Noninvasive Ultra Low Intensity Light Photodynamic Treatment of Glioblastoma with Drug Augmentation: LoGlo PDT Regimen
Abstract
1. Introduction
2. 5-ALA
- 5-ALA allows intraoperative PDT;
- A fluorescent metabolic product of 5-ALA, protoporphyrin IX (PpIX), allows a more complete primary tumor resection, termed fluorescence guided resection.
| PpIX Fluorescence | 5-ALA | LFT Elevation | MAP Decrease | Nausea |
|---|---|---|---|---|
| T1/2 = 10 min * | T1/2 = 1–3 h | 4% | 11% | 15% |
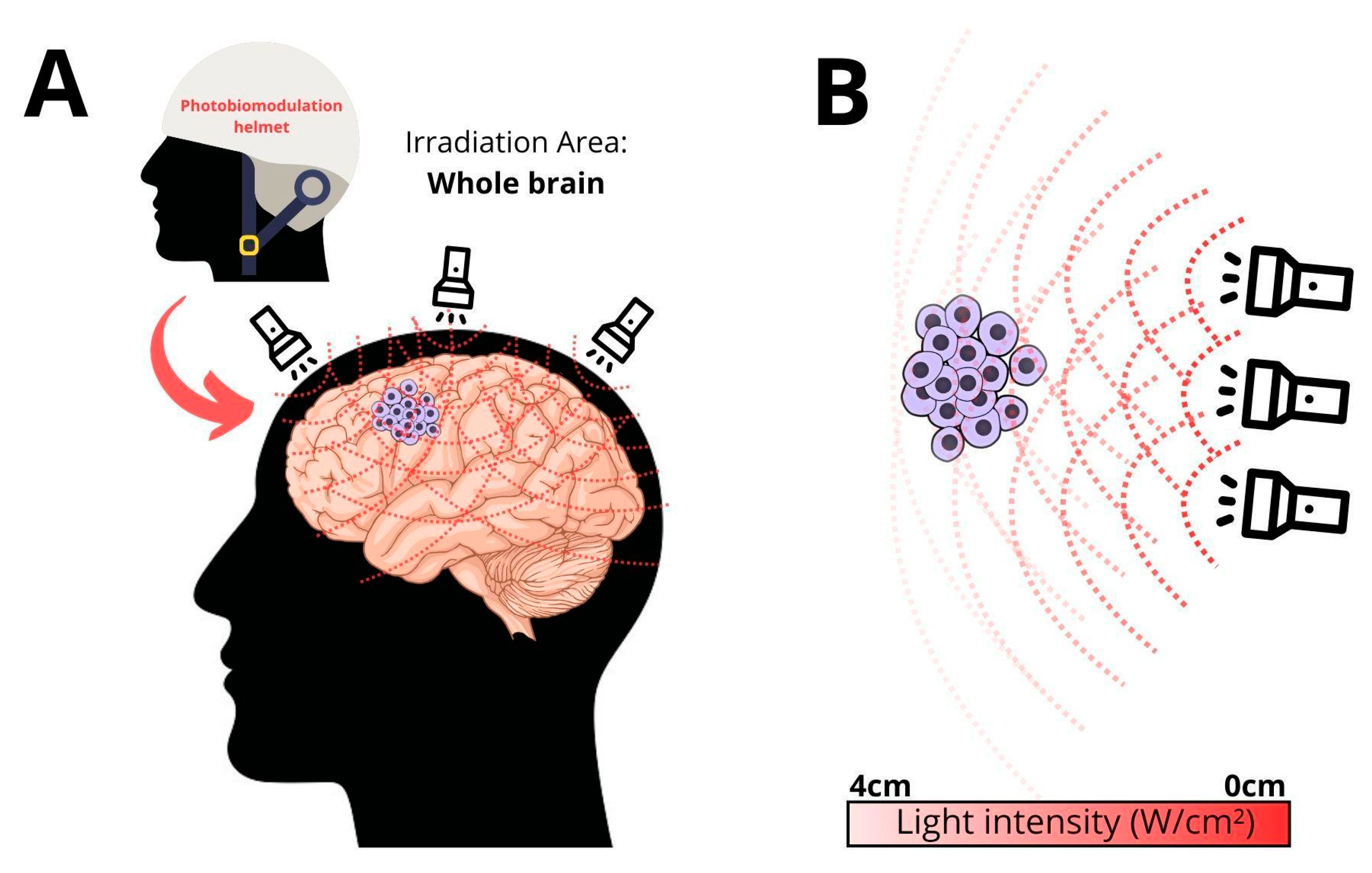
3. Ultra-Low Flux PDT
- We know that at the time of diagnosis, GB cells reside throughout the entire brain. GB is a whole brain disease. Intraoperative PDT is only effective within the first 1 or 2 cm within the resection cavity wall.
- PDT is restricted to a single intraoperative session.
| Locus of Applied External 4 W Light | Range of Light Reaching 4 cm Deep to Skull in 4 Cadavers |
| frontal | 32 to 278 μW/cm2 |
| parietal | 134 to 395 μW/cm2 |
| temporal | 7 to 234 μW/cm2 |
| occipital | 1 to 56 μW/cm2 |
- Repetitive treatments enhance effectiveness.
4. Safety of 635 nm Light
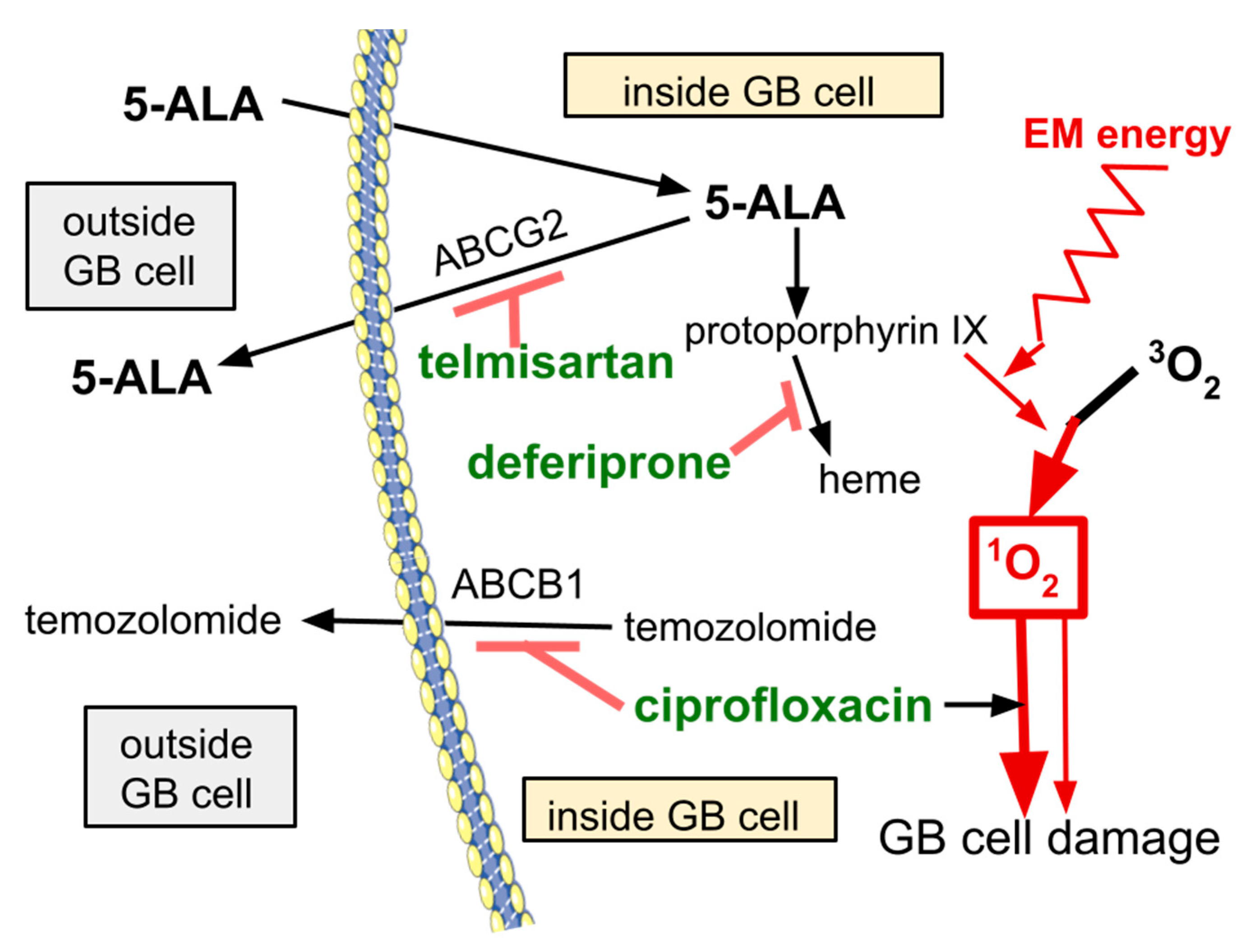
5. The LoGlo PDT Drugs
6. Ciprofloxacin
7. Deferiprone
8. Telmisartan
9. LoGlo PDT and GB Ecosystems
10. Limitations and Caveats
- The uniformity of fatal outcome of GB using all current treatment modalities;
- The established or predicted safety of the individual components of LoGlo PDT;
- The sound rationale predicting LoGlo effectiveness.
11. Discussion and Conclusions
- Low flux transcranial light can be repeatedly and noninvasively delivered sufficient for an effective 24 h PDT.
- Paucicellular colonies of GB anywhere in the brain have potential to be treated by LoGlo PDT.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Ma, R.; Watts, C. Selective 5-aminolevulinic acid-induced protoporphyrin IX fluorescence in Gliomas. Acta Neurochir. 2016, 158, 1935–1941. [Google Scholar] [CrossRef]
- Stepp, H.; Beck, T.; Pongratz, T.; Meinel, T.; Kreth, F.W.; Tonn, J.C.; Stummer, W. ALA and malignant glioma: Fluorescence-guided resection and photodynamic treatment. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.L.; de Souza, M.P. Theranostic Uses of the Heme Pathway in Neuro-Oncology: Protoporphyrin IX (PpIX) and Its Journey from Photodynamic Therapy (PDT) through Photodynamic Diagnosis (PDD) to Sonodynamic Therapy (SDT). Cancers 2024, 16, 740. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, L.E.; Vilchez, M.L.; Caverzán, M.D.; Milla Sanabria, L.N. Understanding the glioblastoma tumor biology to optimize photodynamic therapy: From molecular to cellular events. J. Neurosci. Res. 2021, 99, 1024–1047. [Google Scholar] [CrossRef]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2020, 6, 81. [Google Scholar] [CrossRef]
- Peciu-Florianu, I.; Vannod-Michel, Q.; Vauleon, E.; Bonneterre, M.E.; Reyns, N. Long term follow-up of patients with newly diagnosed glioblastoma treated by intraoperative photodynamic therapy: An update from the INDYGO trial (NCT03048240). J. Neurooncol. 2024, 168, 495–505. [Google Scholar] [CrossRef]
- Hsia, T.; Small, J.L.; Yekula, A.; Batool, S.M.; Escobedo, A.K.; Ekanayake, E.; You, D.G.; Lee, H.; Carter, B.S.; Balaj, L. Systematic Review of Photodynamic Therapy in Gliomas. Cancers 2023, 15, 3918. [Google Scholar] [CrossRef]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neurooncol. 2021, 152, 501–514. [Google Scholar] [CrossRef]
- Bettag, C.; Schatlo, B.; Abboud, T.; Behme, D.; Bock, C.; von der Brelie, C.; Rohde, V.; Mielke, D. Endoscope-enhanced fluorescence guided microsurgery increases survival in patients with glioblastoma. Acta Neurochir. 2023, 165, 4221–4226. [Google Scholar] [CrossRef]
- Picart, T.; Pallud, J.; Berthiller, J.; Dumot, C.; Berhouma, M.; Ducray, F.; Armoiry, X.; Margier, J.; Guerre, P.; Varlet, P.; et al. Members of RESECT study group:. Use of 5-ALA fluorescence-guided surgery versus white-light conventional microsurgery for the resection of newly diagnosed glioblastomas (RESECT study): A French multicenter randomized phase III study. J. Neurosurg. 2023, 140, 987–1000. [Google Scholar] [CrossRef]
- Wong, L.S.; St George, J.; Agyemang, K.; Grivas, A.; Houston, D.; Foo, S.Y.; Mullan, T. Outcomes of Fluorescence-Guided vs. White Light Resection of Glioblastoma in a Single Institution. Cureus 2023, 15, e42695. [Google Scholar] [CrossRef] [PubMed]
- Eatz, T.A.; Eichberg, D.G.; Lu, V.M.; Di, L.; Komotar, R.J.; Ivan, M.E. Intraoperative 5-ALA fluorescence-guided resection of high-grade glioma leads to greater extent of resection with better outcomes: A systematic review. J. Neurooncol. 2022, 156, 233–256. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.B., Jr.; Vasquez, M.W.M.; de Almeida Teixeira, B.C.; Neto, M.C.; Sprenger, F.; Filho, J.L.N.; Almeida-Lopes, L.; Ramina, R. Association of 5-aminolevulinic acid fluorescence guided resection with photodynamic therapy in recurrent glioblastoma: A matched cohort study. Acta Neurochir. 2024, 166, 212. [Google Scholar] [CrossRef] [PubMed]
- Schipmann, S.; Müther, M.; Stögbauer, L.; Zimmer, S.; Brokinkel, B.; Holling, M.; Grauer, O.; Suero Molina, E.; Warneke, N.; Stummer, W. Combination of ALA-induced fluorescence-guided resection and intraoperative open photodynamic therapy for recurrent glioblastoma: Case series on a promising dual strategy for local tumor control. J. Neurosurg. 2020, 134, 426–436. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Siddiqui, S.; Hussain, M.A.B.; Khan, S.; Liu, H.; Akhtar, K.; Hasan, S.A.; Ahmed, I.; Mallidi, S.; Khan, A.P.; et al. Clinical evaluation of a mobile, low-cost system for fluorescence guided photodynamic therapy of early oral cancer in India. Photodiagnosis Photodyn. Ther. 2022, 38, 102843. [Google Scholar] [CrossRef]
- Regula, J.; MacRobert, A.J.; Gorchein, A.; Buonaccorsi, G.A.; Thorpe, S.M.; Spencer, G.M.; Hatfield, A.R.; Bown, S.G. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX—A pilot study. Gut 1995, 36, 67–75. [Google Scholar] [CrossRef]
- Mackenzie, G.D.; Dunn, J.M.; Selvasekar, C.R.; Mosse, C.A.; Thorpe, S.M.; Novelli, M.R.; Bown, S.G.; Lovat, L.B. Optimal conditions for successful ablation of high-grade dysplasia in Barrett’s oesophagus using aminolaevulinic acid photodynamic therapy. Lasers Med. Sci. 2009, 24, 729–734. [Google Scholar] [CrossRef]
- Kelty, C.J.; Ackroyd, R.; Brown, N.J.; Brown, S.B.; Reed, M.W. Comparison of high- vs. low-dose 5-aminolevulinic acid for photodynamic therapy of Barrett’s esophagus. Surg. Endosc. 2004, 18, 452–458. [Google Scholar] [CrossRef]
- Webber, J.; Kessel, D.; Fromm, D. Side effects and photosensitization of human tissues after aminolevulinic acid. J. Surg. Res. 1997, 68, 31–37. [Google Scholar] [CrossRef]
- Miyake, M.; Nakai, Y.; Hori, S.; Morizawa, Y.; Hirao, Y.; Fujimoto, K. Transient liver toxicity as a result of the oral administration of 5-aminolevulinic acid for photodynamic diagnosis in patients with bladder cancer. Int. J. Urol. 2019, 26, 315–317. [Google Scholar] [CrossRef]
- Chung, I.W.; Eljamel, S. Risk factors for developing oral 5-aminolevulinic acid-induced side effects in patients undergoing fluorescence guided resection. Photodiagnosis Photodyn. Ther. 2013, 10, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.W.; Lin, L.T.; Chen, P.H.; Ho, M.H.; Huang, W.T.; Lee, Y.J.; Chiou, S.H.; Hsieh, Y.S.; Dong, C.Y.; Wang, H.W. Low-fluence rate, long duration photodynamic therapy in glioma mouse model using organic light emitting diode (OLED). Photodiagnosis Photodyn. Ther. 2015, 12, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, M.; Bellnier, D.A.; Vaughan, L.A.; Spernyak, J.A.; Mazurchuk, R.; Foster, T.H.; Henderson, B.W. Light delivery over extended time periods enhances the effectiveness of photodynamic therapy. Clin. Cancer Res. 2008, 14, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- James, N.S.; Cheruku, R.R.; Missert, J.R.; Sunar, U.; Pandey, R.K. Measurement of Cyanine Dye Photobleaching in Photosensitizer Cyanine Dye Conjugates Could Help in Optimizing Light Dosimetry for Improved Photodynamic Therapy of Cancer. Molecules 2018, 23, 1842. [Google Scholar] [CrossRef]
- Henderson, B.W.; Busch, T.M.; Snyder, J.W. Fluence rate as a modulator of PDT mechanisms. Lasers Surg. Med. 2006, 38, 489–493. [Google Scholar] [CrossRef]
- Sitnik, T.M.; Henderson, B.W. The effect of fluence rate on tumor and normal tissue responses to photodynamic therapy. Photochem. Photobiol. 1998, 67, 462–466. [Google Scholar] [CrossRef]
- Shin, D.; Nguyen, L.; TLe, M.; Ju, D.; NLe, J.; Berg, K.; Hirschberg, H. The effects of low irradiance long duration photochemical internalization on glioma spheroids. Photodiagnosis Photodyn. Ther. 2019, 26, 442–447. [Google Scholar] [CrossRef]
- van Geel, I.P.; Oppelaar, H.; Marijnissen, J.P.; Stewart, F.A. Influence of fractionation and fluence rate in photodynamic therapy with Photofrin or mTHPC. Radiat. Res. 1996, 145, 602–609. [Google Scholar] [CrossRef]
- Gibson, S.L.; VanDerMeid, K.R.; Murant, R.S.; Raubertas, R.F.; Hilf, R. Effects of various photoradiation regimens on the antitumor efficacy of photodynamic therapy for R3230AC mammary carcinomas. Cancer Res. 1990, 50, 7236–7241. [Google Scholar]
- Liu, Z.; Mela, A.; Argenziano, M.G.; Banu, M.A.; Furnari, J.; Kotidis, C.; Sperring, C.P.; Humala, N.; Mahajan, A.; Bruce, J.N.; et al. Single-cell analysis of 5-aminolevulinic acid intraoperative labeling specificity for glioblastoma. J. Neurosurg. 2023, 140, 968–978. [Google Scholar] [CrossRef]
- Specchia, F.M.C.; Monticelli, M.; Zeppa, P.; Bianconi, A.; Zenga, F.; Altieri, R.; Pugliese, B.; Di Perna, G.; Cofano, F.; Tartara, F.; et al. Let Me See: Correlation between 5-ALA Fluorescence and Molecular Pathways in Glioblastoma: A Single Center Experience. Brain Sci. 2021, 11, 795. [Google Scholar] [CrossRef]
- Bader, N.; Peschmann, C.; Kast, R.E.; Heiland, T.; Merz, T.; McCook, O.; Alfieri, A.; Karpel-Massler, G.; Capanni, F.; Halatsch, M.E. Globus Lucidus: A porcine study of an intracranial implant designed to deliver closed, repetitive photodynamic and photochemical therapy in glioblastoma. Photodiagnosis Photodyn. Ther. 2024, 46, 104059. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.A.; Guérin, L.; Lecomte, F.; Baert, G.; Vignion, A.S.; Mordon, S.; Reyns, N. Is interstitial photodynamic therapy for brain tumors ready for clinical practice? A systematic review. Photodiagnosis Photodyn. Ther. 2021, 36, 102492. [Google Scholar] [CrossRef] [PubMed]
- Bogaards, A.; Varma, A.; Zhang, K.; Zach, D.; Bisland, S.K.; Moriyama, E.H.; Lilge, L.; Muller, P.J.; Wilson, B.C. Fluorescence image-guided brain tumour resection with adjuvant metronomic photodynamic therapy: Pre-clinical model and technology development. Photochem. Photobiol. Sci. 2005, 4, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Komolibus, K.; Fisher, C.; Swartling, J.; Svanberg, S.; Svanberg, K.; Andersson-Engels, S. Perspectives on interstitial photodynamic therapy for malignant tumors. J. Biomed. Opt. 2021, 26, 070604. [Google Scholar] [CrossRef]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef]
- Morse, P.T.; Tuck, S.; Kerns, M.; Goebel, D.J.; Wan, J.; Waddell, T.; Wider, J.M.; Hüttemann, C.L.; Malek, M.H.; Lee, I.; et al. Non-invasive treatment of ischemia/reperfusion injury: Effective transmission of therapeutic near-infrared light into the human brain through soft skin-conforming silicone waveguides. Bioeng. Transl. Med. 2023, 8, e10496. [Google Scholar] [CrossRef]
- Mathews, M.S.; Angell-Petersen, E.; Sanchez, R.; Sun, C.H.; Vo, V.; Hirschberg, H.; Madsen, S.J. The effects of ultra low fluence rate single and repetitive photodynamic therapy on glioma spheroids. Lasers Surg. Med. 2009, 41, 578–584. [Google Scholar] [CrossRef]
- Tedford, C.E.; DeLapp, S.; Jacques, S.; Anders, J. Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg. Med. 2015, 47, 312–322. [Google Scholar] [CrossRef]
- Joshi, H.; Sinha, P.; Bowers, D.; John, J.P. Dose response of transcranial near infrared light stimulation on brain functional connectivity and cognition in older adults—A randomized comparison. J. Biophotonics 2024, 17, e202300215. [Google Scholar] [CrossRef]
- Fradkin, Y.; De Taboada, L.; Naeser, M.; Saltmarche, A.; Snyder, W.; Steingold, E. Transcranial photobiomodulation in children aged 2-6 years: A randomized sham-controlled clinical trial assessing safety, efficacy, and impact on autism spectrum disorder symptoms and brain electrophysiology. Front. Neurol. 2024, 15, 1221193. [Google Scholar] [CrossRef] [PubMed]
- Shahdadian, S.; Wang, X.; Liu, H. Directed physiological networks in the human prefrontal cortex at rest and post transcranial photobiomodulation. Sci. Rep. 2024, 14, 10242. [Google Scholar] [CrossRef] [PubMed]
- Blivet, G.; Roman, F.J.; Lelouvier, B.; Ribière, C.; Touchon, J. Photobiomodulation Therapy: A Novel Therapeutic Approach to Alzheimer’s Disease Made Possible by the Evidence of a Brain-Gut Interconnection. J. Integr. Neurosci. 2024, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Nizamutdinov, D.; Liu, H.; Huang, J.H. Recent Mechanisms of Neurodegeneration and Photobiomodulation in the Context of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 9272. [Google Scholar] [CrossRef]
- Nairuz, T.; Sangwoo-Cho Lee, J.H. Photobiomodulation Therapy on Brain: Pioneering an Innovative Approach to Revolutionize Cognitive Dynamics. Cells 2024, 13, 966. [Google Scholar] [CrossRef]
- Bicknell, B.; Liebert, A.; Herkes, G. Parkinson’s Disease and Photobiomodulation: Potential for Treatment. J. Pers. Med. 2024, 14, 112. [Google Scholar] [CrossRef]
- Lim, L. Traumatic Brain Injury Recovery with Photobiomodulation: Cellular Mechanisms, Clinical Evidence, and Future Potential. Cells 2024, 13, 385. [Google Scholar] [CrossRef]
- Liebert, A.; Bicknell, B.; Laakso, E.L.; Heller, G.; Jalilitabaei, P.; Tilley, S.; Mitrofanis, J.; Kiat, H. Improvements in clinical signs of Parkinson’s disease using photobiomodulation: A prospective proof-of-concept study. BMC Neurol. 2021, 21, 256. [Google Scholar] [CrossRef]
- Fernandes, F.; Oliveira, S.; Monteiro, F.; Gasik, M.; Silva, F.S.; Sousa, N.; Carvalho, Ó.; Catarino, S.O. Devices used for photobiomodulation of the brain-a comprehensive and systematic review. J. Neuroeng. Rehabil. 2024, 21, 53. [Google Scholar] [CrossRef]
- Kashtan, H.; Konikoff, F.; Haddad, R.; Skornick, Y. Photodynamic therapy of cancer of the esophagus using systemic aminolevulinic acid and a non laser light source: A phase I/II study. Gastrointest. Endosc. 1999, 49, 760–764. [Google Scholar] [CrossRef]
- Oh, H.; Park, J.B.; Yoon, H.K.; Lee, H.C.; Park, C.K.; Park, H.P. Effects of preoperative 5-aminolevulinic acid administration on postoperative liver enzymes after brain tumor surgery in patients with elevated preoperative liver enzymes. J. Clin. Neurosci. 2020, 72, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, H.K.; Lee, H.C.; Park, H.P.; Park, C.K.; Dho, Y.S.; Hwang, J.W. Preoperative 5-aminolevulinic acid administration for brain tumor surgery is associated with an increase in postoperative liver enzymes: A retrospective cohort study. Acta Neurochir. 2019, 161, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Offersen, C.M.; Skjoeth-Rasmussen, J. Evaluation of the risk of liver damage from the use of 5-aminolevulinic acid for intraoperative identification and resection in patients with malignant gliomas. Acta Neurochir. 2017, 159, 145–150. [Google Scholar] [CrossRef]
- Kast, R.E.; Skuli, N.; Sardi, I.; Capanni, F.; Hessling, M.; Frosina, G.; Kast, A.P.; Karpel-Massler, G.; Halatsch, M.E. Augmentation of 5-Aminolevulinic Acid Treatment of Glioblastoma by Adding Ciprofloxacin, Deferiprone, 5-Fluorouracil and Febuxostat: The CAALA Regimen. Brain Sci. 2018, 8, 203. [Google Scholar] [CrossRef]
- Schuck, E.L.; Dalhoff, A.; Stass, H.; Derendorf, H. Pharmacokinetic pharmacodynamic (PK/PD) evaluation of a once-daily treatment using ciprofloxacin in an extended release dosage form. Infection 2005, 33 (Suppl. S2), 22–28. [Google Scholar] [CrossRef]
- Hider, R.C.; Hoffbrand, A.V. The Role of Deferiprone in Iron Chelation. N. Engl. J. Med. 2018, 379, 2140–2150. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y.; Chen, X.; Li, R.; Yin, J.; Zhong, D. Pharmacokinetics of telmisartan in healthy Chinese subjects after oral administration of two dosage levels. Arzneimittelforschung 2006, 56, 569–573. [Google Scholar] [CrossRef]
- Alomari, S.; Zhang, I.; Hernandez, A.; Kraft, C.Y.; Raj, D.; Kedda, J.; Tyler, B. Drug Repurposing for Glioblastoma and Current Advances in Drug Delivery—A Comprehensive Review of the Literature. Biomolecules 2021, 11, 1870. [Google Scholar] [CrossRef]
- Ferrario, N.; Marras, E.; Vivona, V.; Randisi, F.; Fallica, A.N.; Marrazzo, A.; Perletti, G.; Gariboldi, M.B. Mechanisms of the Antineoplastic Effects of New Fluoroquinolones in 2D and 3D Human Breast and Bladder Cancer Cell Lines. Cancers 2024, 16, 2227. [Google Scholar] [CrossRef]
- Kloskowski, T.; Fekner, Z.; Szeliski, K.; Paradowska, M.; Balcerczyk, D.; Rasmus, M.; Dąbrowski, P.; Kaźmierski, Ł.; Drewa, T.; Pokrywczyńska, M. Effect of four fluoroquinolones on the viability of bladder cancer cells in 2D and 3D cultures. Front. Oncol. 2023, 13, 1222411. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yang, J.L.; Chen, J.J.; Tai, S.B.; Yeh, Y.H.; Liu, P.F.; Lin, M.W.; Chung, C.L.; Chen, C.L. Fluoroquinolones Suppress TGF-β and PMA-Induced MMP-9 Production in Cancer Cells: Implications in Repurposing Quinolone Antibiotics for Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 11602. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, M.A.A.; Abdel-Aziz, S.A.; Shaykoon, M.S.A.; Abuo-Rahma, G.E.A. Towards anticancer fluoroquinolones: A review article. Arch Pharm. 2019, 352, e1800376. [Google Scholar] [CrossRef] [PubMed]
- Zandi, A.; Zanjani, T.M.; Ziai, S.A.; Poul, Y.K.; Hoseini, M.H. Evaluation of the Cytotoxic Effects of Ciprofloxacin on Human Glioblastoma A-172 Cell Line. Middle East J. Cancer 2017, 8, 119–126. [Google Scholar]
- Gull, H.H.; Karadag, C.; Senger, B.; Sorg, R.V.; Möller, P.; Mellert, K.; Steiger, H.J.; Hänggi, D.; Cornelius, J.F. Ciprofloxacin enhances phototoxicity of 5-aminolevulinic acid mediated photodynamic treatment for chordoma cell lines. Photodiagnosis Photodyn. Ther. 2021, 35, 102346. [Google Scholar] [CrossRef]
- Cornelius, J.F.; Slotty, P.J.; El Khatib, M.; Giannakis, A.; Senger, B.; Steiger, H.J. Enhancing the effect of 5-aminolevulinic acid based photodynamic therapy in human meningioma cells. Photodiagnosis Photodyn. Ther. 2014, 11, 1–6. [Google Scholar] [CrossRef]
- Radtke, L.; Majchrzak-Celińska, A.; Awortwe, C.; Vater, I.; Nagel, I.; Sebens, S.; Cascorbi, I.; Kaehler, M. CRISPR/Cas9-induced knockout reveals the role of ABCB1 in the response to temozolomide, carmustine and lomustine in glioblastoma multiforme. Pharmacol. Res. 2022, 185, 106510. [Google Scholar] [CrossRef]
- de Gooijer, M.C.; de Vries, N.A.; Buckle, T.; Buil, L.C.M.; Beijnen, J.H.; Boogerd, W.; van Tellingen, O. Improved Brain Penetration and Antitumor Efficacy of Temozolomide by Inhibition of ABCB1 and ABCG2. Neoplasia 2018, 20, 710–720. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, Y.; Li, T.; Tan, D.; Fu, B.; Yang, M.; Chen, Y.; Cao, M.; Xuan, C.; Du, Q.; et al. Intercellular adhesion molecule-1 suppresses TMZ chemosensitivity in acquired TMZ-resistant gliomas by increasing assembly of ABCB1 on the membrane. Drug Resist. Updat. 2024, 76, 101112. [Google Scholar] [CrossRef]
- Gupta, P.; Gao, H.L.; Ashar, Y.V.; Karadkhelkar, N.M.; Yoganathan, S.; Chen, Z.S. Ciprofloxacin Enhances the Chemosensitivity of Cancer Cells to ABCB1 Substrates. Int. J. Mol. Sci. 2019, 20, 268. [Google Scholar] [CrossRef]
- Ueta, K.; Yamamoto, J.; Tanaka, T.; Nakano, Y.; Kitagawa, T.; Nishizawa, S. 5-Aminolevulinic acid enhances mitochondrial stress upon ionizing irradiation exposure and increases delayed production of reactive oxygen species and cell death in glioma cells. Int. J. Mol. Med. 2017, 39, 387–398. [Google Scholar] [CrossRef]
- Gera, K.; Cline, C.; Al-Mansour, Z.; Medvec, A.; Lee, J.H.; Galochkina, Z.; Hsu, J.; Hiemenz, J.; Farhadfar, N.; Dean, E.A.; et al. A phase ib clinical trial of oral ciprofloxacin and etoposide in subjects with resistant acute myeloid leukemia. Leuk. Lymphoma 2024, 65, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhou, C.; Zhu, M.; Shi, S.; Zhang, L.; Zhao, Y.; Li, C.; Wang, Y.; Wang, Y. Iron chelation promotes 5-aminolaevulinic acid-based photodynamic therapy against oral tongue squamous cell carcinoma. Photodiagnosis Photodyn. Ther. 2020, 31, 101907. [Google Scholar] [CrossRef] [PubMed]
- Howley, R.; Mansi, M.; Shinde, J.; Restrepo, J.; Chen, B. Analysis of Renal Cell Carcinoma Cell Response to the Enhancement of 5-aminolevulinic Acid-mediated Protoporphyrin IX Fluorescence by Iron Chelator Deferoxamine. Photochem. Photobiol. 2023, 99, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Čunderlíková, B.; Kalafutová, A.; Babál, P.; Mlkvý, P.; Teplický, T. Suppression of resistance to aminolevulinic acid-based photodynamic therapy in esophageal cell lines by administration of iron chelators in collagen type I matrices. Int. J. Radiat. Biol. 2023, 99, 474–487. [Google Scholar] [CrossRef]
- Nomoto, T.; Komoto, K.; Nagano, T.; Ishii, T.; Guo, H.; Honda, Y.; Ogura, S.I.; Ishizuka, M.; Nishiyama, N. Polymeric iron chelators for enhancing 5-aminolevulinic acid-induced photodynamic therapy. Cancer Sci. 2023, 114, 1086–1094. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, H.; Yang, L.; Guo, L.; Feng, M. Desferrioxamine Enhances 5-Aminolaevulinic Acid Induced Protoporphyrin IX Accumulation and Therapeutic Efficacy for Hypertrophic Scar. J. Pharm. Sci. 2023, 112, 1635–1643. [Google Scholar] [CrossRef]
- Piffaretti, D.; Burgio, F.; Thelen, M.; Kaelin-Lang, A.; Paganetti, P.; Reinert, M.; D’Angelo, M.L. Protoporphyrin IX tracer fluorescence modulation for improved brain tumor cell lines visualization. J. Photochem. Photobiol. B 2019, 201, 111640. [Google Scholar] [CrossRef]
- Reburn, C.; Gawthorpe, G.; Perry, A.; Wood, M.; Curnow, A. Novel Iron-Chelating Prodrug Significantly Enhanced Fluorescence Mediated Detection of Glioma Cells Experimentally In Vitro. Pharmaceutics 2023, 15, 2668. [Google Scholar] [CrossRef]
- de Souza, A.L.; Marra, K.; Gunn, J.; Samkoe, K.S.; Kanick, S.C.; Davis, S.C.; Chapman, M.S.; Maytin, E.V.; Hasan, T.; Pogue, B.W. Comparing desferrioxamine and light fractionation enhancement of ALA-PpIX photodynamic therapy in skin cancer. Br. J. Cancer 2016, 115, 805–813. [Google Scholar] [CrossRef]
- Negida, A.; Hassan, N.M.; Aboeldahab, H.; Zain, Y.E.; Negida, Y.; Cadri, S.; Cadri, N.; Cloud, L.J.; Barrett, M.J.; Berman, B. Efficacy of the iron-chelating agent, deferiprone, in patients with Parkinson’s disease: A systematic review and meta-analysis. CNS Neurosci. Ther. 2024, 30, e14607. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The Vital Role Played by Deferiprone in the Transition of Thalassaemia from a Fatal to a Chronic Disease and Challenges in Its Repurposing for Use in Non-Iron-Loaded Diseases. Pharmaceuticals 2023, 16, 1016. [Google Scholar] [CrossRef] [PubMed]
- Deppe, S.; Ripperger, A.; Weiss, J.; Ergün, S.; Benndorf, R.A. Impact of genetic variability in the ABCG2 gene on ABCG2 expression, function, and interaction with AT1 receptor antagonist telmisartan. Biochem. Biophys. Res. Commun. 2014, 443, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Lee, H.K.; To, K.K.; Fok, B.S.; Wo, S.K.; Ho, C.S.; Wong, C.K.; Zuo, Z.; Chan, T.Y.; Chan, J.C.; et al. Telmisartan increases systemic exposure to rosuvastatin after single and multiple doses, and in vitro studies show telmisartan inhibits ABCG2-mediated transport of rosuvastatin. Eur. J. Clin. Pharmacol. 2016, 72, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Ripperger, A.; Krischer, A.; Robaa, D.; Sippl, W.; Benndorf, R.A. Pharmacogenetic Aspects of the Interaction of AT1 Receptor Antagonists with ATP-Binding Cassette Transporter ABCG2. Front. Pharmacol. 2018, 9, 463. [Google Scholar] [CrossRef]
- Weiss, J.; Sauer, A.; Divac, N.; Herzog, M.; Schwedhelm, E.; Böger, R.H.; Haefeli, W.E.; Benndorf, R.A. Interaction of angiotensin receptor type 1 blockers with ATP-binding cassette transporters. Biopharm. Drug Dispos. 2010, 31, 150–161. [Google Scholar] [CrossRef]
- Hagiya, Y.; Endo, Y.; Yonemura, Y.; Takahashi, K.; Ishizuka, M.; Abe, F.; Tanaka, T.; Okura, I.; Nakajima, M.; Ishikawa, T.; et al. Pivotal roles of peptide transporter PEPT1 and ATP-binding cassette (ABC) transporter ABCG2 in 5-aminolevulinic acid (ALA)-based photocytotoxicity of gastric cancer cells in vitro. Photodiagnosis Photodyn. Ther. 2012, 9, 204–214. [Google Scholar] [CrossRef]
- Kobuchi, H.; Moriya, K.; Ogino, T.; Fujita, H.; Inoue, K.; Shuin, T.; Yasuda, T.; Utsumi, K.; Utsumi, T. Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS ONE 2012, 7, e50082. [Google Scholar] [CrossRef]
- Kawai, N.; Hirohashi, Y.; Ebihara, Y.; Saito, T.; Murai, A.; Saito, T.; Shirosaki, T.; Kubo, T.; Nakatsugawa, M.; Kanaseki, T.; et al. ABCG2 expression is related to low 5-ALA photodynamic diagnosis (PDD) efficacy and cancer stem cell phenotype, and suppression of ABCG2 improves the efficacy of PDD. PLoS ONE 2019, 14, e0216503. [Google Scholar] [CrossRef]
- Chandratre, S.; Olsen, J.; Howley, R.; Chen, B. Targeting ABCG2 transporter to enhance 5-aminolevulinic acid for tumor visualization and photodynamic therapy. Biochem. Pharmacol. 2023, 217, 115851. [Google Scholar] [CrossRef]
- Müller, P.; Abdel Gaber, S.A.; Zimmermann, W.; Wittig, R.; Stepp, H. ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells. J. Photochem. Photobiol. B 2020, 210, 111963. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kajimoto, Y.; Inoue, Y.; Ikegami, Y.; Kuroiwa, T. Critical role of ABCG2 in ALA-photodynamic diagnosis and therapy of human brain tumor. Adv. Cancer Res. 2015, 125, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, A.I.; Sucunza, D.; Pedrosa, M.A.; Garrido-Gil, P.; Kulisevsky, J.; Lanciego, J.L.; Labandeira-Garcia, J.L. Angiotensin Type 1 Receptor Antagonists Protect Against Alpha Synuclein Induced Neuroinflammation and Dopaminergic Neuron Death. Neurotherapeutics 2018, 15, 1063–1081. [Google Scholar] [CrossRef] [PubMed]
- Torika, N.; Asraf, K.; Danon, A.; Apte, R.N.; Fleisher-Berkovich, S. Telmisartan Modulates Glial Activation: In Vitro and In Vivo Studies. PLoS ONE 2016, 11, e0155823. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Xu, C.S.; Li, X.C.; Yang, C.; Lan, T.; Wang, M.Y.; Yu, D.H.; Tang, F.; Wang, Z.F.; Li, Z.Q. Telmisartan inhibits microglia-induced neurotoxic A1 astrocyte conversion via PPARγ mediated NF-κB/p65 degradation. Int. Immunopharmacol. 2023, 123, 110761. [Google Scholar] [CrossRef]
- Wang, Z.F.; Li, J.; Ma, C.; Huang, C.; Li, Z.Q. Telmisartan ameliorates Aβ oligomer-induced inflammation via PPARγ/PTEN pathway in BV2 microglial cells. Biochem. Pharmacol. 2020, 171, 113674. [Google Scholar] [CrossRef]
- Paul, S.K.; Guendouzi, A.; Banerjee, A.; Guendouzi, A.; Haldar, R. Identification of approved drugs with ALDH1A1 inhibitory potential aimed at enhancing chemotherapy sensitivity in cancer cells: An in-silico drug repurposing approach. J. Biomol. Struct. Dyn. 2024, 1–15. [Google Scholar] [CrossRef]
- Kumar, U.; Aich, J.; Devarajan, S. Exploring the repurposing potential of telmisartan drug in breast cancer: An in-silico and in-vitro approach. Anticancer. Drugs 2023, 34, 1094–1103. [Google Scholar] [CrossRef]
- Yamana, Y.; Fujihara, S.; Kobara, H.; Oura, K.; Samukawa, E.; Chiyo, T.; Okamura, M.; Yamana, H.; Tadokoro, T.; Fujita, K.; et al. MicroRNA profiles following telmisartan treatment in pancreatic ductal adenocarcinoma cells. J. Cancer Res. Ther. 2022, 18, S305–S312. [Google Scholar] [CrossRef]
- Khorsand, M.; Khajeh, S.; Eslami, M.; Nezafat, N.; Ghasemi, Y.; Razban, V.; Mostafavi-Pour, Z. Telmisartan anti-cancer activities mechanism through targeting N-cadherin by mimicking ADH-1 function. J. Cell Mol. Med. 2022, 26, 2392–2403. [Google Scholar] [CrossRef]
- Tsujiya, Y.; Hasegawa, A.; Yamamori, M.; Okamura, N. Telmisartan Induced Cytotoxicity via G2/M Phase Arrest in Renal Cell Carcinoma Cell Lines. Biol. Pharm. Bull. 2021, 44, 1878–1885. [Google Scholar] [CrossRef]
- Tsujiya, Y.; Yamamori, M.; Hasegawa, A.I.; Yamamoto, Y.; Yashiro, M.; Okamura, N. Telmisartan Exerts Cytotoxicity in Scirrhous Gastric Cancer Cells by Inducing G0/G1 Cell Cycle Arrest. Anticancer Res. 2021, 41, 5461–5468. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Chou, C.H.; Li, Y.F.; Huang, L.C.; Kao, Y.; Hueng, D.Y.; Tsai, C.K. Antiproliferative and apoptotic effects of telmisartan in human glioma cells. Cancer Cell Int. 2023, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, T.; Li, C.; Guo, J.; Xu, B.; Xue, L. Telmisartan attenuates human glioblastoma cells proliferation and oncogenicity by inducing the lipid oxidation. Asia Pac. J. Clin. Oncol. 2022, 18, 217–223. [Google Scholar] [CrossRef]
- Rout, S.; Satapathy, B.S.; Sahoo, R.N.; Pattnaik, S. Telmisartan loaded lipid nanocarrier as a potential repurposing approach to treat glioma: Characterization, apoptosis evaluation in U87MG cells, pharmacokinetic and molecular simulation study. Nanotechnology 2024, 35, 425101. [Google Scholar] [CrossRef]
- Quan, W.; Xu, C.S.; Ma, C.; Chen, X.; Yu, D.H.; Li, Z.Y.; Wang, D.W.; Tang, F.; Wan, G.P.; Wan, J.; et al. Anti-tumor effects of telmisartan in glioma-astrocyte non-contact co-cultures: A critical role of astrocytic IL-6-mediated paracrine growth promotion. Int. Immunopharmacol. 2024, 139, 112707. [Google Scholar] [CrossRef]
- Kast, R.E.; Alfieri, A.; Assi, H.I.; Burns, T.C.; Elyamany, A.M.; Gonzalez-Cao, M.; Karpel-Massler, G.; Marosi, C.; Salacz, M.E.; Sardi, I.; et al. MDACT: A New Principle of Adjunctive Cancer Treatment Using Combinations of Multiple Repurposed Drugs, with an Example Regimen. Cancers 2022, 14, 2563. [Google Scholar] [CrossRef]
- Salvalaggio, A.; Pini, L.; Bertoldo, A.; Corbetta, M. Glioblastoma and brain connectivity: The need for a paradigm shift. Lancet Neurol. 2024, 23, 740–748. [Google Scholar] [CrossRef]
- White, J.; White, M.P.J.; Wickremesekera, A.; Peng, L.; Gray, C. The tumour microenvironment, treatment resistance and recurrence in glioblastoma. J. Transl. Med. 2024, 22, 540. [Google Scholar] [CrossRef]
- Duenas-Gonzalez, A.; Gonzalez-Fierro, A.; Bornstein-Quevedo, L.; Gutierrez-Delgado, F.; Kast, R.E.; Chavez-Blanco, A.; Dominguez-Gomez, G.; Candelaria, M.; Romo-Pérez, A.; Correa-Basurto, J.; et al. Multitargeted polypharmacotherapy for cancer treatment, theoretical concepts and proposals. Expert. Rev. Anticancer Ther. 2024, 24, 665–677. [Google Scholar] [CrossRef]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes. Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef]
- Rumie Vittar, N.B.; Lamberti, M.J.; Pansa, M.F.; Vera, R.E.; Rodriguez, M.E.; Cogno, I.S.; Milla Sanabria, L.N.; Rivarola, V.A. Ecological photodynamic therapy: New trend to disrupt the intricate networks within tumor ecosystem. Biochim. Biophys. Acta 2013, 1835, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Prager, B.C.; Bhargava, S.; Mahadev, V.; Hubert, C.G.; Rich, J.N. Glioblastoma Stem Cells: Driving Resilience through Chaos. Trends Cancer 2020, 6, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, M.L.; Rodríguez, L.B.; Palacios, R.E.; Prucca, C.G.; Caverzán, M.D.; Caputto, B.L.; Rivarola, V.A.; Milla Sanabria, L.N. Isolation and initial characterization of human glioblastoma cells resistant to photodynamic therapy. Photodiagnosis Photodyn. Ther. 2021, 33, 102097. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Aguilar, L.; Vilchez, M.L.; Milla Sanabria, L.N. Targeting glioblastoma stem cells: The first step of photodynamic therapy. Photodiagnosis Photodyn. Ther. 2021, 36, 102585. [Google Scholar] [CrossRef] [PubMed]
- Schimanski, A.; Ebbert, L.; Sabel, M.C.; Finocchiaro, G.; Lamszus, K.; Ewelt, C.; Etminan, N.; Fischer, J.C.; Sorg, R.V. Human glioblastoma stem-like cells accumulate protoporphyrin IX when subjected to exogenous 5-aminolaevulinic acid, rendering them sensitive to photodynamic treatment. J. Photochem. Photobiol. B 2016, 163, 203–210. [Google Scholar] [CrossRef]
- Omura, N.; Nonoguchi, N.; Fujishiro, T.; Park, Y.; Ikeda, N.; Kajimoto, Y.; Hosomi, R.; Yagi, R.; Hiramatsu, R.; Furuse, M.; et al. Ablation efficacy of 5-aminolevulinic acid-mediated photodynamic therapy on human glioma stem cells. Photodiagnosis Photodyn. Ther. 2023, 41, 103119. [Google Scholar] [CrossRef]
- Fujishiro, T.; Nonoguchi, N.; Pavliukov, M.; Ohmura, N.; Kawabata, S.; Park, Y.; Kajimoto, Y.; Ishikawa, T.; Nakano, I.; Kuroiwa, T. 5-Aminolevulinic acid-mediated photodynamic therapy can target human glioma stem-like cells refractory to antineoplastic agents. Photodiagnosis Photodyn. Ther. 2018, 24, 58–68. [Google Scholar] [CrossRef]
- Kiesel, B.; Mischkulnig, M.; Woehrer, A.; Martinez-Moreno, M.; Millesi, M.; Mallouhi, A.; Czech, T.; Preusser, M.; Hainfellner, J.A.; Wolfsberger, S.; et al. Systematic histopathological analysis of different 5-aminolevulinic acid-induced fluorescence levels in newly diagnosed glioblastomas. J. Neurosurg. 2018, 129, 341–353. [Google Scholar] [CrossRef]
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F.W. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Lasers Surg. Med. 2013, 45, 225–234. [Google Scholar] [CrossRef]
- Leroy, H.A.; Vermandel, M.; Vignion-Dewalle, A.S.; Leroux, B.; Maurage, C.A.; Duhamel, A.; Mordon, S.; Reyns, N. Interstitial photodynamic therapy and glioblastoma: Light fractionation in a preclinical model. Lasers Surg. Med. 2017, 49, 506–515. [Google Scholar] [CrossRef]
- Busch, T.M.; Wileyto, E.P.; Emanuele, M.J.; Del Piero, F.; Marconato, L.; Glatstein, E.; Koch, C.J. Photodynamic therapy creates fluence rate-dependent gradients in the intratumoral spatial distribution of oxygen. Cancer Res. 2002, 62, 7273–7279. [Google Scholar]
| Term | Definition | Units |
|---|---|---|
| fluence | an amount, energy delivered per unit area | J/cm2 |
| flux | a rate, energy delivered per unit area per second | W/cm2 |
| Drug | Common Use in General Medicine | Repurposed Use in LoGlo PDT |
|---|---|---|
| ciprofloxacin | as antibiotic | increased PpIX levels |
| deferiprone | for iron chelation | for lowering intracellular iron |
| telmisartan | to treat hypertension | to inhibit ABCG2, to agonize PPAR gamma, to inhibit and angiotensin receptors |
| Drug | Oral Dose | Cmax | Tmax | T1/2 | Reference |
|---|---|---|---|---|---|
| ciprofloxacin | 1200 mg/d | 3 µg/mL | 2–3 h | 4 h | [55] |
| deferiprone | 50 mg/kg/d | 42 µg/mL | 30 min | 2 h | [56] |
| telmisartan | 20–80 mg/d | 160 ng/mL | 2 h | 24 h | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kast, R.E.; Kast, A.P.; Arnhold, J.; Capanni, F.; Sanabria, L.N.M.; Bader, N.; Vieira, B.M.; Alfieri, A.; Karpel-Massler, G.; da Silva, E.B., Jr. Noninvasive Ultra Low Intensity Light Photodynamic Treatment of Glioblastoma with Drug Augmentation: LoGlo PDT Regimen. Brain Sci. 2024, 14, 1164. https://doi.org/10.3390/brainsci14121164
Kast RE, Kast AP, Arnhold J, Capanni F, Sanabria LNM, Bader N, Vieira BM, Alfieri A, Karpel-Massler G, da Silva EB Jr. Noninvasive Ultra Low Intensity Light Photodynamic Treatment of Glioblastoma with Drug Augmentation: LoGlo PDT Regimen. Brain Sciences. 2024; 14(12):1164. https://doi.org/10.3390/brainsci14121164
Chicago/Turabian StyleKast, Richard E., Anton P. Kast, Jürgen Arnhold, Felix Capanni, Laura N. Milla Sanabria, Nicolas Bader, Bruno Marques Vieira, Alex Alfieri, Georg Karpel-Massler, and Erasmo Barros da Silva, Jr. 2024. "Noninvasive Ultra Low Intensity Light Photodynamic Treatment of Glioblastoma with Drug Augmentation: LoGlo PDT Regimen" Brain Sciences 14, no. 12: 1164. https://doi.org/10.3390/brainsci14121164
APA StyleKast, R. E., Kast, A. P., Arnhold, J., Capanni, F., Sanabria, L. N. M., Bader, N., Vieira, B. M., Alfieri, A., Karpel-Massler, G., & da Silva, E. B., Jr. (2024). Noninvasive Ultra Low Intensity Light Photodynamic Treatment of Glioblastoma with Drug Augmentation: LoGlo PDT Regimen. Brain Sciences, 14(12), 1164. https://doi.org/10.3390/brainsci14121164








