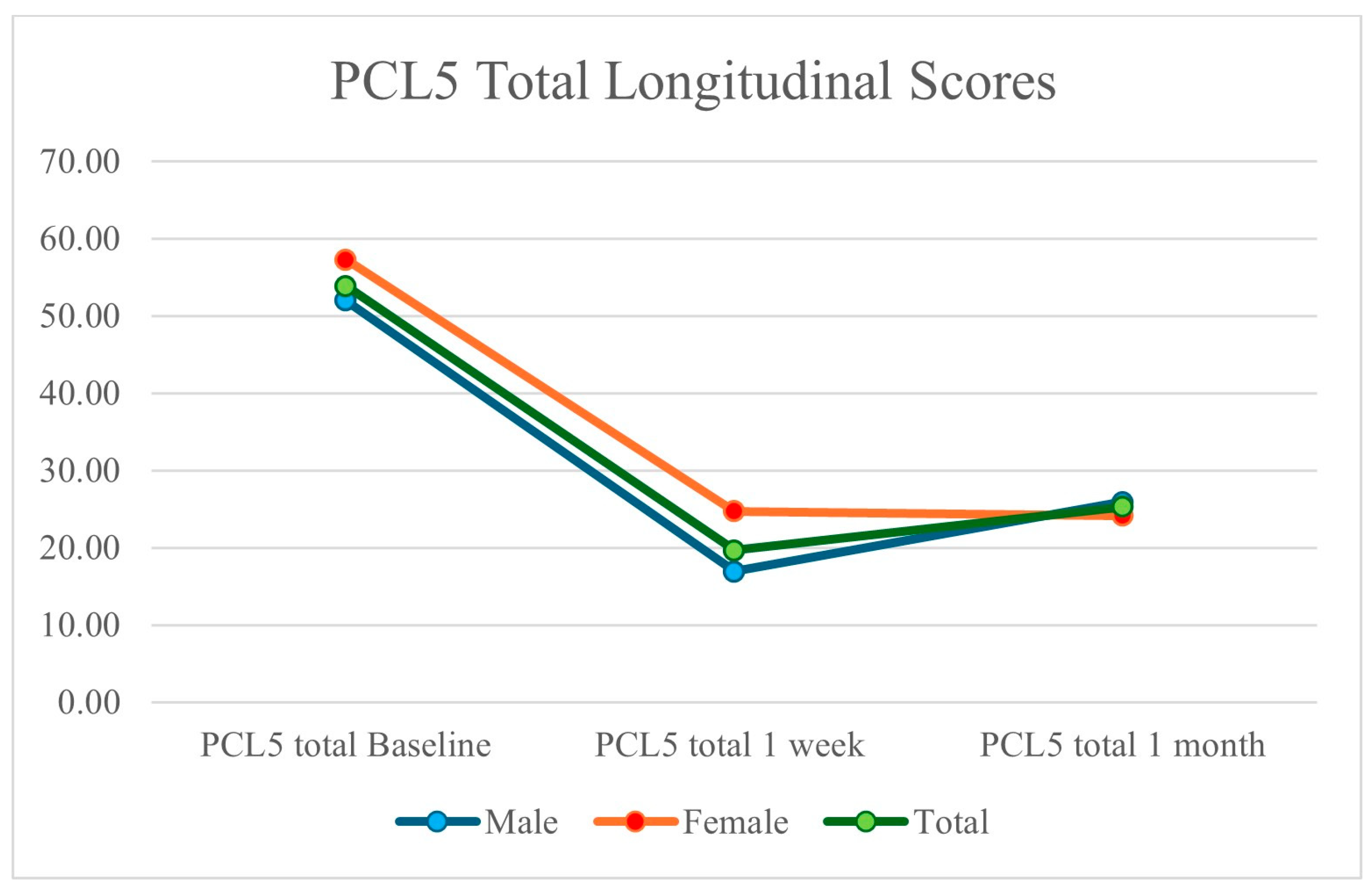

| Male | Female | Total | |
|---|---|---|---|
| Avg. NSI at Baseline | 41.54 | 50.00 | 44.50 |
| Avg. NSI at 1 Month | 18.85 | 29.00 | 22.40 |
| Avg. NSI % Improvement | 54.63% | 42.00% | 49.66% |
| Avg. TBI Sub-Score at Baseline | 11.77 | 22.29 | 15.45 |
| Avg. TBI Sub-Score at 1 Month | 5.69 | 11.71 | 7.80 |
| Avg. TBI Sub-Score % Improvement | 51.63% | 47.44% | 49.51% |
| Avg. PCL-5 at Baseline | 52.00 | 57.29 | 53.85 |
| Avg. PCL-5 at 1 Month | 25.92 | 24.14 | 25.30 |
| Avg. PCL-5 % Improvement | 50.15% | 57.86% | 53.02% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulvaney, S.W.; Lynch, J.H.; Mahadevan, S.; Dineen, K.J.; Rae Olmsted, K.L. The Effect of Bilateral, Two-Level Cervical Sympathetic Chain Blocks on Specific Symptom Clusters for Traumatic Brain Injury, Independent of Concomitant PTSD Symptoms. Brain Sci. 2024, 14, 1193. https://doi.org/10.3390/brainsci14121193
Mulvaney SW, Lynch JH, Mahadevan S, Dineen KJ, Rae Olmsted KL. The Effect of Bilateral, Two-Level Cervical Sympathetic Chain Blocks on Specific Symptom Clusters for Traumatic Brain Injury, Independent of Concomitant PTSD Symptoms. Brain Sciences. 2024; 14(12):1193. https://doi.org/10.3390/brainsci14121193
Chicago/Turabian StyleMulvaney, Sean W., James H. Lynch, Sanjay Mahadevan, Kyle J. Dineen, and Kristine L. Rae Olmsted. 2024. "The Effect of Bilateral, Two-Level Cervical Sympathetic Chain Blocks on Specific Symptom Clusters for Traumatic Brain Injury, Independent of Concomitant PTSD Symptoms" Brain Sciences 14, no. 12: 1193. https://doi.org/10.3390/brainsci14121193
APA StyleMulvaney, S. W., Lynch, J. H., Mahadevan, S., Dineen, K. J., & Rae Olmsted, K. L. (2024). The Effect of Bilateral, Two-Level Cervical Sympathetic Chain Blocks on Specific Symptom Clusters for Traumatic Brain Injury, Independent of Concomitant PTSD Symptoms. Brain Sciences, 14(12), 1193. https://doi.org/10.3390/brainsci14121193








