Coincidence of Concentric Vessel-Wall Contrast Enhancement in Moyamoya Disease and Acute Postoperative Ischemic Stroke During Revascularization Procedures
Abstract
1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. VW-CE MR Imaging Protocol
2.3. Data Processing, Workflow and Statistics
2.4. Severity of PAIS
2.5. Standardized Diagnostic Protocol
2.6. Standard Protocol Approvals, Registrations, and Patient Consent
3. Results
Perioperative Acute Ischemic Stroke After Revascularization
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACA | anterior cerebral artery |
| APT | antiplatelet therapy |
| BMI | body mass index |
| BOLD | blood-oxygenation-level dependent |
| CDC | Clavien–Dindo Classification |
| CT | computed tomography scan |
| DM | diabetes mellitus |
| EC-IC | extracranial–intracranial |
| PAIS | perioperative acute ischemic stroke |
| HDL | high-density lipoprotein |
| HTN | (arterial) hypertension |
| ICA | internal carotid artery |
| MCA | middle cerebral artery |
| MMD | Moyamoya disease |
| MRI | magnetic resonance imaging |
| OR | odds ratio |
| VW-CE | contrast-enhancing vessel wall |
| TIA | transitory ischemic attack |
References
- Yamada, S.; Oki, K.; Itoh, Y.; Kuroda, S.; Houkin, K.; Tominaga, T.; Miyamoto, S.; Hashimoto, N.; Suzuki, N. Effects of Surgery and Antiplatelet Therapy in Ten-Year Follow-Up from the Registry Study of Research Committee on Moyamoya Disease in Japan. J. Stroke Cerebrovasc. Dis. 2016, 25, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kazumata, K.; Ito, M.; Tokairin, K.; Ito, Y.; Houkin, K.; Nakayama, N.; Kuroda, S.; Ishikawa, T.; Kamiyama, H. The frequency of postoperative stroke in moyamoya disease following combined revascularization: A single-university series and systematic review. J. Neurosurg. 2014, 121, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, H.; Tang, J.; Lin, C.; Ni, W.; Gu, Y. Postoperative cerebral infarction after revascularization in patients with moyamoya disease: Incidence and risk factors. Front. Neurol. 2022, 13, 1053193. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Chen, X.; Yu, J.; Li, X.-Q. Risk factors for postoperative stroke in adults patients with moyamoya disease: A systematic review with meta-analysis. BMC Neurol. 2019, 19, 98. [Google Scholar] [CrossRef]
- Hyun, S.-J.; Kim, J.-S.; Hong, S.-C. Prognostic factors associated with perioperative ischemic complications in adult-onset moyamoya disease. Acta Neurochir. 2010, 152, 1181–1188. [Google Scholar] [CrossRef]
- Antonucci, M.; Burns, T.; Pulling, T.; Rosenberg, J.; Marks, M.; Steinberg, G.; Zaharchuk, G. Acute Preoperative Infarcts and Poor Cerebrovascular Reserve Are Independent Risk Factors for Severe Ischemic Complications following Direct Extracranial-Intracranial Bypass for Moyamoya Disease. Am. J. Neuroradiol. 2016, 37, 228–235. [Google Scholar] [CrossRef]
- Jung, Y.J.; Ahn, J.S.; Kwon, D.H.; Kwun, B.D. Ischemic complications occurring in the contralateral hemisphere after surgical treatment of adults with moyamoya disease. J. Korean Neurosurg. Soc. 2011, 50, 492–496. [Google Scholar] [CrossRef]
- Tashiro, R.; Fujimura, M.; Kameyama, M.; Mugikura, S.; Endo, H.; Takeuchi, Y.; Tomata, Y.; Niizuma, K.; Tominaga, T. Incidence and Risk Factors of the Watershed Shift Phenomenon after Superficial Temporal Artery-Middle Cerebral Artery Anastomosis for Adult Moyamoya Disease. Cerebrovasc. Dis. 2019, 47, 178–187. [Google Scholar] [CrossRef]
- Oshima, H.; Katayama, Y.; Hirayama, T. Intracerebral steal phenomenon associated with global hyperemia in moyamoya disease during revascularization surgery. J. Neurosurg. 2000, 92, 949–954. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Zhao, M.; Cao, P.; Liu, X.; Ren, H.; Zhang, D.; Zhang, Y.; Wang, R.; Zhao, J. High variance of intraoperative blood pressure predicts early cerebral infarction after revascularization surgery in patients with Moyamoya disease. Neurosurg. Rev. 2020, 43, 759–769. [Google Scholar] [CrossRef]
- Hara, S.; Nariai, T.; Inaji, M.; Tanaka, Y.; Maehara, T. Imaging Pattern and the Mechanisms of Postoperative Infarction After Indirect Revascularization in Patients with Moyamoya Disease. World Neurosurg. 2021, 155, e510–e521. [Google Scholar] [CrossRef] [PubMed]
- Guzman, R.; Lee, M.; Achrol, A.; Bell-Stephens, T.; Kelly, M.; Do, H.M.; Marks, M.P.; Steinberg, G.K. Clinical outcome after 450 revascularization procedures for moyamoya disease. J. Neurosurg. 2009, 111, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, H.; Liu, D.; Liu, X.; Zhang, L.; Peng, P.; Yuan, F.; Liu, S.; Sheng, F.; Liu, Y.; et al. Association of intracranial vessel wall enhancement and cerebral hemorrhage in moyamoya disease: A high-resolution magnetic resonance imaging study. J. Neurol. 2021, 268, 4768–4777. [Google Scholar] [CrossRef] [PubMed]
- Brinjikji, W.; Mossa-Basha, M.; Huston, J.; Rabinstein, A.A.; Lanzino, G.; Lehman, V.T. Intracranial vessel wall imaging for evaluation of steno-occlusive diseases and intracranial aneurysms. J. Neuroradiol. 2017, 44, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.-Y.; Hauser, T.-K.; Haas, P.; Tellermann, J.; Hurth, H.; Ernemann, U.; Tatagiba, M.; Bender, B.; Khan, N.; Roder, C. Intensity Score of Vessel Wall Contrast Enhancement in MRI Allows Prediction of Disease Progression in Moyamoya Angiopathy. Neurosurgery 2024, 95, 1000–1009. [Google Scholar] [CrossRef]
- Masuda, J.; Ogata, J.; Yutani, C. Smooth muscle cell proliferation and localization of macrophages and T cells in the occlusive intracranial major arteries in moyamoya disease. Stroke 1993, 24, 1960–1967. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, H.; Liu, D.; Hao, F.; Zhang, L.; Peng, P.; Yuan, F.; Liu, S.; Sheng, F.; Liu, Y.; et al. Vessel wall enhancement as a predictor of arterial stenosis progression and poor outcomes in moyamoya disease. Eur. Radiol. 2023, 33, 2489–2499. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Zhou, F.; Li, M.; Liu, R.; Guan, M.; Li, R.; He, L.; Xu, Y.; Zhang, B.; et al. The Contrast Enhancement of Intracranial Arterial Wall on High-resolution MRI and Its Clinical Relevance in Patients with Moyamoya Vasculopathy. Sci. Rep. 2017, 7, 44264. [Google Scholar] [CrossRef]
- Roder, C.; Hauser, T.-K.; Ernemann, U.; Tatagiba, M.; Khan, N.; Bender, B. Arterial wall contrast enhancement in progressive moyamoya disease. J. Neurosurg. 2019, 132, 1845–1853. [Google Scholar] [CrossRef]
- Fujimura, M.; Tominaga, T.; Kuroda, S.; Takahashi, J.C.; Endo, H.; Ogasawara, K.; Miyamoto, S.; Labor Welfare Research Committee on Moyamoya Disease (Spontaneous Occlusion of Circle of Willis) of the Ministry of Health; the Guideline Committee 2021 of the Japan Stroke Society. 2021 Japanese Guidelines for the Management of Moyamoya Disease: Guidelines from the Research Committee on Moyamoya Disease and Japan Stroke Society. Neurol. Med.-Chir. 2022, 62, 165–170. [Google Scholar] [CrossRef]
- Bersano, A.; Khan, N.; Fuentes, B.; Acerbi, F.; Canavero, I.; Tournier-Lasserve, E.; Vajcoczy, P.; Zedde, M.L.; Hussain, S.; Lémeret, S.; et al. European Stroke Organisation (ESO) Guidelines on Moyamoya angiopathy Endorsed by Vascular European Reference Network (VASCERN). Eur. Stroke J. 2023, 8, 55–84. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The clavien-dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.-K.; Seeger, A.; Bender, B.; Klose, U.; Thurow, J.; Ernemann, U.; Tatagiba, M.; Meyer, P.T.; Khan, N.; Roder, C. Hypercapnic BOLD MRI compared to H215O PET/CT for the hemodynamic evaluation of patients with Moyamoya Disease. NeuroImage Clin. 2019, 22, 101713. [Google Scholar] [CrossRef] [PubMed]
- Roder, C.; Klose, U.; Hurth, H.; Brendle, C.; Tatagiba, M.; Ernemann, U.; Khan, N.; Hauser, T.-K. Longitudinal Reproducibility of CO2-Triggered BOLD MRI for the Hemodynamic Evaluation of Adult Patients with Moyamoya Angiopathy. Cerebrovasc. Dis. 2021, 50, 332–338. [Google Scholar] [CrossRef]
- Roder, C.; Bürkle, E.; Ebner, F.H.; Tatagiba, M.; Ernemann, U.; Buck, A.; Meyer, P.T.; Khan, N. Estimation of Severity of Moyamoya Disease with [15O]Water-Positron Emission Tomography Compared with Magnetic Resonance Imaging and Angiography. World Neurosurg. 2018, 117, e75–e81. [Google Scholar] [CrossRef]
- Zerweck, L.; Roder, C.; Hauser, T.-K.; Thurow, J.; Mengel, A.; Tatagiba, M.; Khan, N.; Meyer, P.T.; Ernemann, U.; Klose, U. Hemodynamic evaluation of patients with Moyamoya Angiopathy: Comparison of resting-state fMRI to breath-hold fMRI and [15O]water PET. Neuroradiology 2022, 64, 553–563. [Google Scholar] [CrossRef]
- Zerweck, L.; Pohmann, R.; Klose, U.; Martirosian, P.; Haas, P.; Ernemann, U.; Khan, N.; Roder, C.; Hauser, T.-K.; Hennersdorf, F. Evaluation of the contribution of individual arteries to the cerebral blood supply in patients with Moyamoya angiopathy: Comparison of vessel-encoded arterial spin labeling and digital subtraction angiography. Neuroradiology 2024, 66, 1131–1140. [Google Scholar] [CrossRef]
- Zerweck, L.; Hauser, T.-K.; Roder, C.; Blazhenets, G.; Khan, N.; Ernemann, U.; Meyer, P.T.; Klose, U. Evaluation of the cerebrovascular reactivity in patients with Moyamoya Angiopathy by use of breath-hold fMRI: Investigation of voxel-wise hemodynamic delay correction in comparison to [15O]water PET. Neuroradiology 2023, 65, 539–550. [Google Scholar] [CrossRef]
- Schubert, G.A.; Biermann, P.; Weiss, C.; Seiz, M.; Vajkoczy, P.; Schmiedek, P.; Thomé, C. Risk Profile In Extracranial/Intracranial Bypass Surgery—The Role of Antiplatelet Agents, Disease Pathology, and Surgical Technique In 168 Direct Revascularization Procedures. World Neurosurg. 2014, 82, 672–677. [Google Scholar] [CrossRef]
- Kuroda, S.; Houkin, K.; Nunomura, M.; Abe, H. Frontal lobe infarction due to hemodynamic change after surgical revascularization in moyamoya disease. two case reports. Neurol. Med.-Chir. 2000, 40, 315–320. [Google Scholar] [CrossRef]
- Li, C.; Zhang, N.; Yu, S.; Xu, Y.; Yao, Y.; Zeng, M.; Li, D.; Xia, C. Individualized Perioperative Blood Pressure Management for Adult Moyamoya Disease: Experience from 186 Consecutive Procedures. J. Stroke Cerebrovasc. Dis. 2021, 30, 105413. [Google Scholar] [CrossRef] [PubMed]
- Funaki, T.; Takahashi, J.C.; Takagi, Y.; Kikuchi, T.; Yoshida, K.; Mitsuhara, T.; Kataoka, H.; Okada, T.; Fushimi, Y.; Miyamoto, S. Unstable moyamoya disease: Clinical features and impact on perioperative ischemic complications. J. Neurosurg. 2015, 122, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Choi, J.-U.; Yang, K.-H.; Kim, T.-G.; Kim, D.-S. Risk factors for postoperative ischemic complications in patients with moyamoya disease. J. Neurosurg. Pediatr. 2005, 103, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Deng, X.; Zhang, D.; Wang, S.; Zhang, Y.; Wang, R.; Zhao, J. Risk factors for and outcomes of postoperative complications in adult patients with moyamoya disease. J. Neurosurg. 2019, 130, 531–542. [Google Scholar] [CrossRef]
- Pettersson, S.D.; Olofsson, H.K.; Ali, S.; Szarek, D.; Miękisiak, G.; Ogilvy, C.S. Risk Factors for Ischemic Stroke After Revascularization Surgery in Patients with Moyamoya Disease: An Age-Stratified Comparative Meta-Analysis. World Neurosurg. 2023, 173, 146–157.e14. [Google Scholar] [CrossRef]
- van Sloten, T.T.; Sedaghat, S.; Carnethon, M.R.; Launer, L.J.; Stehouwer, C.D. Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020, 8, 325–336. [Google Scholar] [CrossRef]
- Yu, X.; Ge, P.; Zhai, Y.; Wang, R.; Zhang, Y.; Zhang, D. Hypo-high density lipoproteinemia is a predictor for recurrent stroke during the long-term follow-up after revascularization in adult moyamoya disease. Front. Neurol. 2022, 13, 891622. [Google Scholar] [CrossRef]
- Kim, D.Y.; Son, J.P.; Yeon, J.Y.; Kim, G.M.; Kim, J.S.; Hong, S.C.; Bang, O.Y. Infarct Pattern and Collateral Status in Adult Moyamoya Disease: A Multimodal Magnetic Resonance Imaging Study. Stroke 2017, 48, 111–116. [Google Scholar] [CrossRef]
- Pompsch, M.; Veltkamp, R.; Diehl, R.R.; Kraemer, M. Microembolic signals and antiplatelet therapy in Moyamoya angiopathy. J. Neurol. 2022, 269, 6605–6612. [Google Scholar] [CrossRef]
- Liu, T.; Qin, M.; Xiong, X.; Feng, L.; Lai, X.; Gao, Y. Benefits and risks of antiplatelet therapy for moyamoya disease: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1132339. [Google Scholar] [CrossRef]
- Luo, Y.; Cao, Z.; Ye, H.; Wu, S.; Sun, X. Antiplatelet therapy may improve the prognosis of patients with moyamoya disease: A 12-year retrospective study. J. Neurol. 2023, 270, 3876–3884. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.; Kim, J.; Choi, E.; Kim, Y.; Chung, J.; Saver, J.L.; Bang, O.Y.; Kim, G. Association of Antiplatelet Therapy, Including Cilostazol, With Improved Survival in Patients With Moyamoya Disease in a Nationwide Study. J. Am. Hear. Assoc. 2021, 10, e017701. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Ichiya, Y.; Sasaki, M.; Yoshida, T.; Masuda, K.; Matsushima, T.; Fukui, M. Response to hypercapnia in moyamoya disease. Cerebrovascular response to hypercapnia in pediatric and adult patients with moyamoya disease. Stroke 1997, 28, 701–707. [Google Scholar] [CrossRef] [PubMed]
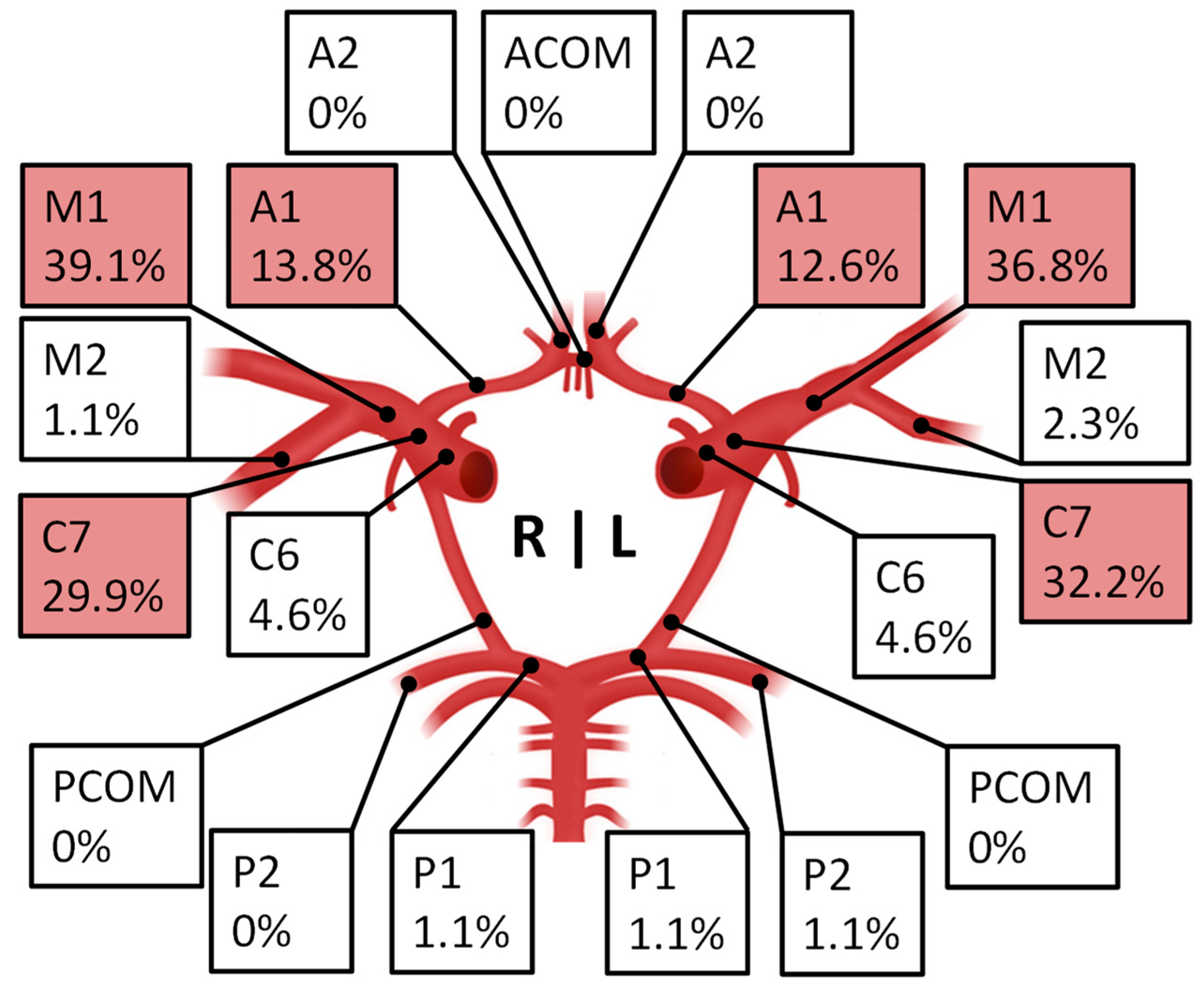

| Patient Characteristics | n (%) |
|---|---|
Sex
| 80 (72.7%) 30 (27.2%) |
| Age (at first surgery) | median 45.1 y (16.6–69.2) |
Ethnicity
| 99 (90.0%) 10 (9.1%) 1 (0.9%) |
Initial MMD onset
| 7 (6.4%) 77 (70.0%) 26 (23.6%) |
Moyamoya disease
| 21 (19.1%) 16 (14.5%) 73 (66.4%) |
Suzuki classification (Right/Left)
| 5/4 11/9 27/34 22/22 16/16 8/9 |
vessel-wall imaging
| 110 (100%) 74 (67.3%) |
| Time interval between VW-CE imaging and the first surgery | mean 86 d ± 82 d |
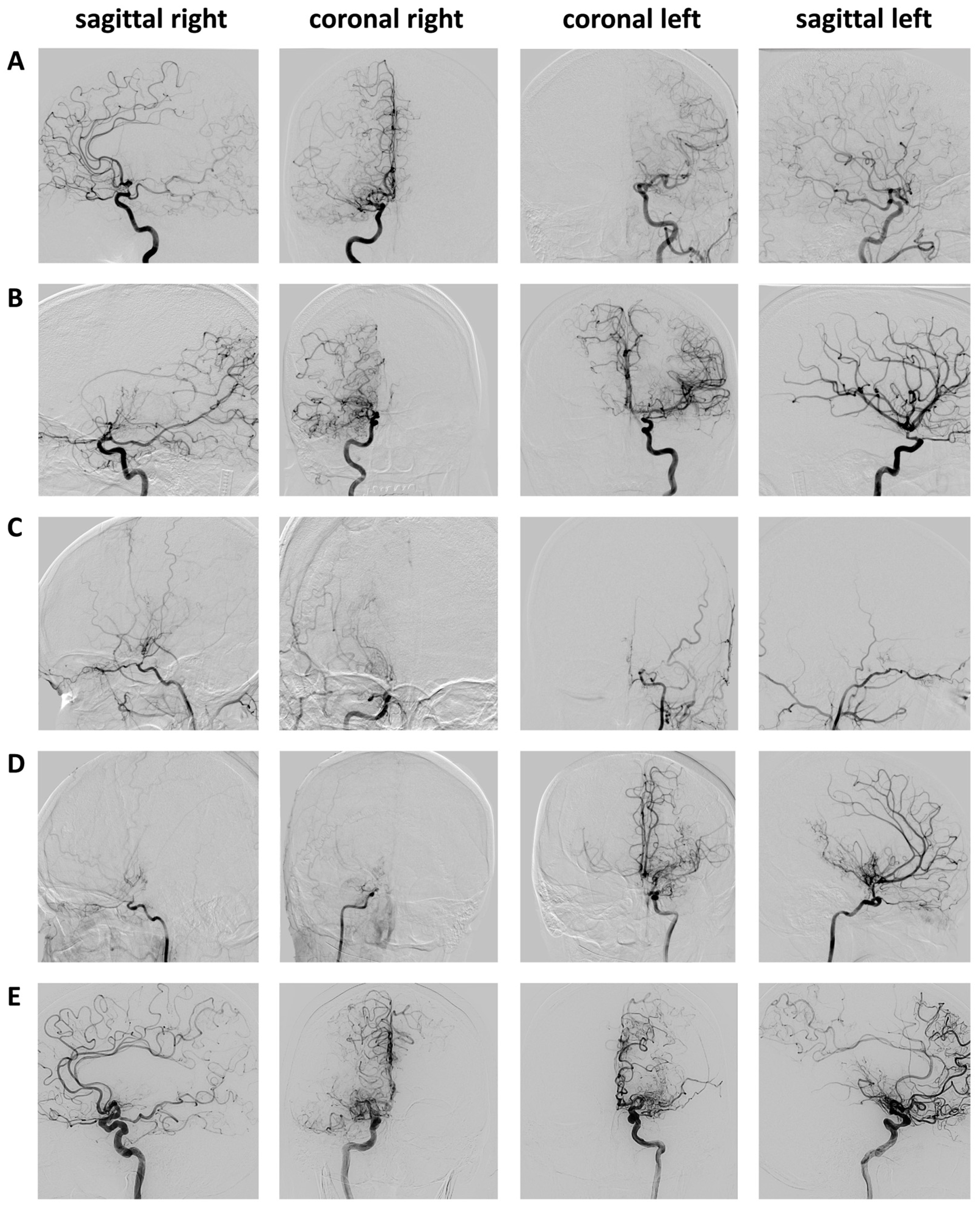
| Case | Age [Years] | Sex | Suzuki (Left/Right) | VW-CE | Revascularization | Ethnicity | BMI | Smoking | HTN | DM | Initial MMD Onset | NIHSS Preop/Postop/6mFU | mRS Preop/Postop/6mFU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 63 | female | 3/3 | C7 right A1 right | STA-MCA right combined | Caucasian | 26.6 | no | yes | no | ischemic | 0/5/3 | 2/3/3 |
| B | 46 | female | 2/4 | C7 left | STA-MCA right combined | Caucasian | 37.3 | no | yes | yes | ischemic | 0/3/3 | 1/1/1 |
| C | 22 | female | 5/3 | C7 right M1 right C7 left | STA-MCA bilaterally combined | Caucasian | 41.9 | no | yes | no | ischemic | 3/4/3 | 2/3/2 |
| D | 46 | female | 4/6 | C7 left M1 left A1 left | STA-MCA bilaterally direct | Caucasian | 29.1 | no | no | no | ischemic | 2/32/ # | 3/5/ # |
| E | 59 | male | 4/4 | P1 left P2 left | STA-MCA bilaterally direct | Caucasian | 29.1 | yes | yes | no | ischemic | 0/4/ * | 1/2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haas, P.; Hauser, T.-K.; Wiggenhauser, L.M.; Zerweck, L.; Tatagiba, M.; Khan, N.; Roder, C. Coincidence of Concentric Vessel-Wall Contrast Enhancement in Moyamoya Disease and Acute Postoperative Ischemic Stroke During Revascularization Procedures. Brain Sci. 2024, 14, 1190. https://doi.org/10.3390/brainsci14121190
Haas P, Hauser T-K, Wiggenhauser LM, Zerweck L, Tatagiba M, Khan N, Roder C. Coincidence of Concentric Vessel-Wall Contrast Enhancement in Moyamoya Disease and Acute Postoperative Ischemic Stroke During Revascularization Procedures. Brain Sciences. 2024; 14(12):1190. https://doi.org/10.3390/brainsci14121190
Chicago/Turabian StyleHaas, Patrick, Till-Karsten Hauser, Lucas Moritz Wiggenhauser, Leonie Zerweck, Marcos Tatagiba, Nadia Khan, and Constantin Roder. 2024. "Coincidence of Concentric Vessel-Wall Contrast Enhancement in Moyamoya Disease and Acute Postoperative Ischemic Stroke During Revascularization Procedures" Brain Sciences 14, no. 12: 1190. https://doi.org/10.3390/brainsci14121190
APA StyleHaas, P., Hauser, T.-K., Wiggenhauser, L. M., Zerweck, L., Tatagiba, M., Khan, N., & Roder, C. (2024). Coincidence of Concentric Vessel-Wall Contrast Enhancement in Moyamoya Disease and Acute Postoperative Ischemic Stroke During Revascularization Procedures. Brain Sciences, 14(12), 1190. https://doi.org/10.3390/brainsci14121190






