Consciousness and Energy Processing in Neural Systems
Abstract
1. Introduction
“All that we know about matter relates to the series of phenomena in which energy is transferred from one portion of matter to another, till in some part of the series our bodies are affected, and we become conscious of a sensation”.
2. Energy Processing in Neural Systems
2.1. Energy Processing in Nervous Tissue
2.2. Energy Processing in Brains
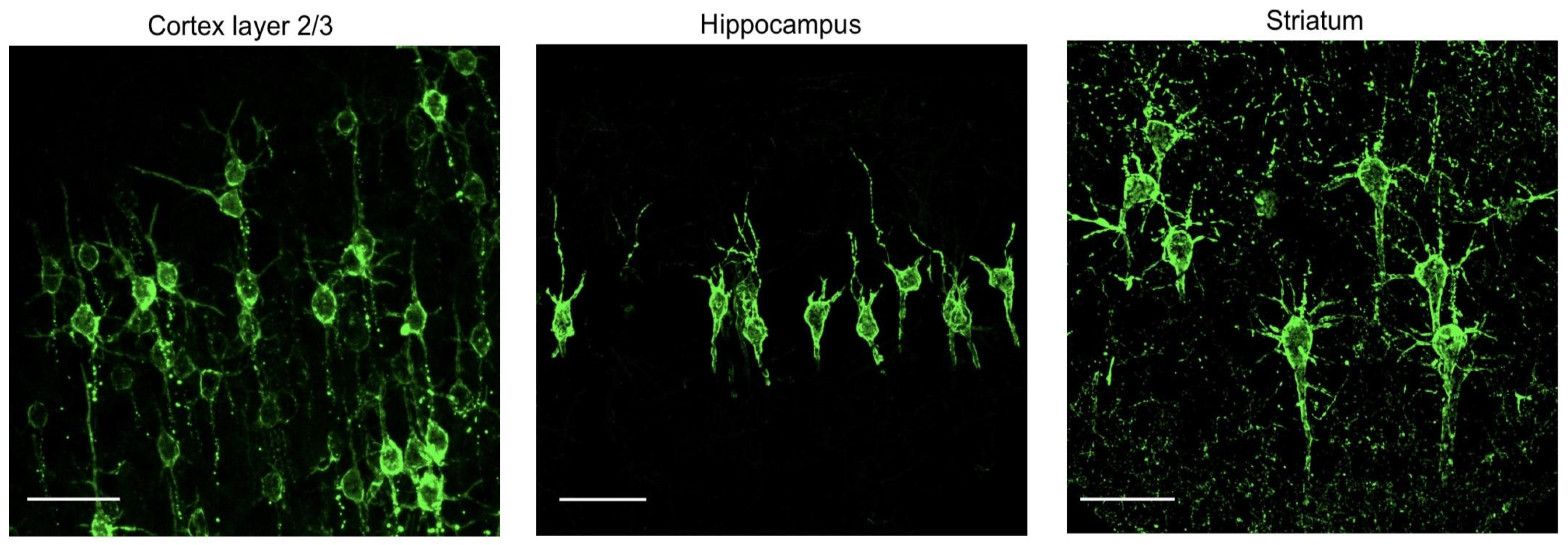
2.3. Energy Processing in Neurons
2.4. Quantum Scale Energy Processing in Neural Structures
2.5. Neural Activity as Biophysical Work
3. Conscious Experience in the Brain
3.1. Defining Conscious Experience
3.2. The Neural Organization of Conscious Experience
“Overall, a large body of work supports the notion that the presence of consciousness is invariably associated with high brain complexity, which, vice versa is found to be consistently decreased during physiological, pharmacological, or pathological-induced loss of consciousness”.
3.3. Differentiation and Integration
4. The Production of Conscious Experience in Neural Systems
4.1. Hypothesis
- When biophysical work is performed in localized portions, or subsystems, of a nervous system without coordination between the subsystems, then the system is differentiated or segregated but not spatially or temporally integrated. Since the biophysical work being performed in the nervous system is insufficiently differentiated and integrated, the system is unconscious: there is nothing ‘it is like’ as the system as a whole and the stimulation of the system elicits a simple linear response.
- When different subsystems at different scales interact by transferring energy between themselves over longer periods in coordinated ways, they become integrated into larger and more diverse subsystems at different spatiotemporal scales. The nature of this larger subsystem is determined by the way it integrates the biophysical work of its subsystems over time. But below a certain critical threshold of differentiation and integration of its activity, it remains unconscious: there is ‘nothing it is like’ as the system and the stimulation of the system elicits a less simple but still linear response.
- Once the nervous system has reached or exceeded a critical level of integration and differentiation of its biophysical work then there is ‘something it is like’ as the system from its intrinsic perspective, and it reaches the threshold necessary to be a consciously experiencing system. The stimulation of the system then elicits a complex nonlinear response, whether neurobiologically or behaviorally. The quantity and complexity of the work being carried out in each system, including all its subsystems, determines the level or kind of conscious experience produced in the system as a whole and the complexity of its response to stimulation.
4.2. Testing and Falsifying the Hypothesis
5. Further Work
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maxwell, J.C. Matter and Motion; Society for Promoting Christian Knowledge: London, UK, 1877. [Google Scholar]
- Northoff, G.; Lamme, V. Neural signs and mechanisms of consciousness: Is there a potential convergence of theories of consciousness in sight? Neurosci. Biobehav. Rev. 2020, 118, 568–587. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; Szczotka, J.; Prentner, R. Explanatory profiles of models of consciousness—Towards a systematic classification. Neurosci. Conscious. 2021, 2021, niab021. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Bayne, T. Theories of consciousness. Nat. Rev. Neurosci. 2022, 23, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.L. A landscape of consciousness: Toward a taxonomy of explanations and implications. Prog. Biophys. Mol. Biol. 2024, 190, 28–169. [Google Scholar] [PubMed]
- Tononi, G.; Boly, M.; Massimini, M.; Koch, C. Integrated information theory: From consciousness to its physical substrate. Nat. Rev. Neurosci. 2016, 17, 450–461. [Google Scholar] [CrossRef]
- Mashour, G.A.; Roelfsema, P.; Changeux, J.P.; Dehaene, S. Conscious Processing and the Global Neuronal Workspace Hypothesis. Neuron 2020, 105, 776–798. [Google Scholar] [CrossRef]
- Sprevak, M.; Smith, R. An Introduction to Predictive Processing Models of Perception and Decision-Making. Top. Cogn. Sci. 2023; early view. [Google Scholar] [CrossRef]
- Brown, R. Integrated Information Theory Doesn’t Address the Hard Problem. 2017. Available online: https://onemorebrown.com/2017/08/13/integrated-information-theory-doesnt-address-the-hard-problem/ (accessed on 14 December 2022).
- Cerullo, M.A. The Problem with Phi: A Critique of Integrated Information Theory. PLoS Comput. Biol. 2015, 11, e1004286. [Google Scholar] [CrossRef]
- Merker, B.; Williford, K.; Rudrauf, D. The integrated information theory of consciousness: A case of mistaken identity. Behav. Brain Sci. 2021, 45, e41. [Google Scholar] [CrossRef]
- Brown, R.; Lau, H.; LeDoux, J.E. Understanding the Higher-Order Approach to Consciousness. Trends Cogn. Sci. 2019, 23, 754–768. [Google Scholar] [CrossRef]
- Schurger, A.; Graziano, M. Consciousness explained or described? Neurosci. Conscious. 2022, 2022, niac001. [Google Scholar] [CrossRef]
- Doerig, A.; Schurger, A.; Herzog, M.H. Hard criteria for empirical theories of consciousness. Cogn. Neurosci. 2021, 12, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Yaron, I.; Melloni, L.; Pitts, M.; Mudrik, L. The ConTraSt database for analysing and comparing empirical studies of consciousness theories. Nat. Hum. Behav. 2022, 6, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Niven, J.E. Neuronal energy consumption: Biophysics, efficiency and evolution. Curr. Opin. Neurobiol. 2016, 41, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.J. The Ecological Approach to Visual Perception; Psychology Press: London, UK, 1979. [Google Scholar]
- Pepperell, R. Vision as an Energy-Driven Process. arXiv 2002, arXiv:2008.00754. [Google Scholar]
- Yoshioka, T.; Sakakibara, M. Physical aspects of sensory transduction on seeing, hearing and smelling. Biophysics 2013, 9, 183–191. [Google Scholar] [CrossRef]
- Manicka, S.; Levin, M. Modeling somatic computation with non-neural bioelectric networks. Sci. Rep. 2019, 9, 18612. [Google Scholar] [CrossRef]
- Swan, M.; dos Santos, R.P.; Witte, F. Quantum Neurobiology. Quantum Rep. 2022, 4, 107–126. [Google Scholar] [CrossRef]
- Hameroff, S. ‘Orch OR’ is the most complete, and most easily falsifiable theory of consciousness. Cogn. Neurosci. 2021, 12, 74–76. [Google Scholar] [CrossRef]
- Bassett, D.S.; Gazzaniga, M.S. Understanding complexity in the human brain. Trends Cogn. Sci. 2011, 15, 200–209. [Google Scholar] [CrossRef]
- Tognoli, E.; Kelso, J.A. Enlarging the scope: Grasping brain complexity. Front. Syst. Neurosci. 2014, 8, 122. [Google Scholar] [CrossRef]
- Forbes, R.M.; Cooper, A.R.; Mitchell, H.H. The composition of the adult human body as determined by chemical analysis. J. Biol. Chem. 1953, 203, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Suzuki, Y. The Metabolic Basis of Inherited Disease; McGraw-Hill: New York, NY, USA, 1972. [Google Scholar]
- Gonzalez-Riano, C.; Garcia, A.; Barbas, C. Metabolomics studies in brain tissue: A review. J. Pharm. Biomed. Anal. 2016, 130, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Kohn, F.P.; Koch, C.; Ritzmann, R. The Effect of Gravity on the Nervous System. In Into Space—A Journey of How Humans Adapt and Live in Microgravity; Russomano, T., Rehnberg, L., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Isakovic, J.; Dobbs-Dixon, I.; Chaudhury, D.; Mitrecic, D. Modeling of inhomogeneous electromagnetic fields in the nervous system: A novel paradigm in understanding cell interactions, disease etiology and therapy. Sci. Rep. 2018, 8, 12909. [Google Scholar] [CrossRef] [PubMed]
- Erecińska, M.; Silver, I.A. ATP and brain function. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 1989, 9, 2–19. [Google Scholar] [CrossRef]
- Attwell, D.; Laughlin, S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef]
- Raichle, M.E.; Gusnard, D.A. Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. USA 2002, 99, 10237–10239. [Google Scholar] [CrossRef]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nature reviews. Neuroscience 2012, 13, 407–420. [Google Scholar]
- Magistretti, P.J.; Allaman, I. A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015, 86, 883–901. [Google Scholar] [CrossRef]
- Raichle, M.E.; Mintun, M.A. Brain work and brain imaging. Annu. Rev. Neurosci. 2006, 29, 449–476. [Google Scholar] [CrossRef]
- Wong-Riley, M.T. Energy metabolism of the visual system. Eye Brain 2010, 2, 99–116. [Google Scholar] [CrossRef]
- Rees, G. Neural correlates of the contents of visual awareness in humans. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2007, 362, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Buxton, R.B. The physics of functional magnetic resonance imaging (fMRI). Rep. Prog. Phys. Phys. Soc. 2013, 76, 096601. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Rees, G. Neuroimaging of visual awareness in patients and normal subjects. Curr. Opin. Neurobiol. 2001, 11, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Rolls, E.T.; Romo, R. Stochastic dynamics as a principle of brain function. Prog. Neurobiol. 2009, 88, 1–16. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc. Natl. Acad. Sci. USA 2012, 109 (Suppl. S1), 10661–10668. [Google Scholar] [CrossRef]
- Peng, H.; Xie, P.; Liu, L.; Kuang, X.; Wang, Y.; Qu, L.; Gong, H.; Jiang, S.; Li, A.; Ruan, Z.; et al. Morphological diversity of single neurons in molecularly defined cell types. Nature 2021, 598, 174–181. [Google Scholar] [CrossRef]
- Fodstad, H. The neuron theory. Stereotact. Funct. Neurosurg. 2001, 77, 20–24. [Google Scholar] [CrossRef]
- Thatcher, J.D. Charged membranes. Sci. Signal. 2013, 6, tr6. [Google Scholar] [CrossRef]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic energy use and supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef]
- Zhang, X.C.; Yang, H.; Liu, Z.; Sun, F. Thermodynamics of voltage-gated ion channels. Biophys. Rep. 2018, 4, 300–319. [Google Scholar] [CrossRef]
- Raghavan, M.; Fee, D.; Barkhaus, P.E. Generation and propagation of the action potential. Handb. Clin. Neurol. 2019, 160, 3–22. [Google Scholar] [PubMed]
- Sibson, N.R.; Dhankhar, A.; Mason, G.F.; Rothman, D.L.; Behar, K.L.; Shulman, R.G. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc. Natl. Acad. Sci. USA 1998, 95, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Kaila, K.; Raichle, M. Inhibition and brain work. Neuron 2007, 56, 771–783. [Google Scholar] [CrossRef]
- Hirokawa, N.; Niwa, S.; Tanaka, Y. Molecular motors in neurons: Transport mechanisms and roles in brain function, development, and disease. Neuron 2010, 68, 610–638. [Google Scholar] [CrossRef] [PubMed]
- Javier-Torrent, M.; Zimmer-Bensch, G.; Nguyen, L. Mechanical Forces Orchestrate Brain Development. Trends Neurosci. 2021, 44, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Anastassiou, C.A.; Koch, C. Ephaptic coupling to endogenous electric field activity: Why bother? Curr. Opin. Neurobiol. 2015, 31, 95–103. [Google Scholar] [CrossRef]
- Chiang, C.C.; Shivacharan, R.S.; Wei, X.; Gonzalez-Reyes, L.E.; Durand, D.M. Slow periodic activity in the longitudinal hippocampal slice can self-propagate non-synaptically by a mechanism consistent with ephaptic coupling. J. Physiol. 2019, 597, 249–269. [Google Scholar] [CrossRef]
- Ruffini, G.; Salvador, R.; Tadayon, E.; Sanchez-Todo, R.; Pascual-Leone, A.; Santarnecchi, E. Realistic modeling of mesoscopic ephaptic coupling in the human brain. PLoS Comput. Biol. 2020, 16, e1007923. [Google Scholar] [CrossRef]
- Scholkmann, F. Two emerging topics regarding long-range physical signaling in neurosystems: Membrane nanotubes and electromagnetic fields. J. Integr. Neurosci. 2015, 14, 135–153. [Google Scholar] [CrossRef]
- Qiu, C.; Shivacharan, R.S.; Zhang, M.; Durand, D.M. Can Neural Activity Propagate by Endogenous Electrical Field? J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 15800–15811. [Google Scholar] [CrossRef]
- Ucar, H.; Watanabe, S.; Noguchi, J.; Morimoto, Y.; Iino, Y.; Yagishita, S.; Takahashi, N.; Kasai, H. Mechanical actions of dendritic-spine enlargement on presynaptic exocytosis. Nature 2021, 600, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Piatkevich, K.D.; Bensussen, S.; Tseng, H.A.; Shroff, S.N.; Lopez-Huerta, V.G.; Park, D.; Jung, E.E.; Shemesh, O.A.; Straub, C.; Gritton, H.J.; et al. Population imaging of neural activity in awake behaving mice. Nature 2019, 574, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Bohr, N. Light and Life. In Atomic Physics and Human Knowledge; Science Editions; Dover Publications, Inc.: Mineola, NY, USA, 1932; pp. 3–12. [Google Scholar]
- Roncaglia, A.; Cerisola, F.; Paz, J. Work Measurement as a Generalized Quantum Measurement. Phys. Rev. Lett. 2014, 113, 250601. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Physics of life: The dawn of quantum biology. Nature 2011, 474, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Marais, A.; Adams, B.; Ringsmuth, A.K.; Ferretti, M.; Gruber, J.M.; Hendrikx, R.; Schuld, M.; Smith, S.L.; Sinayskiy, I.; Krüger, T.P.J.; et al. The future of quantum biology. J. R. Soc. Interface 2018, 15, 20180640. [Google Scholar] [CrossRef]
- Romero, E.; Prior, J.; Chin, A.W.; Morgan, S.E.; Novoderezhkin, V.I.; Plenio, M.B.; van Grondelle, R. Quantum—Coherent dynamics in photosynthetic charge separation revealed by wavelet analysis. Sci. Rep. 2017, 7, 2890. [Google Scholar] [CrossRef]
- Beck, F.; Eccles, J. Quantum aspects of brain activity and the role of consciousness. Proc. Natl. Acad. Sci. USA 1992, 89, 11357–11361. [Google Scholar] [CrossRef]
- Tarlacı, S.; Pregnolato, M. Quantum neurophysics: From non-living matter to quantum neurobiology and psychopathology. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2016, 103, 161–173. [Google Scholar] [CrossRef]
- Hameroff, S.; Penrose, R. Consciousness in the universe: A review of the ‘Orch OR’ theory. Phys. Life Rev. 2014, 11, 39–78. [Google Scholar] [CrossRef]
- Fisher, M. Quantum cognition: The possibility of processing with nuclear spins in the brain. Ann. Phys. 2015, 362, 593–602. [Google Scholar] [CrossRef]
- Sergi, A.; Messina, A.; Vicario, C.M.; Martino, G. A Quantum-Classical Model of Brain Dynamics. Entropy 2023, 25, 592. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Ghosh, S.; Fujita, D.; Bandyopadhyay, A. Live visualizations of single isolated tubulin protein self-assembly via tunneling current: Effect of electromagnetic pumping during spontaneous growth of microtubule. Sci. Rep. 2014, 4, 7303. [Google Scholar] [CrossRef] [PubMed]
- Goodson, H.V.; Jonasson, E.M. Microtubules and Microtubule-Associated Proteins. Cold Spring Harb. Perspect. Biol. 2018, 10, a022608. [Google Scholar] [CrossRef] [PubMed]
- Kalra, A.P.; Benny, A.; Travis, S.M.; Zizzi, E.A.; Morales-Sanchez, A.; Oblinsky, D.G.; Craddock, T.J.A.; Hameroff, S.R.; MacIver, M.B.; Tuszyński, J.A.; et al. Electronic Energy Migration in Microtubules. ACS Cent. Sci. 2023, 9, 352–361. [Google Scholar] [CrossRef]
- Roy, C.; Sherrington, C. On the regulation of the blood supply in the brain. J. Physiol. 1890, 11, 85–108, 158-7–158-17. [Google Scholar] [CrossRef]
- Sokoloff, L. Relation between physiological function and energy metabolism in the central nervous system. J. Neurochem. 1977, 29, 13–26. [Google Scholar] [CrossRef]
- Poznanski, R. The dynamic organicity theory of consciousness: How consciousness arises from the functionality of multiscale complexity in the material brain. J. Multiscale Neurosci. 2024, 3, 68–87. [Google Scholar] [CrossRef]
- Aalling, N.N.; Nedergaard, M.; DiNuzzo, M. Cerebral Metabolic Changes During Sleep. Curr. Neurol. Neurosci. Rep. 2018, 18, 57. [Google Scholar] [CrossRef]
- Shulman, R.; Hyder, F.; Rothman, D. Baseline brain energy supports the state of consciousness. Proc. Natl. Acad. Sci. USA 2009, 106, 11096–11101. [Google Scholar] [CrossRef]
- Dinuzzo, M.; Nedergaard, M. Brain energetics during the sleep–wake cycle. Curr. Opin. Neurobiol. 2017, 47, 65–72. [Google Scholar] [CrossRef]
- Slupe, A.M.; Kirsch, J.R. Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2018, 38, 2192–2208. [Google Scholar] [CrossRef] [PubMed]
- Stender, J.; Mortensen, K.N.; Thibaut, A.; Darkner, S.; Laureys, S.; Gjedde, A.; Kupers, R. The Minimal Energetic Requirement of Sustained Awareness after Brain Injury. Curr. Biol. CB 2016, 26, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Bazzigaluppi, P.; Amini, A.E.; Weisspapier, I.; Stefanovic, B.; Carlen, P. Hungry neurons: Metabolic insights on seizure dynamics. Int. J. Mol. Sci. 2017, 18, 2269. [Google Scholar] [CrossRef] [PubMed]
- Pai, A.; Heining, M. Ketamine. Contin. Educ. Anaesth. Crit. Care Pain 2007, 7, 59–63. [Google Scholar] [CrossRef]
- Juan, E.; Górska, U.; Kozma, C.; Papantonatos, C.; Bugnon, T.; Denis, C.; Kremen, V.; Worrell, G.; Struck, A.F.; Bateman, L.M.; et al. Distinct signatures of loss of consciousness in focal impaired awareness versus tonic-clonic seizures. Brain J. Neurol. 2022, 146, awac291. [Google Scholar] [CrossRef]
- Nagel, T. What is it like to be a bat? Philos. Rev. 1974, 83, 435–450. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Bodart, O.; Gosseries, O.; Wannez, S.; Thibaut, A.; Annen, J.; Boly, M.; Rosanova, M.; Casali, A.G.; Casarotto, S.; Tononi, G.; et al. Measures of metabolism and complexity in the brain of patients with disorders of consciousness. NeuroImage. Clin. 2017, 14, 354–362. [Google Scholar] [CrossRef]
- Jang, H.; Mashour, G.A.; Hudetz, A.G.; Huang, Z. Measuring the dynamic balance of integration and segregation underlying consciousness, anesthesia, and sleep in humans. Nat. Commun. 2024, 15, 9164. [Google Scholar] [CrossRef]
- Casali, A.G.; Gosseries, O.; Rosanova, M.; Boly, M.; Sarasso, S.; Casali, K.R.; Casarotto, S.; Bruno, M.A.; Laureys, S.; Tononi, G.; et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Trans. Med. 2013, 5, 198ra105. [Google Scholar] [CrossRef]
- Casarotto, S.; Comanducci, A.; Rosanova, M.; Sarasso, S. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann. Neurol. 2016, 80, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Mateos, D.M.; Guevara Erra, R.; Wennberg, R.; Perez Velazquez, J.L. Measures of entropy and complexity in altered states of consciousness. Cogn. Neurodyn. 2018, 12, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Comolatti, R.; Pigorini, A.; Casarotto, S.; Fecchio, M.; Faria, G.; Sarasso, S.; Rosanova, M.; Gosseries, O.; Boly, M.; Bodart, O.; et al. A fast and general method to empirically estimate the complexity of brain responses to transcranial and intracranial stimulations. Brain Stimul. 2019, 12, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Edelman, G.M. Consciousness and complexity. Science 1998, 282, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Izhikevich, E.; Reeke, G.N.; Edelman, G.M. Theories and measures of consciousness: An extended framework. Proc. Natl. Acad. Sci. USA 2006, 103, 10799–10804. [Google Scholar] [CrossRef]
- Sarasso, S. Adenauer Girardi Casali, Silvia Casarotto, Mario Rosanova, Corrado Sinigaglia, Marcello Massimini, Consciousness and complexity: A consilience of evidence. Neurosci. Conscious. 2021, 2021, niab023. [Google Scholar] [CrossRef]
- Noel, J.P.; Simon, D.; Thelen, A.; Maier, A.; Blake, R.; Wallace, M.T. Probing Electrophysiological Indices of Perceptual Awareness across Unisensory and Multisensory Modalities. J. Cogn. Neurosci. 2018, 30, 814–828. [Google Scholar] [CrossRef]
- van Vugt, B.; Dagnino, B.; Vartak, D.; Safaai, H.; Panzeri, S.; Dehaene, S.; Roelfsema, P.R. The threshold for conscious report: Signal loss and response bias in visual and frontal cortex. Science 2018, 360, 537–542. [Google Scholar] [CrossRef]
- Malach, R. Local neuronal relational structures underlying the contents of human conscious experience. Neurosci. Conscious. 2021, 2021, niab028. [Google Scholar] [CrossRef]
- Moutard, C.; Dehaene, S.; Malach, R. Spontaneous Fluctuations and Non-linear Ignitions: Two Dynamic Faces of Cortical Recurrent Loops. Neuron 2015, 88, 194–206. [Google Scholar] [CrossRef]
- Mashour, G.A.; Palanca, B.J.; Basner, M.; Li, D.; Wang, W.; Blain-Moraes, S.; Lin, N.; Maier, K.; Muench, M.; Tarnal, V.; et al. Recovery of consciousness and cognition after general anesthesia in humans. eLife 2021, 10, e59525. [Google Scholar] [CrossRef] [PubMed]
- Edelman, G.M.; Gally, J.A. Reentry: A key mechanism for integration of brain function. Front. Integr. Neurosci. 2013, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Lamme, V.A.; Roelfsema, P.R. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000, 23, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Hameroff, S.R.; Craddock, T.J.; Tuszynski, J.A. Quantum effects in the understanding of consciousness. J. Integr. Neurosci. 2014, 13, 229–252. [Google Scholar] [CrossRef]
- Georgiev, D.D.; Glazebrook, J.F. The quantum physics of synaptic communication via the SNARE protein complex. Prog. Biophys. Mol. Biol. 2018, 135, 16–29. [Google Scholar] [CrossRef]
- Kerskens, C.; Pérez, D. Experimental indications of non-classical brain functions. J. Phys. Commun. 2022, 6, 105001. [Google Scholar] [CrossRef]
- Chalmers, D.J. Facing Up to the Problem of Consciousness. In The Character of Consciousness; Oxford University: Oxford, UK, 2010. [Google Scholar]
- Demertzi, A.; Tagliazucchi, E.; Dehaene, S.; Deco, G.; Barttfeld, P.; Raimondo, F.; Martial, C.; Fernández-Espejo, D.; Rohaut, B.; Voss, H.U.; et al. Human consciousness is supported by dynamic complex patterns of brain signal coordination. Sci. Adv. 2019, 5, eaat7603. [Google Scholar] [CrossRef]
- Kent, L.; Wittmann, M. Special Issue: Consciousness science and its theories Time consciousness: The missing link in theories of consciousness. Neurosci. Conscious. 2021, 2021, niab011. [Google Scholar] [CrossRef]
- Bressler, S.L.; Kelso, J.A. Coordination Dynamics in Cognitive Neuroscience. Front. Neurosci. 2016, 10, 397. [Google Scholar] [CrossRef]
- Vatansever, D.; Schröter, M.; Adapa, R.M.; Bullmore, E.T.; Menon, D.K.; Stamatakis, E.A. Reorganisation of Brain Hubs across Altered States of Consciousness. Sci. Rep. 2020, 10, 3402. [Google Scholar] [CrossRef]
- Zahedi, A.; Lynn, S.J.; Sommer, W. Cognitive simulation along with neural adaptation explain effects of suggestions: A novel theoretical framework. Front. Psychol. 2024, 15, 1388347. [Google Scholar] [CrossRef] [PubMed]
- Deco, G.; Tononi, G.; Boly, M.; Kringelbach, M.L. Rethinking segregation and integration: Contributions of whole-brain modelling. Nat. Rev. Neurosci. 2015, 16, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Fair, D.A.; Dosenbach, N.U.; Church, J.A.; Cohen, A.L.; Brahmbhatt, S.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. USA 2007, 104, 13507–13512. [Google Scholar] [CrossRef] [PubMed]
- Rizkallah, J.; Annen, J.; Modolo, J.; Gosseries, O.; Benquet, P.; Mortaheb, S.; Amoud, H.; Cassol, H.; Mheich, A.; Thibaut, A.; et al. Decreased integration of EEG source-space networks in disorders of consciousness. NeuroImage. Clin. 2019, 23, 101841. [Google Scholar] [CrossRef]
- Lord, L.D.; Stevner, A.B.; Deco, G.; Kringelbach, M.L. Understanding principles of integration and segregation using whole-brain computational connectomics: Implications for neuropsychiatric disorders. Philos. Trans. R. Soc. 2017, 375, 20160283. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Li, Y. Investigating cognitive flexibility deficit in schizophrenia using task-based whole-brain functional connectivity. Front. Psychiatry 2022, 13, 1069036. [Google Scholar] [CrossRef]
- Koch, C.; Massimini, M.; Boly, M.; Tononi, G. Neural correlates of consciousness: Progress and problems. Nat. Rev. Neurosci. 2016, 17, 307–321. [Google Scholar] [CrossRef]
- Tononi, G.; Sporns, O.; Edelman, G.M. A measure for brain complexity: Relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. USA 1994, 91, 5033–5037. [Google Scholar] [CrossRef]
- Del Pozo, S.M.; Laufs, H.; Bonhomme, V.; Laureys, S.; Balenzuela, P.; Tagliazucchi, E. Unconsciousness reconfigures modular brain network dynamics. Chaos 2021, 31, 093117. [Google Scholar] [CrossRef]
- Song, C. Brain structural complexity and consciousness. Philos. Mind Sci. 2021, 2, 6. [Google Scholar] [CrossRef]
- Cabanac, M.; Cabanac, A.J.; Parent, A. The emergence of consciousness in phylogeny. Behav. Brain Res. 2009, 198, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Mason, G.J.; Lavery, J.M. What Is It Like to Be a Bass? Red Herrings, Fish Pain and the Study of Animal Sentience. Front. Vet. Sci. 2022, 9, 788289. [Google Scholar] [CrossRef] [PubMed]
- Lewin, R. Is your brain really necessary? Science 1980, 210, 1232–1234. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.F.; Cai, X.; Qiao, J.; Switzer, B.; Baun, J.; Morrison, T.; Iriah, S.; Madularu, D.; Sinkevicius, K.W.; Kulkarni, P. Life without a brain: Neuroradiological and behavioral evidence of neuroplasticity necessary to sustain brain function in the face of severe hydrocephalus. Sci. Rep. 2019, 9, 16479. [Google Scholar] [CrossRef] [PubMed]
- Sprengers, M.; Vonck, K.; Carrette, E.; Marson, A.G.; Boon, P. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst. Rev. 2017, 7, CD008497. [Google Scholar] [CrossRef]
- Burke, M.J.; Fried, P.J.; Pascual-Leone, A. Transcranial magnetic stimulation: Neurophysiological and clinical applications. Handb. Clin. Neurol. 2019, 163, 73–92. [Google Scholar]
- Berglund, K.; Stern, M.A.; Gross, R.E. Bioluminescence-Optogenetics. Adv. Exp. Med. Biol. 2021, 1293, 281–293. [Google Scholar]
- Vakani, R.; Nair, D.R. Electrocorticography and functional mapping. Handb. Clin. Neurol. 2019, 160, 313–327. [Google Scholar]
- Müller-Putz, G.R. Electroencephalography. Handb. Clin. Neurol. 2020, 168, 249–262. [Google Scholar]
- Wang, Y.; Wang, R.; Xu, X. Neural Energy Supply-Consumption Properties Based on Hodgkin-Huxley Model. Neural Plast 2017, 2017, 6207141. [Google Scholar] [CrossRef]
- Nath, S. Energy landscapes and dynamics of ion translocation through membrane transporters: A meeting ground for physics, chemistry, and biology. J. Biol. Phys. 2021, 47, 401–433. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wang, R.; Pan, X.; Zhu, Z. Energy expenditure computation of a single bursting neuron. Cogn. Neurodyn. 2019, 13, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Liput, D.J.; Nguyen, T.A.; Augustin, S.M.; Lee, J.O.; Vogel, S.S. A Guide to Fluorescence. Lifetime Microscopy and Förster’s Resonance Energy Transfer in Neuroscience. Curr. Protoc. Neurosci. 2020, 94, e108. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.B.; Seth, A.K. Practical measures of integrated information for time-series data. PLoS Comput. Biol. 2011, 7, e1001052. [Google Scholar] [CrossRef] [PubMed]
- Sanz Perl, Y.; Bocaccio, H.; Pallavicini, C.; Pérez-Ipiña, I.; Laureys, S.; Laufs, H.; Kringelbach, M.; Deco, G.; Tagliazucchi, E. Nonequilibrium brain dynamics as a signature of consciousness. Phys. Rev. E 2021, 104, 014411. [Google Scholar] [CrossRef]
- Wutzl, B.; Golaszewski, S.M.; Leibnitz, K.; Langthaler, P.B.; Kunz, A.B.; Leis, S.; Schwenker, K.; Thomschewski, A.; Bergmann, J.; Trinka, E. Narrative Review: Quantitative EEG in Disorders of Consciousness. Brain Sci. 2021, 11, 697. [Google Scholar] [CrossRef]
- Bassett, D.S.; Sporns, O. Network neuroscience. Nat. Neurosci. 2017, 20, 353–364. [Google Scholar] [CrossRef]
- Bartlett, G. Does integrated information theory make testable predictions about the role of silent neurons in consciousness? Neurosci. Conscious. 2022, 2022, niac015. [Google Scholar] [CrossRef]
- Pockett, S. The electromagnetic field theory of consciousness: A testable hypothesis about the characteristics of conscious as opposed to non-conscious fields. J. Conscious. Stud. 2012, 19, 191–223. [Google Scholar]
- McFadden, J. Integrating information in the brain’s EM field: The cemi field theory of consciousness. Neurosci. Conscious. 2020, 2020, niaa016. [Google Scholar] [CrossRef]
- Hunt, T.; Schooler, J.W. The Easy Part of the Hard Problem: A Resonance Theory of Consciousness. Front. Hum. Neurosci. 2019, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Solms, M. The Hard Problem of Consciousness and the Free Energy Principle. Front. Psychol. 2019, 9, 2714. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Wiese, W.; Hobson, J.A. Sentience and the Origins of Consciousness: From Cartesian Duality to Markovian Monism. Entropy 2020, 22, 516. [Google Scholar] [CrossRef]
- Pepperell, R. Consciousness as a physical process caused by the organisation of energy in the brain. Front. Psychol. 2018, 9, 2091. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepperell, R. Consciousness and Energy Processing in Neural Systems. Brain Sci. 2024, 14, 1112. https://doi.org/10.3390/brainsci14111112
Pepperell R. Consciousness and Energy Processing in Neural Systems. Brain Sciences. 2024; 14(11):1112. https://doi.org/10.3390/brainsci14111112
Chicago/Turabian StylePepperell, Robert. 2024. "Consciousness and Energy Processing in Neural Systems" Brain Sciences 14, no. 11: 1112. https://doi.org/10.3390/brainsci14111112
APA StylePepperell, R. (2024). Consciousness and Energy Processing in Neural Systems. Brain Sciences, 14(11), 1112. https://doi.org/10.3390/brainsci14111112




