Radiological Outcome of Middle Meningeal Artery Embolization in Relation to Chronic Subdural Hematoma Cause and Architecture
Abstract
1. Introduction
2. Materials and Methods
- Patients diagnosed with cSDH, treated with MMAE and followed up for at least 1 month;
- Available clinical data, procedural details, pre-procedural and follow-up CT scans.
- Insufficient DICOM (Digital Imaging and Communications in Medicine);
- Post-MMAE surgical evacuation.
2.1. MMAE Procedural Details
2.2. Imaging Assessment
2.3. Statistics
2.4. Ethics, Informed Consent and Confidentiality
3. Results
3.1. cSDH Volume and Thickness Correlation
3.2. cSDH Cause Groups
3.3. cSDH Architecture Groups
4. Discussion
4.1. Imaging Parameters for Post-MMAE cSDH Change Assessment
4.2. cSDH Cause
4.3. cSDH Architecture
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miranda, L.B.; Braxton, E.; Hobbs, J.; Quigley, M.R. Chronic Subdural Hematoma in the Elderly: Not a Benign Disease: Clinical Article. J. Neurosurg. 2011, 114, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Feghali, J.; Yang, W.; Huang, J. Updates in Chronic Subdural Hematoma: Epidemiology, Etiology, Pathogenesis, Treatment, and Outcome. World Neurosurg. 2020, 141, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Weigel, R.; Schilling, L.; Schmiedek, P. Specific Pattern of Growth Factor Distribution in Chronic Subdural Hematoma (CSH): Evidence for an Angiogenic Disease. Acta Neurochir. 2001, 143, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Ducruet, A.F.; Grobelny, B.T.; Zacharia, B.E.; Hickman, Z.L.; DeRosa, P.L.; Anderson, K.; Sussman, E.; Carpenter, A.; Connolly, E.S. The Surgical Management of Chronic Subdural Hematoma. Neurosurg. Rev. 2012, 35, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Sato, M.; Yoshida, K.; Toda, M. History and Current Progress of Chronic Subdural Hematoma. J. Neurol. Sci. 2021, 429, 118066. [Google Scholar] [CrossRef]
- Yang, W.; Huang, J. Chronic Subdural Hematoma. Neurosurg. Clin. N. Am. 2017, 28, 205–210. [Google Scholar] [CrossRef]
- Kolias, A.G.; Chari, A.; Santarius, T.; Hutchinson, P.J. Chronic Subdural Haematoma: Modern Management and Emerging Therapies. Nat. Rev. Neurol. 2014, 10, 570–578. [Google Scholar] [CrossRef]
- Blaauw, J.; Jacobs, B.; Den Hertog, H.M.; Van Der Gaag, N.A.; Jellema, K.; Dammers, R.; Lingsma, H.F.; Naalt, J.V.D.; Kho, K.H.; Groen, R.J.M. Neurosurgical and Perioperative Management of Chronic Subdural Hematoma. Front. Neurol. 2020, 11, 550. [Google Scholar] [CrossRef]
- Foppen, M.; Bandral, H.V.; Slot, K.-A.M.; Vandertop, W.P.; Verbaan, D. Success of Conservative Therapy for Chronic Subdural Hematoma Patients: A Systematic Review. Front. Neurol. 2023, 14, 1249332. [Google Scholar] [CrossRef]
- Link, T.W.; Rapoport, B.I.; Paine, S.M.; Kamel, H.; Knopman, J. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: Endovascular Technique and Radiographic Findings. Interv. Neuroradiol. 2018, 24, 455–462. [Google Scholar] [CrossRef]
- Srivatsan, A.; Mohanty, A.; Nascimento, F.A.; Hafeez, M.U.; Srinivasan, V.M.; Thomas, A.; Chen, S.R.; Johnson, J.N.; Kan, P. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: Meta-Analysis and Systematic Review. World Neurosurg. 2019, 122, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Fiorella, D.; Arthur, A.S. Middle Meningeal Artery Embolization for the Management of Chronic Subdural Hematoma. J. Neurointerv. Surg. 2019, 11, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Haldrup, M.; Ketharanathan, B.; Debrabant, B.; Schwartz, O.S.; Mikkelsen, R.; Fugleholm, K.; Poulsen, F.R.; Jensen, T.S.R.; Thaarup, L.V.; Bergholt, B. Embolization of the Middle Meningeal Artery in Patients with Chronic Subdural Hematoma—A Systematic Review and Meta-Analysis. Acta Neurochir. 2020, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Ishihara, S.; Kohyama, S.; Yamane, F.; Ogawa, M.; Sato, A.; Matsutani, M. Experience in Endovascular Treatment of Recurrent Chronic Subdural Hematoma. Interv. Neuroradiol. 2007, 13, 141–144. [Google Scholar] [CrossRef]
- Ban, S.P.; Hwang, G.; Byoun, H.S.; Kim, T.; Lee, S.U.; Bang, J.S.; Han, J.H.; Kim, C.-Y.; Kwon, O.-K.; Oh, C.W. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma. Radiology 2018, 286, 992–999. [Google Scholar] [CrossRef]
- Catapano, J.S.; Ducruet, A.F.; Nguyen, C.L.; Baranoski, J.F.; Cole, T.S.; Majmundar, N.; Wilkinson, D.A.; Fredrickson, V.L.; Cavalcanti, D.D.; Albuquerque, F.C. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: An Institutional Technical Analysis. J. Neurointerv. Surg. 2021, 13, 657–660. [Google Scholar] [CrossRef]
- Ironside, N.; Nguyen, C.; Do, Q.; Ugiliweneza, B.; Chen, C.-J.; Sieg, E.P.; James, R.F.; Ding, D. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: A Systematic Review and Meta-Analysis. J. Neurointerv. Surg. 2021, 13, 951–957. [Google Scholar] [CrossRef]
- Gebel, J.M.; Sila, C.A.; Sloan, M.A.; Granger, C.B.; Weisenberger, J.P.; Green, C.L.; Topol, E.J.; Mahaffey, K.W. Comparison of the ABC/2 Estimation Technique to Computer-Assisted Volumetric Analysis of Intraparenchymal and Subdural Hematomas Complicating the GUSTO-1 Trial. Stroke 1998, 29, 1799–1801. [Google Scholar] [CrossRef]
- Robinson, R.G. Chronic Subdural Hematoma: Surgical Management in 133 Patients. J. Neurosurg. 1984, 61, 263–268. [Google Scholar] [CrossRef]
- Lee, K.-S. ReviewNatural History of Chronic Subdural Haematoma. Brain Inj. 2004, 18, 351–358. [Google Scholar] [CrossRef]
- Nakaguchi, H.; Tanishima, T.; Yoshimasu, N. Factors in the Natural History of Chronic Subdural Hematomas That Influence Their Postoperative Recurrence. J. Neurosurg. 2001, 95, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Tennant, B.C. Hepatic Function. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 327–352. ISBN 978-0-12-396305-5. [Google Scholar]
- Schoenborn, C.A.; Adams, P.E. Health Behaviors of Adults: United States, 2005–2007. In Vital Health Statistics; Series 10; Number 245; U.S. Department of Health and Human Services: Washington, DC, USA, 2010; pp. 1–132. [Google Scholar]
- World Health Organization (Ed.) The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines; World Health Organization: Geneva, Switzerland, 1992; ISBN 978-92-4-154422-1. [Google Scholar]
- Omura, Y.; Ishiguro, T. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: A Systematic Review. Front. Neurol. 2023, 14, 1259647. [Google Scholar] [CrossRef] [PubMed]
- Levitt, M.R.; Hirsch, J.A.; Chen, M. Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: An Effective Treatment with a Bright Future. J. Neurointerv. Surg. 2024, 16, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Won, S.-Y.; Zagorcic, A.; Dubinski, D.; Quick-Weller, J.; Herrmann, E.; Seifert, V.; Konczalla, J. Excellent Accuracy of ABC/2 Volume Formula Compared to Computer-Assisted Volumetric Analysis of Subdural Hematomas. PLoS ONE 2018, 13, e0199809. [Google Scholar] [CrossRef] [PubMed]
- Bechstein, M.; McDonough, R.; Fiehler, J.; Zanolini, U.; Rai, H.; Siddiqui, A.; Shotar, E.; Rouchaud, A.; Goyal, M.; Gellissen, S. Radiological Evaluation Criteria for Chronic Subdural Hematomas: Review of the Literature. Clin. Neuroradiol. 2022, 32, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Hirashima, Y.; Hamada, H.; Hayashi, N.; Origasa, H.; Endo, S. Independent Predictors of Recurrence of Chronic Subdural Hematoma: Results of Multivariate Analysis Performed Using a Logistic Regression Model. J. Neurosurg. 2003, 98, 1217–1221. [Google Scholar] [CrossRef]
- Stanišić, M.; Hald, J.; Rasmussen, I.A.; Pripp, A.H.; Ivanović, J.; Kolstad, F.; Sundseth, J.; Züchner, M.; Lindegaard, K.-F. Volume and Densities of Chronic Subdural Haematoma Obtained from CT Imaging as Predictors of Postoperative Recurrence: A Prospective Study of 107 Operated Patients. Acta Neurochir. 2013, 155, 323–333; discussion 333. [Google Scholar] [CrossRef]
- Edlmann, E.; Giorgi-Coll, S.; Whitfield, P.C.; Carpenter, K.L.H.; Hutchinson, P.J. Pathophysiology of Chronic Subdural Haematoma: Inflammation, Angiogenesis and Implications for Pharmacotherapy. J. Neuroinflamm. 2017, 14, 108. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Turner, R.C.; Josiah, D.; Knotts, C.; Bhatia, S. Do Age and Anticoagulants Affect the Natural History of Acute Subdural Hematomas? Arch. Emerg. Med. Crit. Care 2016, 1, 1010. [Google Scholar]
- Weigel, R.; Schilling, L.; Krauss, J.K. The Pathophysiology of Chronic Subdural Hematoma Revisited: Emphasis on Aging Processes as Key Factor. Geroscience 2022, 44, 1353–1371. [Google Scholar] [CrossRef]
- Holl, D.C.; Volovici, V.; Dirven, C.M.F.; Peul, W.C.; van Kooten, F.; Jellema, K.; van der Gaag, N.A.; Miah, I.P.; Kho, K.H.; den Hertog, H.M.; et al. Pathophysiology and Nonsurgical Treatment of Chronic Subdural Hematoma: From Past to Present to Future. World Neurosurg. 2018, 116, 402–411.e2. [Google Scholar] [CrossRef] [PubMed]
- Edlmann, E.; Whitfield, P.C.; Kolias, A.; Hutchinson, P.J. Pathogenesis of Chronic Subdural Hematoma: A Cohort Evidencing De Novo and Transformational Origins. J. Neurotrauma 2021, 38, 2580–2589. [Google Scholar] [CrossRef] [PubMed]
- Chon, K.-H.; Lee, J.-M.; Koh, E.-J.; Choi, H.-Y. Independent Predictors for Recurrence of Chronic Subdural Hematoma. Acta Neurochir. 2012, 154, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Miah, I.P.; Tank, Y.; Rosendaal, F.R.; Peul, W.C.; Dammers, R.; Lingsma, H.F.; den Hertog, H.M.; Jellema, K.; van der Gaag, N.A.; Dutch Chronic Subdural Hematoma Research Group. Radiological Prognostic Factors of Chronic Subdural Hematoma Recurrence: A Systematic Review and Meta-Analysis. Neuroradiology 2021, 63, 27–40. [Google Scholar] [CrossRef]
- Uttam, B.K.; Yuanyuan, L.; Bizhan, A.; Thorsten, F.R.; Mazhar, K.; Marco, C.; Dheeraj, G. Short-Term Follow-up Pilot Study of Sole Middle Meningeal Artery Embolization for Chronic Subdural Hematoma: Influence of Internal Architecture on the Radiological Outcomes. Neuroradiology 2023, 65, 1143–1153. [Google Scholar] [CrossRef]
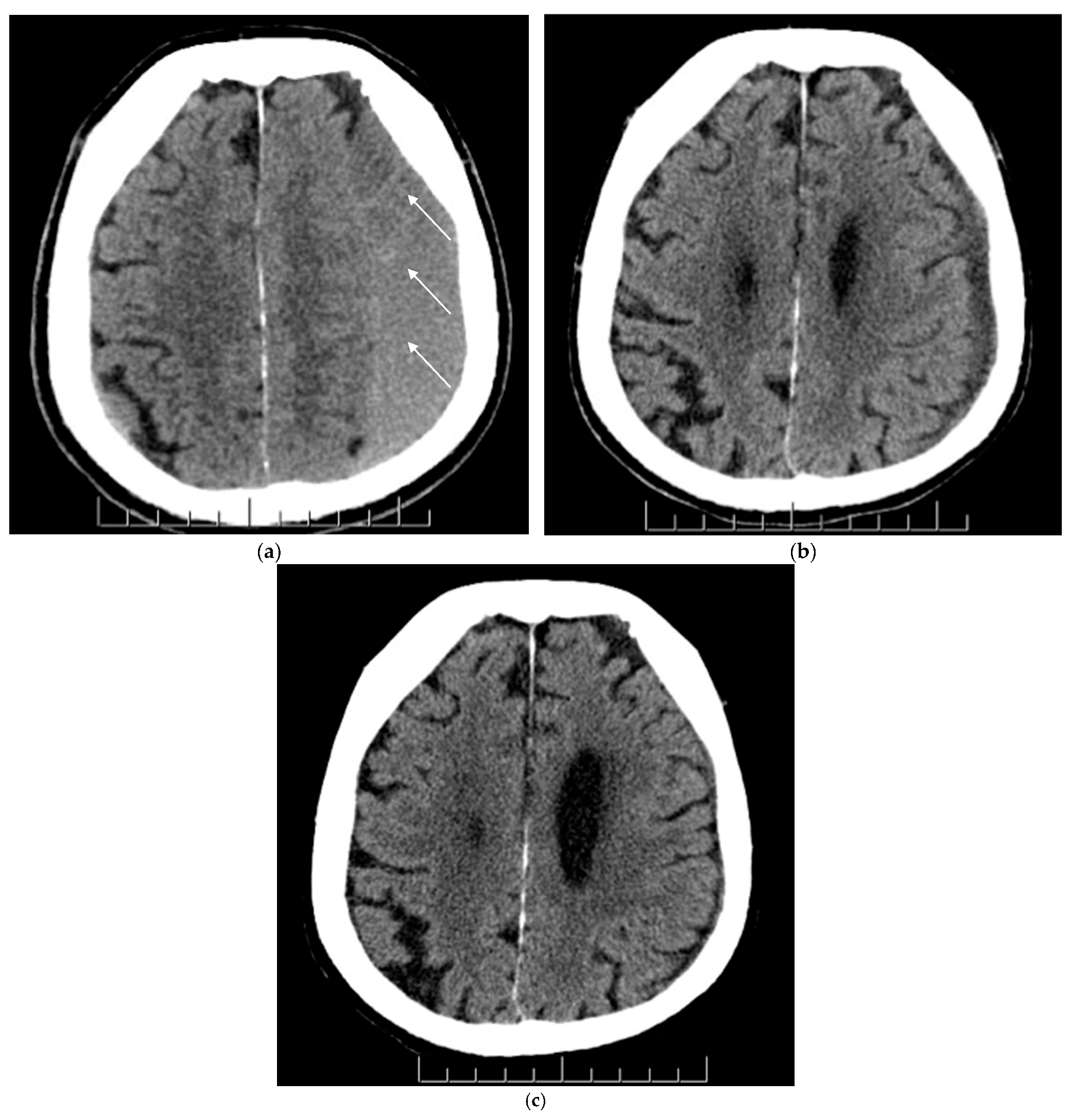
| (a) | ||||
| Thickness (T) and Volume (V) at Admission (0) and at Interval Follow-Up | T-Test Significance (p < 0.05) | |||
| cSDH Cause | cSDH Architecture | |||
| Spontaneous | Traumatic | Non-Mature | Mature | |
| T 0—at 1–3 months (mm) | <0.001 | <0.001 | <0.001 | <0.001 |
| V 0—at 1–3 months (cm3) | <0.001 | <0.001 | <0.001 | <0.001 |
| T 0—at 3–6 months (mm) | <0.001 | <0.001 | <0.001 | <0.001 |
| V 0—at 3–6 months (cm3) | 0.001 | <0.001 | <0.001 | <0.001 |
| T 0—at 6–12 months (mm) | <0.001 | <0.001 | <0.001 | 0.025 |
| V 0—at 6–12 months (cm3) | <0.001 | <0.001 | <0.001 | 0.003 |
| (b) | ||||
| Thickness (T) and Volume (V) at Admission (0) and at Interval Follow-Up | Wilcoxon Signed-Rank Test Significance (p < 0.05) | |||
| cSDH Cause | cSDH Architecture | |||
| Spontaneous | Traumatic | Non-Mature | Mature | |
| T 0—at 1–3 months (mm) | <0.001 | <0.001 | <0.001 | <0.001 |
| V 0—at 1–3 months (cm3) | <0.001 | <0.001 | <0.001 | <0.001 |
| T 0—at 3–6 months (mm) | 0.012 | <0.001 | 0.008 | 0.001 |
| V 0—at 3–6 months (cm3) | 0.012 | <0.001 | 0.008 | 0.001 |
| T 0—at 6–12 months (mm) | 0.002 | 0.068 | 0.008 | 0.018 |
| V 0—at 6–12 months (cm3) | 0.002 | 0.068 | 0.008 | 0.018 |
| Spontaneous–Traumatic cSDH Groups Differences | Mean Difference | 95% CI | Significance (p < 0.05) | ||||
|---|---|---|---|---|---|---|---|
| Lower | Upper | T-Test | MWU * | ANCOVA | GLM * | ||
| T Difference at 1–3 Months (mm) * | −0.37 | −5.13 | 4.40 | 0.438 | 0.910 | 0.145 | 0.119 |
| V Difference at 1–3 Months (cm3) * | −1.68 | −32.37 | 29.01 | 0.456 | 0.860 | 0.187 | 0.172 |
| T Difference at 3–6 months (mm) | −7.32 | −13 | −1.63 | 0.007 | 0.044 | 0.329 | 0.536 |
| V Difference at 3–6 Months (cm3) | −34.21 | −74.07 | 5.63 | 0.044 | 0.048 | 0.559 | 0.670 |
| T Difference at 6–12 Months (mm) | 0.67 | −8.84 | 10.18 | 0.44 | 0.626 | 0.253 | 0.138 |
| V Difference at 6–12 Months (cm3) | 18.46 | −37.88 | 74.8 | 0.247 | 0.716 | 0.467 | 0.354 |
| Hematoma Type 1– Type 2 Groups Differences | Mean Difference | 95% CI | Significance (p < 0.05) | ||
|---|---|---|---|---|---|
| Lower | Upper | T-Test | MWU * | ||
| T Difference at 1–3 Months (mm) * | 0.1 | −4.52 | 4.76 | 0.479 | 0.816 |
| V Difference at 1–3 Months (cm3) * | 2.8 | −27.06 | 32.68 | 0.425 | 0.864 |
| T Difference at 3–6 Months (mm) | 4 | −2.97 | 11.01 | 0.122 | 0.198 |
| V Difference at 3–6 Months (cm3) | 23 | −17.06 | 63.06 | 0.122 | 0.356 |
| T Difference at 6–12 Months (mm) | 5.7 | −1.92 | 13.35 | 0.065 | 0.089 |
| V Difference at 6–12 Months (cm3) | 7.7 | −42.13 | 57.56 | 0.372 | 0.368 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelghafar, A.; Falzon, A.; Hendriks, E.J.; Radovanovic, I.; Andrade, H.; Schaafsma, J.D.; Mosimann, P.J. Radiological Outcome of Middle Meningeal Artery Embolization in Relation to Chronic Subdural Hematoma Cause and Architecture. Brain Sci. 2024, 14, 1097. https://doi.org/10.3390/brainsci14111097
Abdelghafar A, Falzon A, Hendriks EJ, Radovanovic I, Andrade H, Schaafsma JD, Mosimann PJ. Radiological Outcome of Middle Meningeal Artery Embolization in Relation to Chronic Subdural Hematoma Cause and Architecture. Brain Sciences. 2024; 14(11):1097. https://doi.org/10.3390/brainsci14111097
Chicago/Turabian StyleAbdelghafar, Ahmed, Andrew Falzon, Eef J. Hendriks, Ivan Radovanovic, Hugo Andrade, Joanna D. Schaafsma, and Pascal J. Mosimann. 2024. "Radiological Outcome of Middle Meningeal Artery Embolization in Relation to Chronic Subdural Hematoma Cause and Architecture" Brain Sciences 14, no. 11: 1097. https://doi.org/10.3390/brainsci14111097
APA StyleAbdelghafar, A., Falzon, A., Hendriks, E. J., Radovanovic, I., Andrade, H., Schaafsma, J. D., & Mosimann, P. J. (2024). Radiological Outcome of Middle Meningeal Artery Embolization in Relation to Chronic Subdural Hematoma Cause and Architecture. Brain Sciences, 14(11), 1097. https://doi.org/10.3390/brainsci14111097





