Stress-Induced Ultrasonic Vocalization in Laboratory Rats and Mice: A Scoping Review
Abstract
1. Introduction
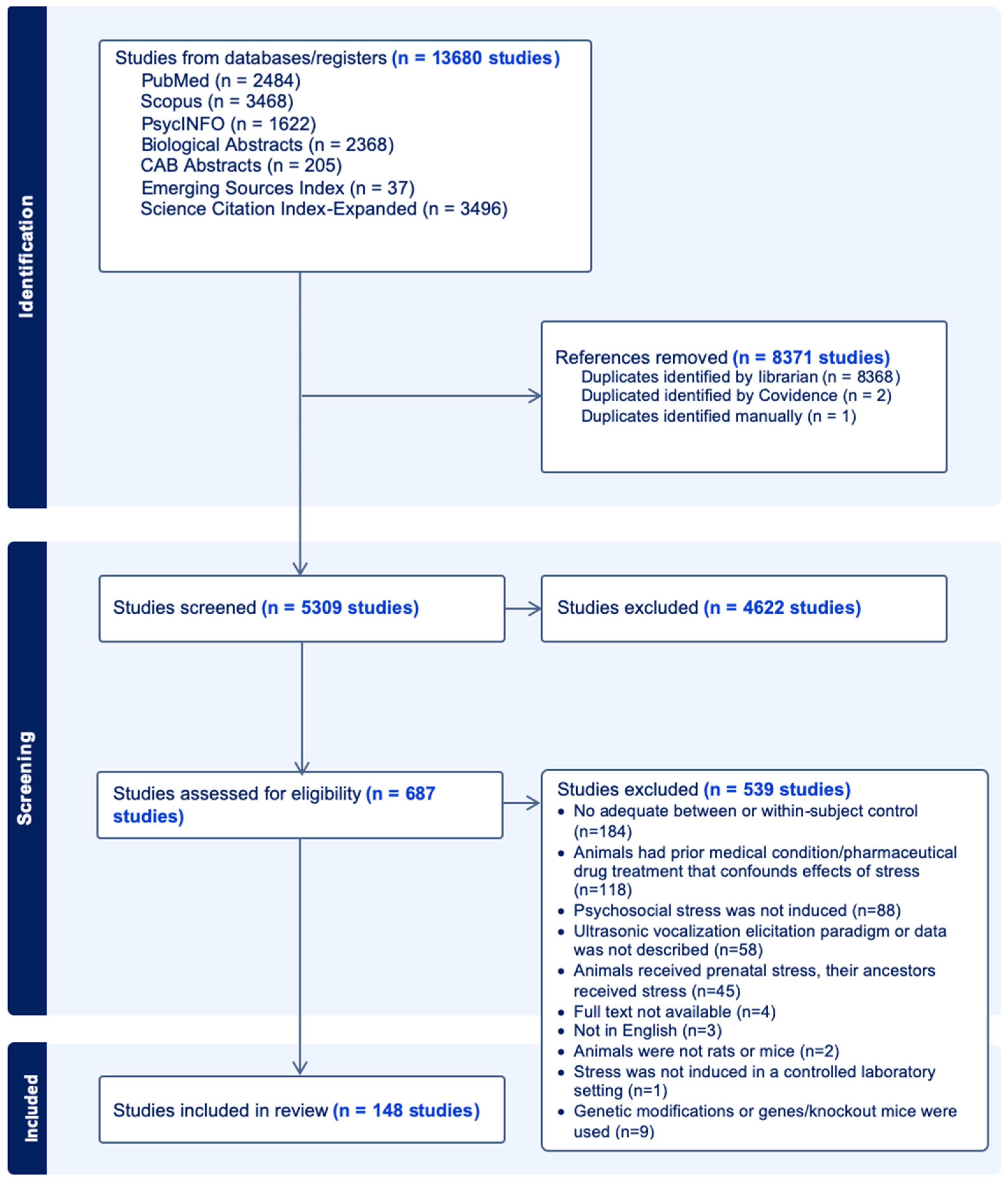
2. Methods
2.1. Screening Procedures
2.2. Eligibility Criteria for Study Inclusion
2.3. Data Extraction and Synthesis of the Results
3. Results
3.1. Frequency of Reporting of Different Stress Paradigms
3.2. Effect of Age, Sex, and Species-Related Variables on Stress-Induced USVs
- (a)
- Species and strain-related changes in USVs:
- (b)
- Age-related changes in USVs:
- (c)
- Sex-related changes in stress-altered USVs:
3.3. Effect of Type and Duration of Stress Paradigm on USVs
- (a)
- Restraint Stress: Four studies involved restraint stress. The type of restraint used for inducing stress varied, but most involved a customized modification of a conical-shaped tube [70,71]. Overall, the average number of positive affective USVs was reduced by restraint stress on each of the 7 days that rats were restrained when compared to baseline (~17–30 calls/15 s post-stress compared to 35–45 calls/15 s pre-stress) [70,71]. C56BL/7 mice had increased amplitude, bandwidth [72], and number of calls [35] with restraint.
- (b)
- Predator/Predator Odor: Eleven studies used predator/predator odor [66,73,74,75,76,77]. Six studies report an increase in the duration of time spent vocalizing in response to either predator exposure (cat) or predator odor (ferret bedding) for 20–25 min total (~20–60% time vocalizing with exposure compared to ~10% without exposure) [75,76]. This response habituates over time [75]. Neonates have a decreased number of USVs from 200–250 calls to positive affective calls in response to predator odor [77]. In one study, the number of pups per litter affected USV response to a 5 min exposure to predator odor (three-pup litters decreased call amplitude, and two-pup litters increased call amplitude) [66].
- (c)
- Chronic Variable Stress: Four studies used chronic variables for stress in rats, which is a combination of different stressors. The stressors differed in duration (4–6 weeks) and type (wet bedding, loud noise, light exposure, water deprivation, cage tilting, novel housing environment, restraint, cage tilt, forced swim, and elevated platform) [45,78,79,80]. In all included studies, the number of 50 kHz calls decreased, and the number of 22 kHz calls increased in response to stress [45,78,79,80].
- (d)
- Cold Exposure: A total of 21 studies used cold exposure; this stress paradigm was more common in pups [81,82]. Of note, C56BL/7 mice that are 3 days to 14 weeks of age, as well as Wistar pups, did not emit alarm-related USVs following cold stress at 2–10 °C for 7–12 days, but Sprague Dawley and albino rat pups increase USV production in response to cold (e.g., 87–205 calls/2 min) [33,35,81,83,84,85,86,87,88]. Sprague Dawley pups experienced a habituation effect (i.e., USVs reduce the number of calls by 21 days when stress is administered from 3 days) [89],
- (e)
- (f)
- Novel Social or Housing Environment: Fourteen studies involved novel social and housing environments. Light source (dim), increased familiarity with social conspecifics, and prior history or current isolation can negatively affect the number of USVs (increase aversive calls and decrease positive affective calls) [66,81,95,96,97,98,99,100].
- (g)
- Maternal Separation: Thirty-four studies employed maternal separation [12,13,31,42,53,57,58,61,65,88,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119] in a temperature-controlled incubator to reduce attrition [12,13,31,42,53,57,58,61,65,102,103,104,105,106,107,108,109]. In some cases, USVs are measured during the separation period (2–13 days) or when an anesthetized dam or littermates are reintroduced back into the cage [12,13,31,42,53,57,58,61,65,102,103,104,105,106,107,108,109]. Overall, pups increase their vocalization rate (calls/min) at aversive calls (24–250 calls in 1–10 min intervals) [12,13,31,42,53,57,58,61,65,102,103,104,105,106,107,108,109]. Three report a decrease in the number of aversive calls [56]. Dams also increased the number of positive affective calls in response to maternal separation [65,105,120]. Pups reduce their USV response to stress once reunited with their mother [120]. The results on call duration and call repertoire varied across studies, strains, and breeding lineage [56,120].
- (h)
- Footshock: Twenty-nine studies paired electric footshock with acoustic tone, an odor, or a treat (e.g., grape) for fear conditioning in rodents [22,24,43,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137]. Varied doses of current and duration of footshock are reported in the literature (0.04–3 mA of current, administered for 1–2 s, at various intervals for up to 70 trials or 40 min). All studies report a decrease in the number and duration of aversive calls [22,24,43,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136].
3.4. Effect of Elicitation Paradigm on Stress-Induced USVs
3.5. Aversive Calls
- (a)
- Acoustic startle/Air puff [147]: Four studies utilized auditory/acoustic stress in order to elicit USVs. Two of these studies utilized acoustic startle elicitation in conjunction with a maternal separation stress paradigm [65,105]. Two studies utilized startle-inducing acoustic stimuli and found that there were continuous USVs throughout testing [148,149], with a potential habituation effect. Ten additional studies utilized a fear conditioning paradigm with an acoustic/auditory cue, air puff, either with USV playback, noise, or tone, with inconsistent effects due to confounders [121,124,127,128,133,134,150,151,152,153].
- (b)
- (c)
- Stressor: Eighty articles utilized the stressor as the elicitation paradigm. In response to the stressor, 75% (24 of 32 studies) reported an increase in lower frequency calls in infant rodents. The effect on the 22 kHz USVs appeared to be dependent on the stressor and could be confounded by many other variables, including age and sex, as discussed above.
3.6. Positive Affective Calls
- (a)
- Social contact: Seven studies utilized some variation of social contact as the elicitation paradigm for positive affective USVs. The types of social contact included a mating paradigm (where USVs were measured post-ejaculation and during male-female interaction) [22,160], same-sex exposure (juvenile rough and tumble behavior) [5,6,7,8], or tickling [37,161].
- (b)
- Isolation: Only five studies utilized isolation as the elicitation procedure for 40–50 kHz USVs. The magnitude and change in isolation-emitted 40–50 kHz vocalization reported in response to stress varied across studies, with some rodent studies reporting an increased number of higher frequency USVs (~40–50 kHz for rat pups and 55 kHz in mice) [38,53].
- (c)
- Stressor: Twenty-nine studies utilized the stressor as the elicitation paradigm. The effect on positive affective USVs is variable depending on the stressor. Sixty-six percent of these studies (10/15) reported an increase in the number of higher-frequency calls in infant rodents.
3.7. Effect of Stress Persistence on USV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schwarting, R.K.W.; Wöhr, M. On the relationships between ultrasonic calling and anxiety-related behavior in rats. Braz. J. Med. Biol. Res. 2012, 45, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W. The Production of Ultrasonic Sounds by Laboratory Rats and Other Mammals. Science 1954, 119, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Simola, N. Rat Ultrasonic Vocalizations and Behavioral Neuropharmacology: From the Screening of Drugs to the Study of Disease. Curr. Neuropharmacol. 2015, 13, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Portfors, C.V. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 28–34. [Google Scholar]
- Lefebvre, E.; Granon, S.; Chauveau, F. Social context increases ultrasonic vocalizations during restraint in adult mice. Anim. Cogn. 2020, 23, 351–359. [Google Scholar] [CrossRef]
- Tornatzky, W.; Miczek, K.A. Behavioral and autonomic responses to intermittent social stress: Differential protection by clonidine and metoprolol. Psychopharmacology 1994, 116, 346–356. [Google Scholar] [CrossRef]
- Wen, F.; Xu, L. Effects of isolation after sexual experience on anxiety-like, depressive-like behaviors and affective states in male rats. Chin. Sci. Bull. 2010, 55, 4136–4142. [Google Scholar] [CrossRef]
- Hamed, A.; Jaroszewski, T.; Maciejak, P.; Szyndler, J.; Lehner, M.; Kamecka, I.; Olczak, M.; Kuzinska, U.; Taracha, E.; Płaźnik, A. The effects of buspirone and diazepam on aversive context- and social isolation-induced ultrasonic vocalisation. Physiol. Behav. 2009, 98, 474–480. [Google Scholar] [CrossRef]
- Walker, F.R.; Naicker, S.; Hinwood, M.; Dunn, N.; Day, T.A. Strain differences in coping behaviour, novelty seeking behaviour, and susceptibility to socially conditioned fear: A comparison between Wistar and Sprague Dawley rats. Stress 2009, 12, 507–516. [Google Scholar] [CrossRef]
- Litvin, Y.; Blanchard, D.C.; Blanchard, R.J. Rat 22kHz ultrasonic vocalizations as alarm cries. Behav. Brain Res. 2007, 182, 166–172. [Google Scholar] [CrossRef]
- Boeluekbas, I.; Mundorf, A.; Freund, N. Maternal separation in rats induces neurobiological and behavioral changes on the maternal side. Sci. Rep. 2020, 10, 22431. [Google Scholar] [CrossRef]
- Shair, H.N.; Rupert, D.D.; Rosko, L.M.; Hofer, M.A.; Myers, M.M.; Welch, M.G. Effects of maternal deprivation and the duration of reunion time on rat pup ultrasonic vocalization responses to isolation: Possible implications for human infant studies: Effects of Maternal Reunion Time on USV Responses to Isolation. Dev. Psychobiol. 2015, 57, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, L.; Xia, Y.; Cheng, Q.; Yuan, J.; Yang, Y.; Wang, Z.; Wang, H.; Dong, J.; Ding, Y.; et al. Maternal Deprivation Influences Pup Ultrasonic Vocalizations of C57BL/6J Mice. PLoS ONE 2016, 11, e0160409. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, J.; Jiang, W.; Xue, Q.; Zheng, X.; Ding, M.; Agarwal, A.A.; Elemans, C.P.H. Aerodynamics and motor control of ultrasonic vocalizations for social communication in mice and rats. BMC Biol. 2022, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Riede, T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J. Neurophysiol. 2011, 106, 2580–2592. [Google Scholar] [CrossRef]
- Riede, T. Stereotypic laryngeal and respiratory motor patterns generate different call types in rat ultrasound vocalization. J. Exp. Zool. A Ecol. Genet. Physiol. 2013, 319, 213–224. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Emission of 22 kHz vocalizations in rats as an evolutionary equivalent of human crying: Relationship to depression. Behav. Brain Res. 2019, 363, 1–12. [Google Scholar] [CrossRef]
- Wright, J.M.; Gourdon, J.C.; Clarke, P.B.S. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: Effects of amphetamine and social context. Psychopharmacology 2010, 211, 1–13. [Google Scholar] [CrossRef]
- Okabe, S.; Kanno, K. Acoustic Properties and Biological Significance of Ultrasonic Vocalizations in Rodents: Emotional Expressions. In Acoustic Communication in Animals; Seki, Y., Ed.; Springer Nature: Singapore, 2023; pp. 153–173. [Google Scholar] [CrossRef]
- Kelm-Nelson, C.A.; Brauer, A.F.L.; Barth, K.J.; Lake, J.M.; Sinnen, M.L.; Stehula, F.J.; Muslu, C.; Marongiu, R.; Kaplitt, M.G.; Ciucci, M.R. Characterization of early-onset motor deficits in the Pink1−/− mouse model of Parkinson disease. Brain Res. 2018, 1680, 1–12. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Communication of Adult Rats by Ultrasonic Vocalization: Biological, Sociobiological, and Neuroscience Approaches. ILAR J. 2009, 50, 43–50. [Google Scholar] [CrossRef]
- Choi, J.S.; Brown, T.H. Central Amygdala Lesions Block Ultrasonic Vocalization and Freezing as Conditional But Not Unconditional Responses. J. Neurosci. 2003, 23, 8713–8721. [Google Scholar] [CrossRef] [PubMed]
- Moskal, J.R.; Burgdorf, J. Ultrasonic Vocalizations in Rats as a Measure of Emotional Responses to Stress: Models of Anxiety and Depression. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 413–421. [Google Scholar] [CrossRef]
- Kassai, F.; Gyertyán, I. Effects of Selective Serotonin Reuptake Inhibitors on the Shock-Induced Ultrasonic Vocalization of Rats in Different Experimental Designs. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 309–316. [Google Scholar] [CrossRef]
- Hoffmeister, J.D.; Kelm-Nelson, C.A.; Ciucci, M.R. Quantification of brainstem norepinephrine relative to vocal impairment and anxiety in the Pink1−/− rat model of Parkinson disease. Behav. Brain Res. 2021, 414, 113514. [Google Scholar] [CrossRef] [PubMed]
- Krasko, M.N.; Hoffmeister, J.D.; Schaen-Heacock, N.E.; Welsch, J.M.; Kelm-Nelson, C.A.; Ciucci, M.R. Rat Models of Vocal Deficits in Parkinson’s Disease. Brain Sci. 2021, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Branchi, I.; Santucci, D.; Vitale, A.; Alleva, E. Ultrasonic vocalizations by infant laboratory mice: A preliminary spectrographic characterization under different conditions. Dev. Psychobiol. 1998, 33, 249–256. [Google Scholar] [CrossRef]
- Heckman, J.; McGuinness, B.; Celikel, T.; Englitz, B. Determinants of the mouse ultrasonic vocal structure and repertoire. Neurosci. Biobehav. Rev. 2016, 65, 313–325. [Google Scholar] [CrossRef]
- Lenell, C.; Johnson, A.M. Sexual dimorphism in laryngeal muscle fibers and ultrasonic vocalizations in the adult rat: Sexual Dimorphism of the Adult Rat Larynx. Laryngoscope 2017, 127, E270–E276. [Google Scholar] [CrossRef]
- Takahashi, N.; Kashino, M.; Hironaka, N. Structure of Rat Ultrasonic Vocalizations and Its Relevance to Behavior. PLoS ONE 2010, 5, e14115. [Google Scholar] [CrossRef]
- Lemasson, M.; Delbé, C.; Gheusi, G.; Vincent, J.D.; Lledo, P.M. Use of ultrasonic vocalizations to assess olfactory detection in mouse pups treated with 3-methylindole. Behav. Process. 2005, 68, 13–23. [Google Scholar] [CrossRef]
- Nitschke, W. Classical conditioning of vocalizations in the rat: Acoustic characteristics of the CR and UCR. Anim. Learn. Behav. 1979, 7, 457–460. [Google Scholar] [CrossRef]
- Allin, J.T.; Banks, E.M. Effects of temperature on ultrasound production by infant albino rats. Dev. Psychobiol. 1971, 4, 149–156. [Google Scholar] [CrossRef]
- Zimmerman, E.C.; Bellaire, M.; Ewing, S.G.; Grace, A.A. Abnormal Stress Responsivity in a Rodent Developmental Disruption Model of Schizophrenia. Neuropsychopharmacology 2013, 38, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Yoshioka, T.; Yamada, D.; Hamano, T.; Ohashi, M.; Matsumoto, M.; Iio, K.; Ikeda, M.; Kamei, M.; Otsuki, T.; et al. Cold-Restraint Stress-Induced Ultrasonic Vocalization as a Novel Tool to Measure Anxiety in Mice. Biol. Pharm. Bull. 2022, 45, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M. Effect of social odor context on the emission of isolation-induced ultrasonic vocalizations in the BTBR T+tf/J mouse model for autism. Front. Neurosci. 2015, 9, 73. [Google Scholar] [CrossRef]
- Kosten, T.; Miserendino, M.; Bombace, J.; Lee, H.; Kim, J. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav. Brain Res. 2005, 157, 235–244. [Google Scholar] [CrossRef]
- Motomura, N.; Shimizu, K.; Shimizu, M.; Aoki-Komori, S.; Taniguchi, K.; Serizawa, I.; Saito, T.R. A Comparative Study of Isolation-Induced Ultrasonic Vocalization in Rodent Pups. Exp. Anim. 2002, 51, 187–190. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Bramer, W.M.; Giustini, D.; De Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–242. [Google Scholar] [CrossRef]
- Emmerson, M.G.; Spencer, K.A.; Brown, G.R. Social experience during adolescence in female rats increases 50 kHz ultrasonic vocalizations in adulthood, without affecting anxiety-like behavior. Dev. Psychobiol. 2020, 62, 212–223. [Google Scholar] [CrossRef]
- Hefner, K.; Cameron, H.A.; Karlsson, R.M.; Holmes, A. Short-term and long-term effects of postnatal exposure to an adult male in C57BL/6J mice. Behav. Brain Res. 2007, 182, 344–348. [Google Scholar] [CrossRef]
- Parsana, A.J.; Moran, E.E.; Brown, T.H. Rats learn to freeze to 22-kHz ultrasonic vocalizations through autoconditioning. Behav. Brain Res. 2012, 232, 395–399. [Google Scholar] [CrossRef]
- Marini, F.; Pozzato, C.; Andreetta, V.; Jansson, B.; Arban, R.; Domenici, E.; Carboni, L. Single exposure to social defeat increases corticotropin-releasing factor and glucocorticoid receptor mRNA expression in rat hippocampus. Brain Res. 2006, 1067, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Maello, T.; Matrov, D.; Koiv, K.; Harro, J. Effect of Chronic Stress on Behavior and Cerebral Oxidative Metabolism in Rats with High or Low Positive Affect. Neuroscience 2009, 164, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Szyndler, J.; Wierzba-Bobrowicz, T.; Skórzewska, A.; Maciejak, P.; Walkowiak, J.; Lechowicz, W.; Turzyńska, D.; Bidziński, A.; Płaźnik, A. Behavioral, biochemical and histological studies in a model of pilocarpine-induced spontaneous recurrent seizures. Pharmacol. Biochem. Behav. 2005, 81, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Drugan, R.C.; Christianson, J.P.; Stine, W.W.; Soucy, D.P. Swim stress-induced ultrasonic vocalizations forecast resilience in rats. Behav. Brain Res. 2009, 202, 142–145. [Google Scholar] [CrossRef]
- Takahashi, L.K. Stimulus control of behavioral inhibition in the preweanling rat. Physiol. Behav. 1994, 55, 717–721. [Google Scholar] [CrossRef]
- Takahashi, L.K. Ontogeny of behavioral inhibition induced by unfamiliar adult male conspecifics in preweanling rats. Physiol. Behav. 1992, 52, 493–498. [Google Scholar] [CrossRef]
- Takahashi, L.K.; Turner, J.G.; Kalin, N.H. Development of stress-induced responses in preweanling rats. Dev. Psychobiol. 1991, 24, 341–360. [Google Scholar] [CrossRef]
- Rao, R.M.; Sadananda, M. Strain- and context-based 50 kHz ultrasonic vocalizations and anxiety behaviour in the Wistar-Kyoto rat. J. Biosci. 2015, 40, 561–570. [Google Scholar] [CrossRef]
- Carney, R.S.E. Ultrasonic Vocalizations Emitted during Defensive Behavior Alter the Influence of the Respiratory Rhythm on Brain Oscillatory Dynamics in the Fear Circuit of Rats. eNeuro 2019, 6, ENEURO.0280-19.2019. [Google Scholar] [CrossRef]
- Kaidbey, J.H.; Ranger, M.; Myers, M.M.; Anwar, M.; Ludwig, R.J.; Schulz, A.M.; Barone, J.L.; Kolacz, J.; Welch, M.G. Early Life Maternal Separation and Maternal Behaviour Modulate Acoustic Characteristics of Rat Pup Ultrasonic Vocalizations. Sci. Rep. 2019, 9, 19012. [Google Scholar] [CrossRef]
- Insel, T.R.; Harbaugh, C.R. Central administration of corticotropin releasing factor alters rat pup isolation calls. Pharmacol. Biochem. Behav. 1989, 32, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Kraebel, K.S.; Brasser, S.M.; Campbell, J.O.; Spear, L.P.; Spear, N.E. Developmental differences in temporal patterns and potentiation of isolation-induced ultrasonic vocalizations: Influence of temperature variables. Dev. Psychobiol. 2002, 40, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Freeborn, J.; Venna, V.R.; Roos, S.; Rhoads, J.M.; Liu, Y. Lactobacillus reuteri effects on maternal separation stress in newborn mice. Pediatr. Res. 2021, 90, 980–988. [Google Scholar] [CrossRef]
- Granata, L.; Valentine, A.; Hirsch, J.L.; Honeycutt, J.; Brenhouse, H. Trajectories of Mother-Infant Communication: An Experiential Measure of the Impacts of Early Life Adversity. Front. Hum. Neurosci. 2021, 15, 632702. [Google Scholar] [CrossRef]
- Muller, J.M.; Brunelli, S.A.; Moore, H.; Myers, M.M.; Shair, H.N. Maternally modulated infant separation responses are regulated by D2-family dopamine receptors. Behav. Neurosci. 2005, 119, 1384–1388. [Google Scholar] [CrossRef]
- Moles, A.; Kieffer, B.L.; D’Amato, F.R. Deficit in Attachment Behavior in Mice Lacking the µ-Opioid Receptor Gene. Science 2004, 304, 1983–1986. [Google Scholar] [CrossRef]
- Fride, E.; Suris, R.; Weidenfeld, J.; Mechoulam, R. Differential response to acute and repeated stress in cannabinoid CB1 receptor knockout newborn and adult mice. Behav. Pharmacol. 2005, 16, 431–440. [Google Scholar] [CrossRef]
- Zimmerberg, B.; Germeyan, S.C. Effects of neonatal fluoxetine exposure on behavior across development in rats selectively bred for an infantile affective trait: Effects of Neonatal Fluoxetine Exposure. Dev. Psychobiol. 2015, 57, 141–152. [Google Scholar] [CrossRef]
- Brunelli, S.A.; Myers, M.M.; Asekoff, S.L.; Hofer, M.A. Effects of selective breeding for infant rat ultrasonic vocalization on cardiac responses to isolation. Behav. Neurosci. 2002, 116, 612–623. [Google Scholar] [CrossRef]
- Zimmerberg, B.; Kim, J.H.; Davidson, A.N.; Rosenthal, A.J. Early Deprivation Alters the Vocalization Behavior of Neonates Directing Maternal Attention in a Rat Model of Child Neglect. Ann. N. Y. Acad. Sci. 2003, 1008, 308–313. [Google Scholar] [CrossRef]
- Ise, S.; Nagano, N.; Okuda, S.; Ohta, H. Corticotropin-releasing factor modulates maternal separation-induced ultrasonic vocalization in rat pups via activation of CRF1 receptor. Brain Res. 2008, 1234, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kalinichev, M.; Easterling, K.W.; Holtzman, S.G. Periodic postpartum separation from the offspring results in long-lasting changes in anxiety-related behaviors and sensitivity to morphine in Long-Evans mother rats. Psychopharmacology 2000, 152, 431–439. [Google Scholar] [CrossRef]
- Wilson, K.M.; Wagner, V.A.; Saltzman, W. Specificity of California mouse pup vocalizations in response to olfactory stimuli. Dev. Psychobiol. 2022, 64, e22261. [Google Scholar] [CrossRef]
- Inagaki, H. Sex Differences in Ultrasonic Vocal Expression of Negative Emotional States in Rats. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 337–344. [Google Scholar] [CrossRef]
- Waddell, J.; Yang, T.; Ho, E.; Wellmann, K.; Mooney, S. Prenatal Ethanol Exposure and Whisker Clipping Disrupt Ultrasonic Vocalizations and Play Behavior in Adolescent Rats. Brain Sci. 2016, 6, 43. [Google Scholar] [CrossRef]
- Petroni, V.; Subashi, E.; Premoli, M.; Wöhr, M.; Crusio, W.E.; Lemaire, V.; Pietropaolo, S. Autistic-like behavioral effects of prenatal stress in juvenile Fmr1 mice: The relevance of sex differences and gene-environment interactions. Sci. Rep. 2022, 12, 7269. [Google Scholar] [CrossRef]
- Popik, P.; Potasiewicz, A.; Pluta, H.; Zieniewicz, A. High-frequency ultrasonic vocalizations in rats in response to tickling: The effects of restraint stress. Behav. Brain Res. 2012, 234, 223–227. [Google Scholar] [CrossRef]
- Popik, P.; Kos, T.; Pluta, H.; Nikiforuk, A.; Rojek, K.; Ryguła, R. Inhibition of the glucocorticoid synthesis reverses stress-induced decrease in rat’s 50-kHz ultrasonic vocalizations. Behav. Brain Res. 2014, 260, 53–57. [Google Scholar] [CrossRef]
- Weiner, B.; Hertz, S.; Perets, N.; London, M. Social Ultrasonic Vocalization in Awake Head-Restrained Mouse. Front. Behav. Neurosci. 2016, 10, 236. [Google Scholar] [CrossRef]
- Fendt, M.; Brosch, M.; Wernecke, K.E.A.; Willadsen, M.; Wöhr, M. Predator odour but not TMT induces 22-kHz ultrasonic vocalizations in rats that lead to defensive behaviours in conspecifics upon replay. Sci. Rep. 2018, 8, 11041. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Blanchard, D.C.; Rodgers, J.; Weiss, S.M. The characterization and modelling of antipredator defensive behavior. Neurosci. Biobehav. Rev. 1990, 14, 463–472. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Blanchard, D.C.; Agullana, R.; Weiss, S.M. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol. Behav. 1991, 50, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Shionoya, K.; Hegoburu, C.; Brown, B.L.; Sullivan, R.M.; Doyère, V.; Mouly, A.M. It’s time to fear! Interval timing in odor fear conditioning in rats. Front. Behav. Neurosci. 2013, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Stockman, S.L.; McCarthy, M.M. Predator odor exposure of rat pups has opposite effects on play by juvenile males and females. Pharmacol. Biochem. Behav. 2017, 152, 20–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dönmez, R.A.; Kaya, F.D.; DeriNöz, O.; Emmez, Ö.H.; Candansayar, S.; Bolay Belen, H. Behavioural and neurobiological consequences of 2 different chronic stressors in rats. Turk. J. Med. Sci. 2014, 44, 955–966. [Google Scholar] [CrossRef]
- Yee, N.; Schwarting, R.K.W.; Fuchs, E.; Wöhr, M. Juvenile stress potentiates aversive 22-kHz ultrasonic vocalizations and freezing during auditory fear conditioning in adult male rats. Stress 2012, 15, 533–544. [Google Scholar] [CrossRef]
- Furlanetti, L.L.; Coenen, V.A.; Aranda, I.A.; Döbrössy, M.D. Chronic deep brain stimulation of the medial forebrain bundle reverses depressive-like behavior in a hemiparkinsonian rodent model. Exp. Brain Res. 2015, 233, 3073–3085. [Google Scholar] [CrossRef][Green Version]
- Hofer, M.A.; Shair, H.N. Ultrasonic vocalization by rat pups during recovery from deep hypothermia. Dev. Psychobiol. 1992, 25, 511–528. [Google Scholar] [CrossRef]
- Hofer, M.A.; Shair, H.N. Independence of ultrasonic vocalization and thermogenic responses in infant rats. Behav. Neurosci. 1991, 105, 41–48. [Google Scholar] [CrossRef]
- Nitschke, W.; Bell, R.W.; Zachman, T. Distress vocalizations of young in three inbred strains of mice. Dev. Psychobiol. 1972, 5, 363–370. [Google Scholar] [CrossRef]
- Bell, R.W.; Nitschke, W.; Bell, N.J.; Zachman, T.A. Early experience, ultrasonic vocalizations, and maternal responsiveness in rats. Dev. Psychobiol. 1974, 7, 235–242. [Google Scholar] [CrossRef]
- Blumberg, M.S.; Alberts, J.R. Ultrasonic vocalizations by rat pups in the cold: An acoustic by-product of laryngeal braking? Behav. Neurosci. 1990, 104, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, M.S.; Sokoloff, G.; Kent, K.J. Cardiovascular concomitants in ultrasound production during cold exposure in infant rats. Behav. Neurosci. 1999, 113, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.E.; Schanz, N. The effects of cold, rotation, and genotype on the production of ultrasonic calls in infant mice. Behav. Genet. 2002, 32, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Tamborski Harvey, A.; Hennessy, M.B. Corticotropin-releasing factor modulation of the ultrasonic vocalization rate of isolated rat pups. Dev. Brain Res. 1995, 87, 125–134. [Google Scholar] [CrossRef]
- Hashimoto, H.; Moritani, N.; Katou, M.; Nishiya, T.; Kromkhun, P.; Yokosuka, M.; Tanaka, M.; Saito, T.R. Ontogenetic Changes of Ultrasonic Vocalizations Emitted from Infant Rats. Exp. Anim. 2007, 56, 315–318. [Google Scholar] [CrossRef][Green Version]
- Panksepp, J.; Burgdorf, J.; Beinfeld, M.C.; Kroes, R.A.; Moskal, J.R. Brain regional neuropeptide changes resulting from social defeat. Behav. Neurosci. 2007, 121, 1364–1371. [Google Scholar] [CrossRef]
- Lumley, L.A.; Sipos, M.L.; Charles, R.C.; Charles, R.F.; Meyerhoff, J.L. Social Stress Effects on Territorial Marking and Ultrasonic Vocalizations in Mice. Physiol. Behav. 1999, 67, 769–775. [Google Scholar] [CrossRef]
- Becker, C.; Thiébot, M.H.; Touitou, Y.; Hamon, M.; Cesselin, F.; Benoliel, J.J. Enhanced Cortical Extracellular Levels of Cholecystokinin-Like Material in a Model of Anticipation of Social Defeat in the Rat. J. Neurosci. 2001, 21, 262–269. [Google Scholar] [CrossRef]
- Becker, C.; Andre, J.; Zeau, B.; Rettori, M.-C.; Guardiola-Lemaitre, B.; Hamon, M.; Benoliel, J.-J. Melatonin MT1/2 receptor stimulation reduces cortical overflow of cholecystokinin-like material in a model of anticipation of social defeat in the rat. Neuropharmacology 2004, 46, 1158–1167. [Google Scholar] [CrossRef]
- Vivian, J.A.; Miczek, K.A. Interactions between social stress and morphine in the periaqueductal gray: Effects on affective vocal and reflexive pain responses in rats. Psychopharmacology 1999, 146, 153–161. [Google Scholar] [CrossRef]
- Wöhr, M.; Houx, B.; Schwarting, R.K.W.; Spruijt, B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol. Behav. 2008, 93, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Keesom, S.M.; Finton, C.J.; Sell, G.L.; Hurley, L.M. Early-Life Social Isolation Influences Mouse Ultrasonic Vocalizations during Male-Male Social Encounters. PLoS ONE 2017, 12, e0169705. [Google Scholar] [CrossRef] [PubMed]
- Chabout, J.; Serreau, P.; Ey, E.; Bellier, L.; Aubin, T.; Bourgeron, T.; Granon, S. Adult male mice emit context-specific ultrasonic vocalizations that are modulated by prior isolation or group rearing environment. PLoS ONE 2012, 7, e29401. [Google Scholar] [CrossRef] [PubMed]
- Conely, L.; Bell, R.W. Neonatal ultrasounds elicited by odor cues. Dev. Psychobiol. 1978, 11, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.A.; Shair, H.N. Isolation distress in two-week-old rats: Influence of home cage, social companions, and prior experience with littermates. Dev. Psychobiol. 1987, 20, 465–476. [Google Scholar] [CrossRef]
- Wiedenmayer, C.P.; Lyo, D.; Barr, G.A. Rat pups reduce ultrasonic vocalization after exposure to an adult male rat. Dev. Psychobiol. 2003, 42, 386–391. [Google Scholar] [CrossRef]
- Ricceri, L.; Cutuli, D.; Venerosi, A.; Scattoni, M.L.; Calamandrei, G. Neonatal basal forebrain cholinergic hypofunction affects ultrasonic vocalizations and fear conditioning responses in preweaning rats. Behav. Brain Res. 2007, 183, 111–117. [Google Scholar] [CrossRef]
- Hofer, M.A.; Masmela, J.R.; Brunelli, S.A.; Shair, H.N. Behavioral mechanisms for active maternal potentiation of isolation calling in rat pups. Behav. Neurosci. 1999, 113, 51–61. [Google Scholar] [CrossRef]
- Shoemaker, W.J.; Kehoe, P. Effect of isolation conditions on brain regional enkephalin and β-endorphin levels and vocalizations in 10-day-old rat pups. Behav. Neurosci. 1995, 109, 117–122. [Google Scholar] [CrossRef]
- Rupniak, N.M.J.; Carlson, E.C.; Harrison, T.; Bellier, L.; Aubin, T.; Bourgeron, T.; Granon, S. Pharmacological blockade or genetic deletion of substance P (NK1) receptors attenuates neonatal vocalisation in guinea-pigs and mice. Neuropharmacology 2000, 39, 1413–1421. [Google Scholar] [CrossRef]
- Kalinichev, M.; Easterling, K.W.; Plotsky, P.M.; Holtzman, S.G. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long–Evans rats. Pharmacol. Biochem. Behav. 2002, 73, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Dirks, A.; Fish, E.W.; Kikusui, T.; van der Gugten, J.; Groenink, L.; Olivier, B.; Miczek, K.A. Effects of corticotropin-releasing hormone on distress vocalizations and locomotion in maternally separated mouse pups. Pharmacol. Biochem. Behav. 2002, 72, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Ploj, K.; Roman, E.; Nylander, I. Long-term effects of short and long periods of maternal separation on brain opioid peptide levels in male Wistar rats. Neuropeptides 2003, 37, 149–156. [Google Scholar] [CrossRef]
- Ognibene, E.; Adriani, W.; Macrì, S.; Laviola, G. Neurobehavioural disorders in the infant reeler mouse model: Interaction of genetic vulnerability and consequences of maternal separation. Behav. Brain Res. 2007, 177, 142–149. [Google Scholar] [CrossRef]
- Dimatelis, J.J.; Stein, D.J.; Russell, V.A. Behavioral changes after maternal separation are reversed by chronic constant light treatment. Brain Res. 2012, 1480, 61–71. [Google Scholar] [CrossRef]
- Carden, S.E.; Hofer, M.A. The effects of opioid and benzodiazepine antagonists on dam-induced reductions in rat pup isolation distress. Dev. Psychobiol. 1990, 23, 797–808. [Google Scholar] [CrossRef]
- Carden, S.E.; Hofer, M.A. Socially mediated reduction of isolation distress in rat pups is blocked by naltrexone but not by Ro 15-1788. Behav. Neurosci. 1990, 104, 457–463. [Google Scholar] [CrossRef]
- Delwig, A.; Logan, A.M.; Copenhagen, D.R.; Ahn, A.H. Light Evokes Melanopsin-Dependent Vocalization and Neural Activation Associated with Aversive Experience in Neonatal Mice. PLoS ONE 2012, 7, e43787. [Google Scholar] [CrossRef]
- Hofer, M.A.; Shair, H. Ultrasonic vocalization during social interaction and isolation in 2-weeek-old rats. Dev. Psychobiol. 1978, 11, 495–504. [Google Scholar] [CrossRef]
- Hofer, M.A.; Brunelli, S.A.; Shair, H.N. Potentiation of isolation-induced vocalization by brief exposure of rat pups to maternal cues. Dev. Psychobiol. 1994, 27, 503–517. [Google Scholar] [CrossRef]
- Hofer, M.A.; Shair, H.N.; Murowchick, E. Isolation distress and maternal comfort responses of two-week-old rat pups reared in social isolation. Dev. Psychobiol. 1989, 22, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Shair, H.N. Acquisition and expression of a socially mediated separation response. Behav. Brain Res. 2007, 182, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.M.; Ali, N.; Weller, A.; Brunelli, S.A.; Tu, A.Y.; Hofer, M.A.; Shair, H.N. Brief maternal interaction increases number, amplitude, and bout size of isolation-induced ultrasonic vocalizations in infant rats (Rattus norvegicus). J. Comp. Psychol. 2004, 118, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Isobe, A.; Kawaguchi, M. Relationship between motor function and ultrasonic vocalizations induced by maternal separation in rat pups. J. Vet. Med. Sci. 2019, 81, 287–293. [Google Scholar] [CrossRef]
- Zimmerberg, B.; Rackow, S.H.; George-Friedman, K.P. Sex-Dependent Behavioral Effects of the Neurosteroid Allopregnanolone (3α,5α-THP) in Neonatal and Adult Rats after Postnatal Stress. Pharmacol. Biochem. Behav. 1999, 64, 717–724. [Google Scholar] [CrossRef]
- Burenkova, O.V.; Averkina, A.A.; Aleksandrova, E.A.; Zarayskaya, I.Y. Brief but enough: 45-min maternal separation elicits behavioral and physiological responses in neonatal mice and changes in dam maternal behavior. Physiol. Behav. 2020, 222, 112877. [Google Scholar] [CrossRef]
- Mead, A.; Li, M.; Kapur, S. Clozapine and olanzapine exhibit an intrinsic anxiolytic property in two conditioned fear paradigms: Contrast with haloperidol and chlordiazepoxide. Pharmacol. Biochem. Behav. 2008, 90, 551–562. [Google Scholar] [CrossRef]
- Weber, M.; Paxinos, G.; Richardson, R. Conditioned changes in ultrasonic vocalizations to an aversive olfactory stimulus are lateralized in 6-day-old rats. Dev. Psychobiol. 2000, 37, 121–128. [Google Scholar] [CrossRef]
- Kikusui, T.; Nishizawa, D.; Takeuchi, Y.; Mori, Y. Conditioned fear-related ultrasonic vocalizations are emitted as an emotional response. J. Vet. Med. Sci. 2003, 65, 1299–1305. [Google Scholar] [CrossRef][Green Version]
- Ko, S.W.; Chatila, T.; Zhuo, M. Contribution of CaMKIV to Injury and Fear-Induced Ultrasonic Vocalizations in Adult Mice. Mol. Pain. 2005, 1, 1744–8069. [Google Scholar] [CrossRef]
- Finn, D.P.; Jhaveri, M.D.; Beckett, S.R.G.; Madjd, A.; Kendall, D.; Marsden, C.; Chapman, V. Behavioral, central monoaminergic and hypothalamo–pituitary–adrenal axis correlates of fear-conditioned analgesia in rats. Neuroscience 2006, 138, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Swiergiel, A.; Zhou, Y.; Dunn, A. Effects of chronic footshock, restraint and corticotropin-releasing factor on freezing, ultrasonic vocalization and forced swim behavior in rats. Behav. Brain Res. 2007, 183, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, E.S.; Covey, E.; Kim, J.J. Social Transmission of Fear in Rats: The Role of 22-kHz Ultrasonic Distress Vocalization. PLoS ONE 2010, 5, e15077. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; He, W.; Hu, G.; Li, M. Anxiolytic-like property of risperidone and olanzapine as examined in multiple measures of fear in rats. Pharmacol. Biochem. Behav. 2010, 95, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Y.; Li, S.; Kirouac, G.J. Early fear as a predictor of avoidance in a rat model of post-traumatic stress disorder. Behav. Brain Res. 2012, 226, 112–117. [Google Scholar] [CrossRef]
- Bonasera, S.J.; Schenk, A.K.; Luxenberg, E.J.; Wang, X.; Basbaum, A.; Tecott, L.H. Mice Lacking Serotonin 2C Receptors Have increased Affective Responses to Aversive Stimuli. PLoS ONE 2015, 10, e0142906. [Google Scholar] [CrossRef]
- Rojas-Carvajal, M.; Brenes, J.C. Acute stress differentially affects grooming subtypes and ultrasonic vocalisations in the open-field and home-cage test in rats. Behav. Process. 2020, 176, 104140. [Google Scholar] [CrossRef]
- Karwicka, W.; Wiatrowska, M.; Kondrakiewicz, K.; Knapska, E.; Kursa, M.B.; Hamed, A. Relaying Aversive Ultrasonic Alarm Calls Depends on Previous Experience. Empathy, Social Buffering, or Panic? Brain Sci. 2021, 11, 759. [Google Scholar] [CrossRef]
- Fendt, M.; Gonzalez-Guerrero, C.P.; Kahl, E. Observational Fear Learning in Rats: Role of Trait Anxiety and Ultrasonic Vocalization. Brain Sci. 2021, 11, 423. [Google Scholar] [CrossRef]
- Jelen, P.; Soltysik, S.; Zagrodzka, J. 22-kHz ultrasonic vocalization in rats as an index of anxiety but not fear: Behavioral and pharmacological modulation of affective state. Behav. Brain Res. 2003, 141, 63–72. [Google Scholar] [CrossRef]
- Portavella, M.; Depaulis, A.; Vergnes, M. 22-28 kHz ultrasonic vocalizations associated with defensive reactions in male rats do not result from fear or aversion. Psychopharmacology 1993, 111, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Han, J.S.; Kim, J.J. Selective Neurotoxic Lesions of Basolateral and Central Nuclei of the Amygdala Produce Differential Effects on Fear Conditioning. J. Neurosci. 2004, 24, 7654–7662. [Google Scholar] [CrossRef] [PubMed]
- Pertsov, S.S.; Koplik, E.V.; Karkishchenko, N.N.; Sudakov, K.V. Ultrasonic vocalization of rats in various motivational and emotional states. Bull. Exp. Biol. Med. 2012, 153, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Iku, A.; Harness, A. Activity of cholinergic neurons in the laterodorsal tegmental nucleus during emission of 22 kHz vocalization in rats. Behav. Brain Res. 2011, 225, 276–283. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Kirouac, G.J. Blocking of corticotrophin releasing factor receptor-1 during footshock attenuates context fear but not the upregulation of prepro-orexin mRNA in rats. Pharmacol. Biochem. Behav. 2014, 120, 1–6. [Google Scholar] [CrossRef]
- Choi, E.A.; Leman, S.; Vianna, D.M.L.; Waite, P.M.E.; Carrive, P. Expression of cardiovascular and behavioural components of conditioned fear to context in T4 spinally transected rats. Auton. Neurosci. 2005, 120, 26–34. [Google Scholar] [CrossRef]
- Tomazini, F.M.; Reimer, A.; Albrechet-Souza, L.; Brandão, M.L. Opposite effects of short- and long-duration isolation on ultrasonic vocalization, startle and prepulse inhibition in rats. J. Neurosci. Methods 2006, 153, 114–120. [Google Scholar] [CrossRef]
- Smith, K.L.; Ford, G.K.; Jessop, D.S.; Finn, D.P. Behavioural, neurochemical and neuroendocrine effects of the endogenous β-carboline harmane in fear-conditioned rats. J. Psychopharmacol. 2013, 27, 162–170. [Google Scholar] [CrossRef]
- Inagaki, H.; Kuwahara, M.; Kikusui, T.; Tsubone, H. The influence of social environmental condition on the production of stress-induced 22 kHz calls in adult male Wistar rats. Physiol. Behav. 2005, 84, 17–22. [Google Scholar] [CrossRef]
- Nunes Mamede Rosa, M.L.; Nobre, M.J.; Ribeiro Oliveira, A.; Brandão, M.L. Isolation-induced changes in ultrasonic vocalization, fear-potentiated startle and prepulse inhibition in rats. Neuropsychobiology 2005, 51, 248–255. [Google Scholar] [CrossRef]
- Iacobucci, P.; Colonnello, V.; Fuchs, T.; D’Antuono, L.; Panksepp, J. Differential ultrasonic indices of separation distress in the presence and absence of maternal cues in infant rats bred for high and low positive social affect. Acta Neuropsychiatr. 2013, 25, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M. Ultrasonic calls of rats as indicator variables of negative or positive states: Acetylcholine–dopamine interaction and acoustic coding. Behav. Brain Res. 2007, 182, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Holland, G. Acoustic characteristics of air puff-induced 22-kHz alarm calls in direct recordings. Neurosci. Biobehav. Rev. 2005, 29, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Kaltwasser, M.T. Startle-inducing acoustic stimuli evoke ultrasonic vocalization in the rat. Physiol. Behav. 1990, 48, 13–17. [Google Scholar] [CrossRef]
- Webber, E.S.; Mankin, D.E.; McGraw, J.J.; Beckwith, T.J.; Cromwell, H.C. Ultrasonic vocalizations, predictability and sensorimotor gating in the rat. Behav. Brain Res. 2013, 253, 32–41. [Google Scholar] [CrossRef][Green Version]
- Olszyński, K.H.; Polowy, R.; Wardak, A.D.; Grymanowska, A.W.; Filipkowski, R.K. Increased Vocalization of Rats in Response to Ultrasonic Playback as a Sign of Hypervigilance Following Fear Conditioning. Brain Sci. 2021, 11, 970. [Google Scholar] [CrossRef]
- Atsak, P.; Orre, M.; Bakker, P.; Cerliani, L.; Roozendaal, B.; Gazzola, V.; Moita, M.; Keysers, C. Experience Modulates Vicarious Freezing in Rats: A Model for Empathy. PLoS ONE 2011, 6, e21855. [Google Scholar] [CrossRef]
- Brasser, S.M.; Spear, N.E. A sensory-enhanced context facilitates learning and multiple measures of unconditioned stimulus processing in the preweanling rat. Behav. Neurosci. 1998, 112, 126–140. [Google Scholar] [CrossRef]
- Browning, J.; Whiteman, A.C.; Deng-Bryant, Y.; Shear, D.A. Air-Puff Induced Vocalizations: A Novel Approach to Detecting Negative Affective State Following Concussion in Rats. J. Neurotrauma 2016, 33, A102. [Google Scholar] [CrossRef]
- Johnson, A.M. Social isolation alters ultrasonic vocalizations but not thyroarytenoid neuromuscular junctions in old rats. Laryngoscope 2019, 129, E9–E14. [Google Scholar] [CrossRef]
- Hennessy, M.B.; Davis, H.N.; McCREA, A.E.; Harvey, A.T.; Williams, M.T. Short- and Long-term Consequences of Corticotropin-releasing Factor in Early Development. Ann. N. Y. Acad. Sci. 1999, 897, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.; Ostrum, R. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol. Neurobiol. 2003, 23, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, M.; Campolongo, P.; Mangieri, R.A.; Scattoni, M.L.; Frau, R.; Trezza, V.; La Rana, G.; Russo, R.; Calignano, A.; Gessa, G.L.; et al. Anxiolytic-Like Properties of the Anandamide Transport Inhibitor AM404. Neuropsychopharmacology 2006, 31, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Bassi, G.S.; Nobre, M.J.; Carvalho, M.C.; Brandão, M.L. Substance P injected into the dorsal periaqueductal gray causes anxiogenic effects similar to the long-term isolation as assessed by ultrasound vocalizations measurements. Behav. Brain Res. 2007, 182, 301–307. [Google Scholar] [CrossRef]
- Blass, E.M.; Fitzgerald, E. Milk-induced analgesia and comforting in 10-day-old rats: Opioid mediation. Pharmacol. Biochem. Behav. 1988, 29, 9–13. [Google Scholar] [CrossRef]
- Grimsley, J.M.S.; Sheth, S.; Vallabh, N.; Grimsley, C.A.; Bhattal, J.; Latsko, M.; Jasnow, A.; Wenstrup, J.J. Contextual Modulation of Vocal Behavior in Mouse: Newly Identified 12 kHz “Mid-Frequency” Vocalization Emitted during Restraint. Front. Behav. Neurosci. 2016, 10, 38. [Google Scholar] [CrossRef]
- Hansen, S. Effect of clonidine on the responsiveness of infant rats to maternal stimuli. Psychopharmacology 1993, 111, 78–84. [Google Scholar] [CrossRef]
- Granata, L.E.; Valentine, A.; Hirsch, J.L.; Brenhouse, H.C. Infant ultrasonic vocalizations predict adolescent social behavior in rats: Effects of early life adversity. Dev. Psychobiol. 2022, 64, e22260. [Google Scholar] [CrossRef]
- Lee, J.H.; Kimm, S.; Han, J.S.; Choi, J.S. Chasing as a model of psychogenic stress: Characterization of physiological and behavioral responses. Stress 2018, 21, 323–332. [Google Scholar] [CrossRef]
- Kropp, D.R.; Rainville, J.R.; Glover, M.E.; Tsyglakova, M.; Samanta, R.; Hage, T.R.; Carlson, A.E.; Clinton, S.M.; Hodes, G.E. Chronic variable stress leads to sex specific gut microbiome alterations in mice. Brain Behav. Immun. Health 2024, 37, 100755. [Google Scholar] [CrossRef]
- US Animal and Plant Health Inspection Service. Animal Care Tech Note: Categorizing Animal Pain or Distress in Research Facility Annual Reports. Animal Welfare Care Act. Published Online 2023. Available online: https://www.aphis.usda.gov/sites/default/files/ac-tech-note-categorizing-animal-pain-or-distress.pdf (accessed on 12 September 2022).
- Burgdorf, J.; Brudzynski, S.; Moskal, J. Using rat ultrasonic vocalization to study the neurobiology of emotion: From basic science to the development of novel therapeutics for affective disorders. Curr. Opin. Neurobiol. 2020, 60, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, C.; Carrive, P.; Camus, F.; Bénoliel, J.J.; Similowski, T.; Sévoz-Couche, C. Long-lasting bradypnea induced by repeated social defeat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R352–R364. [Google Scholar] [CrossRef] [PubMed]
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar]
- Burke, K.; Screven, L.A.; Dent, M.L. CBA/CaJ mouse ultrasonic vocalizations depend on prior social experience. PLoS ONE 2018, 13, e0197774. [Google Scholar] [CrossRef]
- Boulanger-Bertolus, J.; Mouly, A.M. Ultrasonic Vocalizations Emission across Development in Rats: Coordination with Respiration and Impact on Brain Neural Dynamics. Brain Sci. 2021, 11, 616. [Google Scholar] [CrossRef]
- Lenell, C.; Broadfoot, C.K.; Schaen-Heacock, N.E.; Ciucci, M.R. Biological and Acoustic Sex Differences in Rat Ultrasonic Vocalization. Brain Sci. 2021, 11, 459. [Google Scholar] [CrossRef]
- Bondarenko, E.; Hodgson, D.M.; Nalivaiko, E. Amygdala mediates respiratory responses to sudden arousing stimuli and to restraint stress in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R951–R959. [Google Scholar] [CrossRef]
- Riede, T.; Schaefer, C.; Stein, A. Role of deep breaths in ultrasonic vocal production of Sprague-Dawley rats. J. Neurophysiol. 2020, 123, 966–979. [Google Scholar] [CrossRef]
- Tenorio-Lopes, L.; Kinkead, R. Sex-Specific Effects of Stress on Respiratory Control: Plasticity, Adaptation, and Dysfunction. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 2097–2134. [Google Scholar] [CrossRef]
- Hofer, M.A.; Shair, H.N. Ultrasonic vocalization, laryngeal braking, and thermogenesis in rat pups: A reappraisal. Behav. Neurosci. 1993, 107, 354–362. [Google Scholar] [CrossRef]

| Author, Year | Age | Strain/s | Stress Paradigm | USV Elicitation Technique | Stress-Induced USV Outcomes | |
|---|---|---|---|---|---|---|
| Mice | ||||||
| Nitschke, 1972 [33] | Infant | C57BL/6J, BALB/cJ, C3H/Hej mice | Cold exposure | Cold exposure | C56BL/6J | Reduced USV call rate over time, with no calls after 6 days of stress |
| BALB/cJ | Reduced USV call rate over time, with no calls 12 days of stress | |||||
| C3H/Hej | Maintained relatively high USV call rate over time, even after 12 days of stress | |||||
| Woehr, 2015 [32] | Infant | BTBR T+tf/J, C57BL/6J | Isolation in a novel context (soiled and clean bedding) | Isolation | BTBR T+tf/J | Higher # of USVs in soiled and clean bedding compared to C57BL/6J Reduced # of USVs with soiled bedding compared to clean |
| C57BL/6J | No change with bedding type | |||||
| Rats | ||||||
| Kassai, 2018 [34] | Adult | Sprague Dawley, Wister, Long Evans Lister-Hooded | Footshock (single session) | Footshock | Sprague Dawley | Lowest USV call duration |
| Wister | Higher USV call duration compared to Sprague Dawley | |||||
| Long Evans | Same as Wister rats, higher USV call duration than Sprague Dawley | |||||
| Lister Hooded | Highest USV call duration | |||||
| Walker, 2009 [8] | Adult | Sprague Dawley, Wister | Social Defeat | Social Defeat | Sprague Dawley | Increased # of USVs compared to Wistar rats and non- stressed controls |
| Wister | No change in # of USVs compared to non-stressed controls | |||||
| Woehr, 2008 [35] | Adult | Long Evans, Wistar | Isolation (novel cage) | Isolation | Wistar | Decreased # of USVs in novel cage compared to home cage Increased # of USVs compared to Long Evans |
| Long Evans | Decreased # of USVs in novel cage compared to home cage | |||||
| Rao, 2015 [36] | Adult | Wistar Kyoto, Wistar | Isolation and Elevated Plus Maze | Isolation and Elevated Plus Maze | Wistar Kyoto | Produced longer call duration in isolation compared to maze No change in # of USV between isolation and maze |
| Wistar | Produced longer call duration in isolation compared to maze Decreased # of USV in maze compared to isolation | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatraman, A.; Bretl, M.; Kim, S.-i.; Christensen, L.; Kelm-Nelson, C.A.; Ciucci, M.R.; Thibeault, S.L. Stress-Induced Ultrasonic Vocalization in Laboratory Rats and Mice: A Scoping Review. Brain Sci. 2024, 14, 1109. https://doi.org/10.3390/brainsci14111109
Venkatraman A, Bretl M, Kim S-i, Christensen L, Kelm-Nelson CA, Ciucci MR, Thibeault SL. Stress-Induced Ultrasonic Vocalization in Laboratory Rats and Mice: A Scoping Review. Brain Sciences. 2024; 14(11):1109. https://doi.org/10.3390/brainsci14111109
Chicago/Turabian StyleVenkatraman, Anumitha, Michelle Bretl, Se-in Kim, Leslie Christensen, Cynthia A. Kelm-Nelson, Michelle R. Ciucci, and Susan L. Thibeault. 2024. "Stress-Induced Ultrasonic Vocalization in Laboratory Rats and Mice: A Scoping Review" Brain Sciences 14, no. 11: 1109. https://doi.org/10.3390/brainsci14111109
APA StyleVenkatraman, A., Bretl, M., Kim, S.-i., Christensen, L., Kelm-Nelson, C. A., Ciucci, M. R., & Thibeault, S. L. (2024). Stress-Induced Ultrasonic Vocalization in Laboratory Rats and Mice: A Scoping Review. Brain Sciences, 14(11), 1109. https://doi.org/10.3390/brainsci14111109






