Mental Stress Classification Based on Selected Electroencephalography Channels Using Correlation Coefficient of Hjorth Parameters
Abstract
:1. Introduction
- We introduce an innovative channel selection approach leveraging the correlation coefficient of Hjorth parameters. This method not only identifies, but also ranks universally significant EEG channels across different subjects, ensuring that the classification accuracy remains uncompromised.
- We introduce a new methodology to extract important features from the general optimal channels.
- We validate and compare the effectiveness of the proposed method with the state-of-the-art channel selection methods.
2. Materials and Methods
2.1. EEG Dataset
2.2. EEG Data Annotation
3. Hjorth Multi-Correlation Coefficient
Correlation Coefficient Measures
| Algorithm 1: Channel selection algorithm based on the correlation coefficient of Hjorth’s parameters. |
Input: = number of participants, K = number of channels = number of trials. Activation . X = NULL, subset channels that highly correlated with class and low correlated to other channels. U = NULL, is general ranked channel set among participants. Result: General Optimal Ranked Channel Set Method: 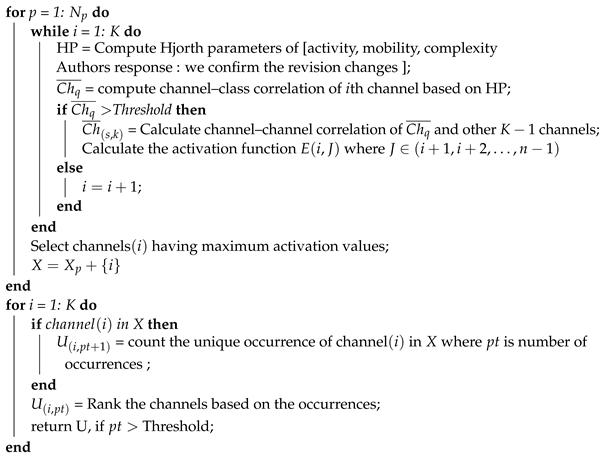 |
4. Feature Extraction
5. Classification
6. Result Analysis and Classification
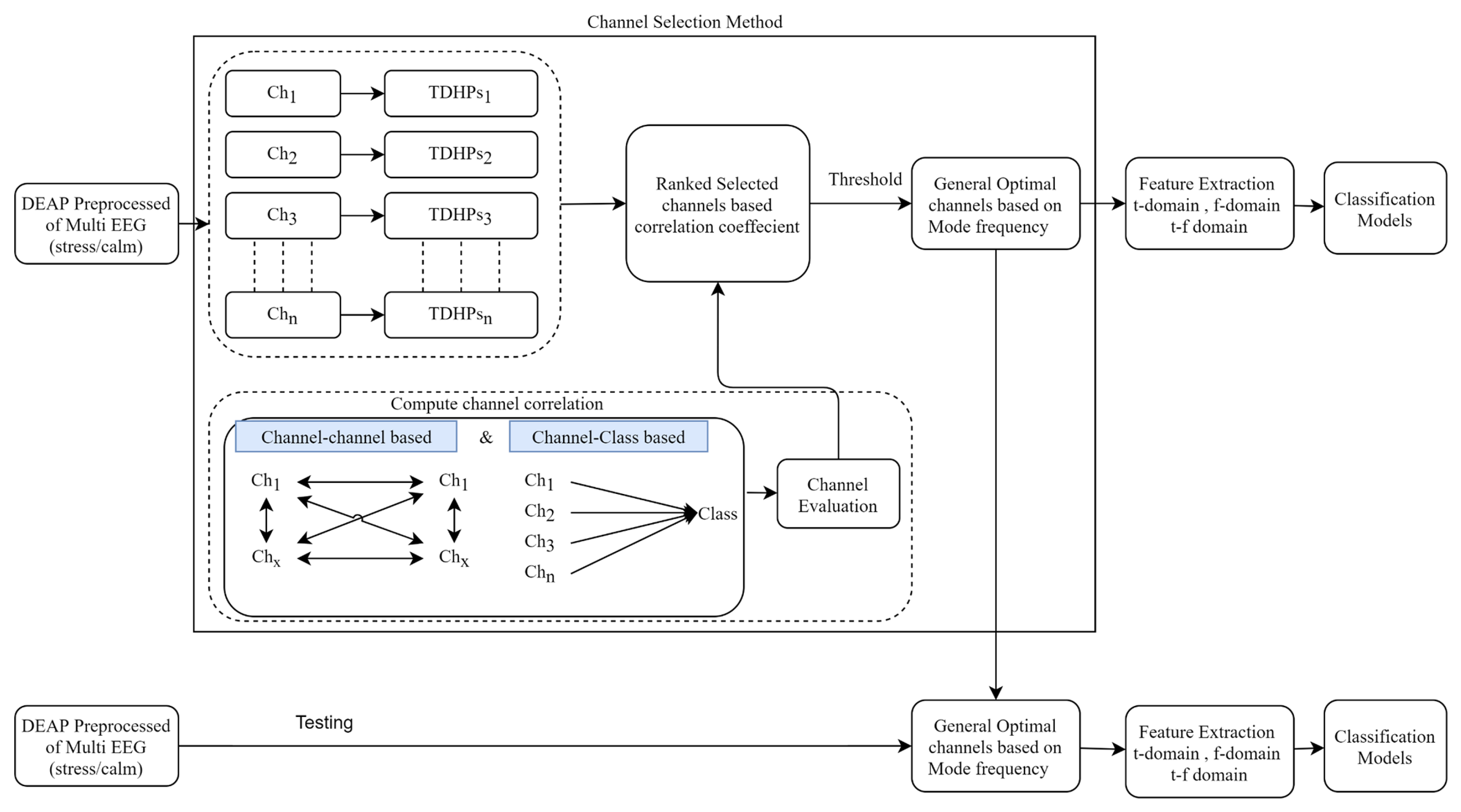
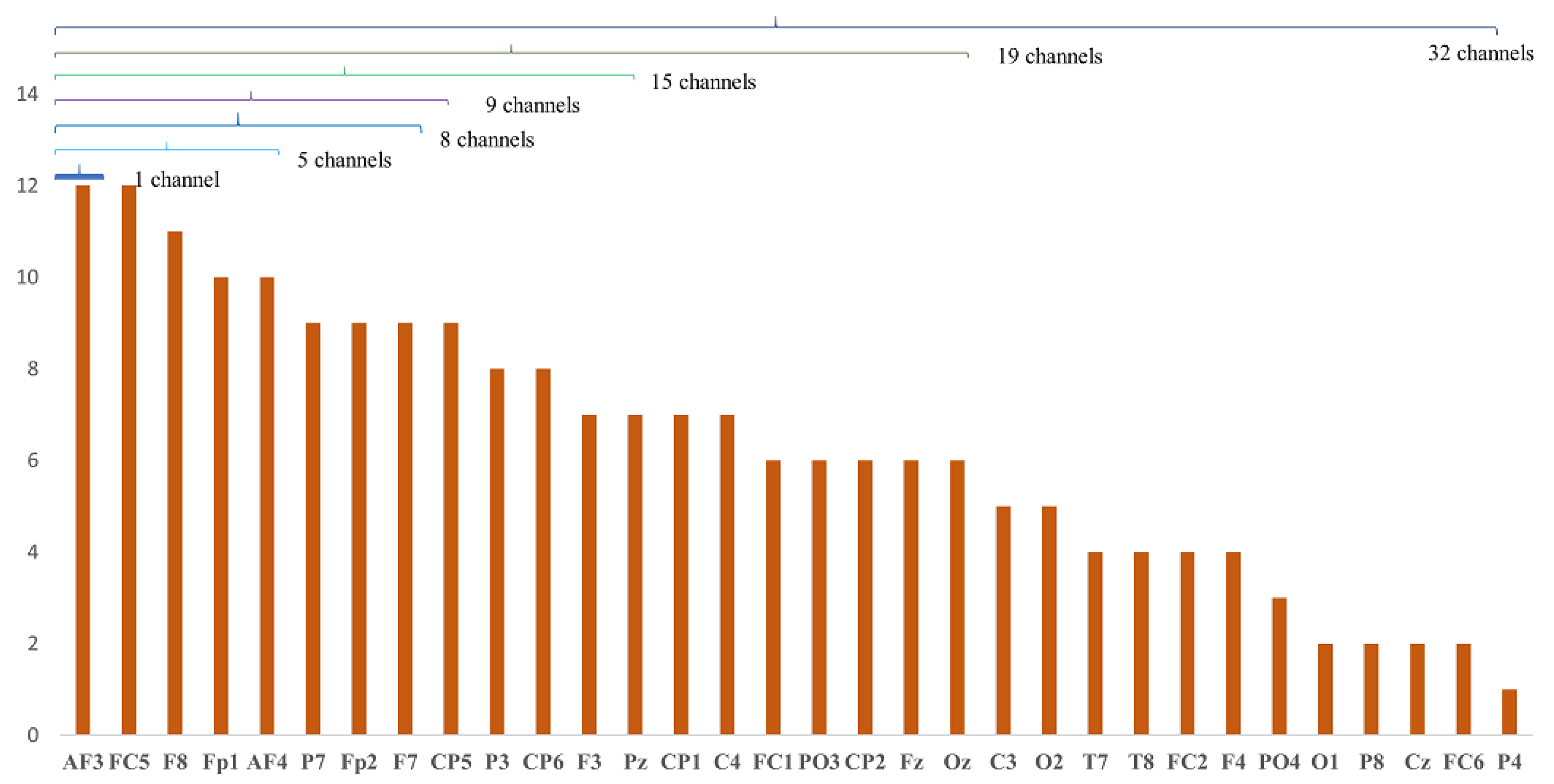
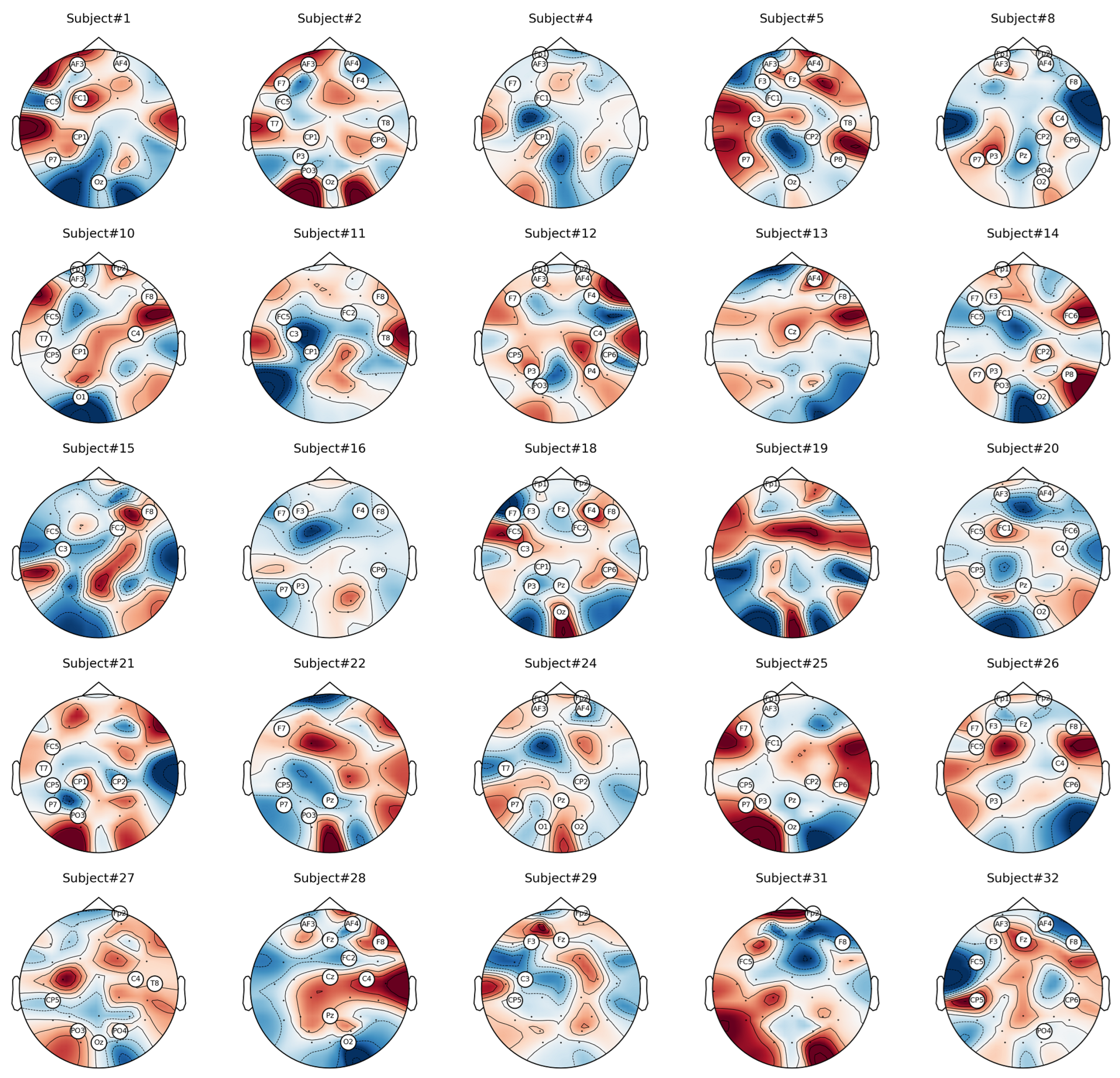
6.1. Analysis of Channel Selection
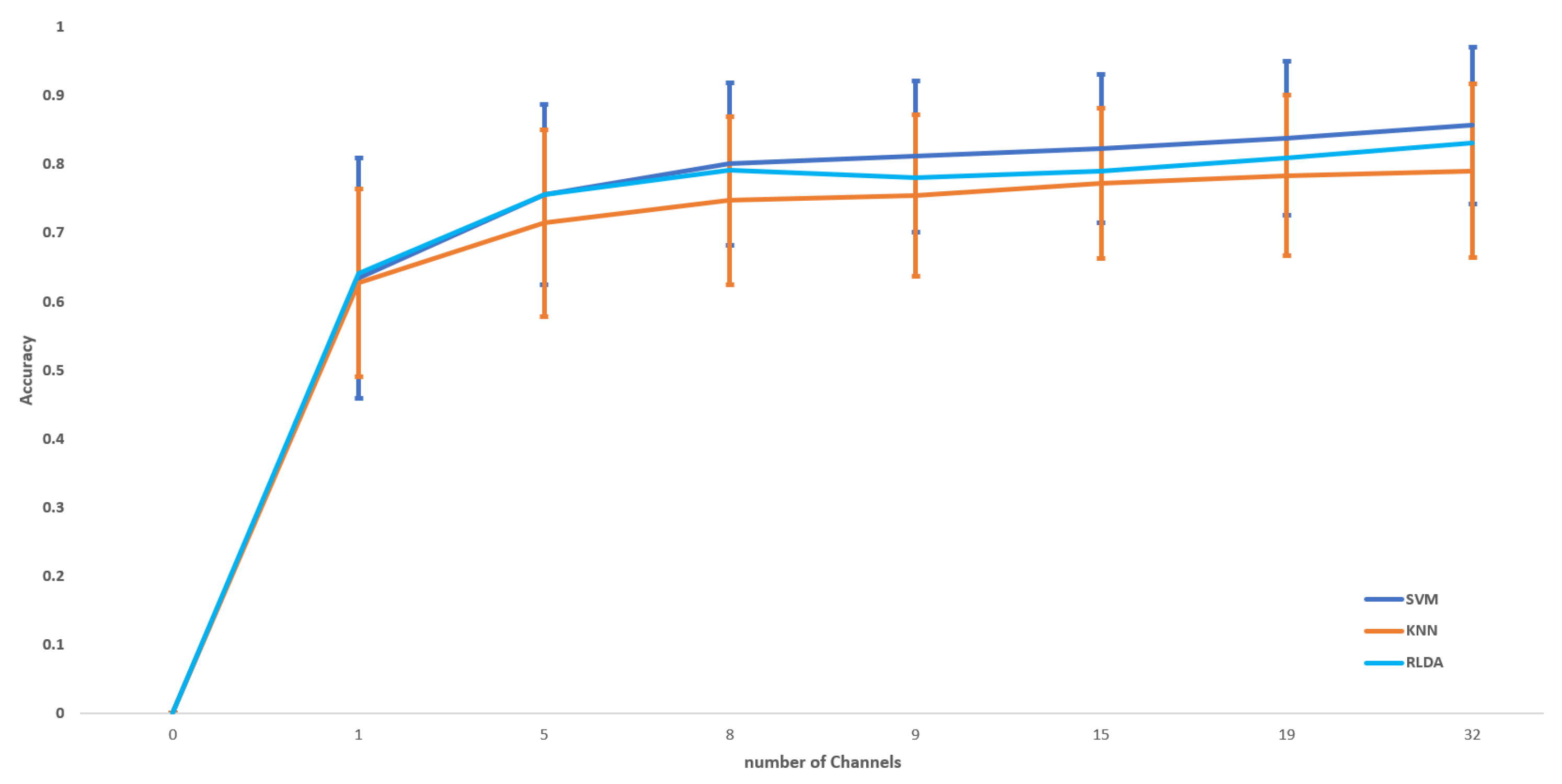
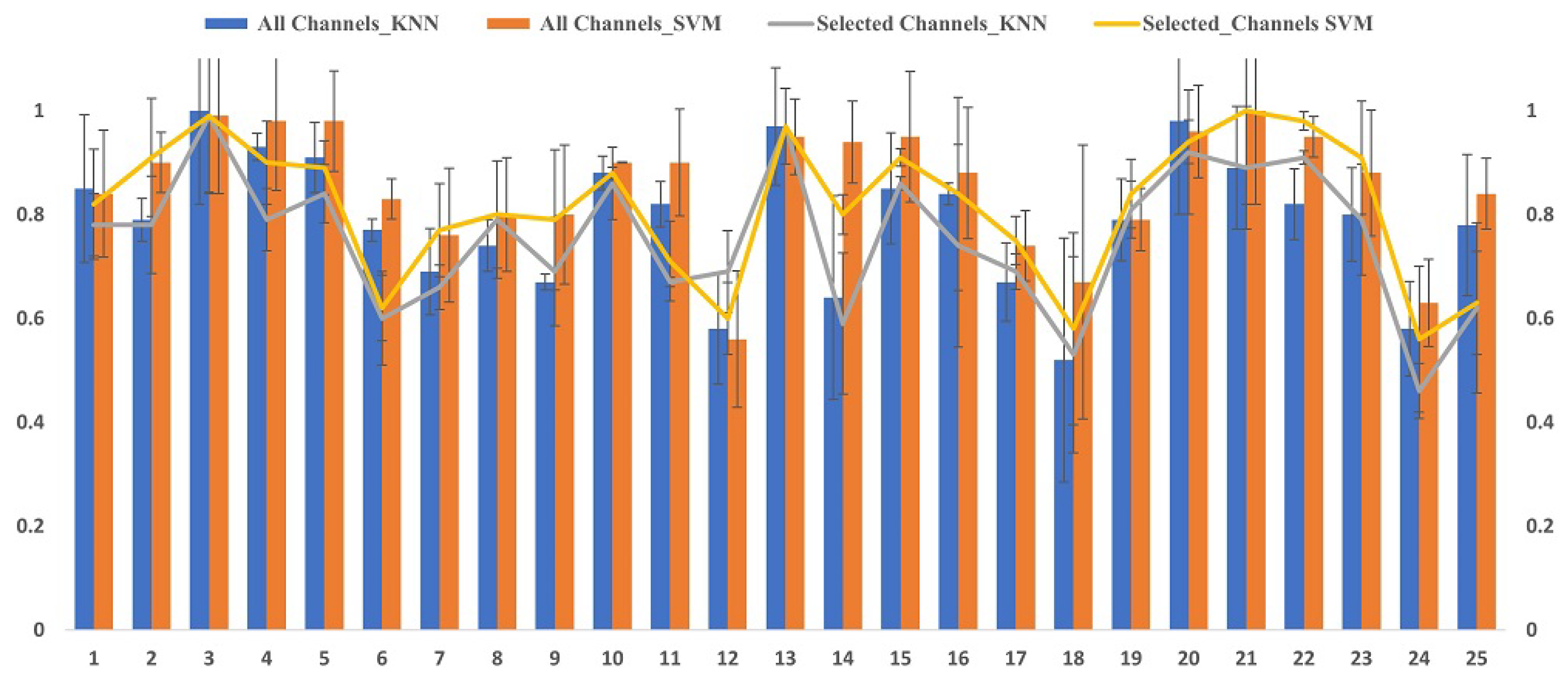
6.2. Classification Results
6.3. Performance Comparison of Mental Stress with Existing Methods in DEAP Dataset
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCHP | Correlation Coefficient of Hjorth Parameter | A novel approach used to determine the correlation between Hjorth parameters for EEG channel selection |
| DEAP | Dataset for Emotion Analysis using Physiological Signals | A widely used dataset for emotion recognition tasks |
| CS | Channel Selection | Technique used to select the most informative EEG channels |
| GOCs | General Optimal Channels | The EEG channels that are found most effective across different subjects |
| PFC | Prefrontal Cortex | The front part of the brain, known to be responsive to stress and involved in cognitive functions |
References
- Dedovic, K.; Renwick, R.; Mahani, N.K.; Engert, V.; Lupien, S.J.; Pruessner, J.C. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry Neurosci. 2005, 30, 319. [Google Scholar] [PubMed]
- Al-Shargie, F.; Tang, T.B.; Kiguchi, M. Stress Assessment Based on Decision Fusion of EEG and fNIRS Signals. IEEE Access 2017, 5, 19889–19896. [Google Scholar] [CrossRef]
- Halim, Z.; Rehan, M. On identification of driving-induced stress using electroencephalogram signals: A framework based on wearable safety-critical scheme and machine learning. Inf. Fusion 2020, 53, 66–79. [Google Scholar] [CrossRef]
- Aspiotis, V.; Miltiadous, A.; Kalafatakis, K.; Tzimourta, K.D.; Giannakeas, N.; Tsipouras, M.G.; Peschos, D.; Glavas, E.; Tzallas, A.T. Assessing Electroencephalography as a Stress Indicator: A VR High-Altitude Scenario Monitored through EEG and ECG. Sensors 2022, 22, 5792. [Google Scholar] [CrossRef] [PubMed]
- Peksa, J.; Mamchur, D. State-of-the-Art on Brain-Computer Interface Technology. Sensors 2023, 23, 6001. [Google Scholar] [CrossRef]
- Sharif, M.S.; Raj Theeng Tamang, M.; Fu, C.H.Y.; Baker, A.; Alzahrani, A.I.; Alalwan, N. An Innovative Random-Forest-Based Model to Assess the Health Impacts of Regular Commuting Using Non-Invasive Wearable Sensors. Sensors 2023, 23, 3274. [Google Scholar] [CrossRef]
- Xin, Q.; Hu, S.; Liu, S.; Zhao, L.; Zhang, Y.D. An Attention-Based Wavelet Convolution Neural Network for Epilepsy EEG Classification. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 957–966. [Google Scholar] [CrossRef]
- Hashempour, S.; Boostani, R.; Mohammadi, M.; Sanei, S. Continuous Scoring of Depression From EEG Signals via a Hybrid of Convolutional Neural Networks. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 176–183. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Y.; Jiang, J.; Lucas, M.V.; Fonzo, G.A.; Rolle, C.E.; Cooper, C.; Chin-Fatt, C.; Krepel, N.; Cornelssen, C.A.; et al. An electroencephalographic signature predicts antidepressant response in major depression. Nat. Biotechnol. 2020, 38, 439–447. [Google Scholar] [CrossRef]
- Song, Y.; Yin, Y.; Xu, P. A Customized ECA-CRNN Model for Emotion Recognition Based on EEG Signals. Electronics 2023, 12, 2900. [Google Scholar] [CrossRef]
- Al-Shargie, F.; Tang, T.B.; Badruddin, N.; Kiguchi, M. Towards multilevel mental stress assessment using SVM with ECOC: An EEG approach. Med. Biol. Eng. Comput. 2018, 56, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Hag, A.; Handayani, D.; Pillai, T.; Mantoro, T.; Kit, M.H.; Al-Shargie, F. A wearable single EEG channel analysis for mental stress state detection. In Proceedings of the 2021 IEEE 7th International Conference on Computing, Engineering and Design (ICCED), Sukabumi, Indonesia, 5–6 August 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Hag, A.; Handayani, D.; Altalhi, M.; Pillai, T.; Mantoro, T.; Kit, M.H.; Al-Shargie, F. Enhancing EEG-Based Mental Stress State Recognition Using an Improved Hybrid Feature Selection Algorithm. Sensors 2021, 21, 8370. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Yin, E.; Jiang, J.; Zhou, Z.; Hu, D. An Asynchronous Hybrid Spelling Approach Based on EEG–EOG Signals for Chinese Character Input. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Chung, W. Optimal Channel Selection Using Correlation Coefficient for CSP Based EEG Classification. IEEE Access 2020, 8, 111514–111521. [Google Scholar] [CrossRef]
- Narayanan, A.M.; Patrinos, P.; Bertrand, A. Optimal versus Approximate Channel Selection Methods for EEG Decoding with Application to Topology-Constrained Neuro-Sensor Networks. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 92–102. [Google Scholar] [CrossRef]
- Gaur, P.; McCreadie, K.; Pachori, R.B.; Wang, H.; Prasad, G. An automatic subject specific channel selection method for enhancing motor imagery classification in EEG-BCI using correlation. Biomed. Signal Process. Control 2021, 68, 102574. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, X.; Huang, X.; Wu, M.; Gao, J.; Lu, D.; Ding, Z.; Hu, B. An Optimal Channel Selection for EEG-Based Depression Detection via Kernel-Target Alignment. IEEE J. Biomed. Health Inform. 2021, 25, 2545–2556. [Google Scholar] [CrossRef]
- Yin, X.; Meng, M.; She, Q.; Gao, Y.; Luo, Z. Optimal channel-based sparse time-frequency blocks common spatial pattern feature extraction method for motor imagery classification. Math. Biosci. Eng. 2021, 18, 4247–4263. [Google Scholar] [CrossRef]
- Qi, F.; Wu, W.; Yu, Z.L.; Gu, Z.; Wen, Z.; Yu, T.; Li, Y. Spatiotemporal-Filtering-Based Channel Selection for Single-Trial EEG Classification. IEEE Trans. Cybern. 2021, 51, 558–567. [Google Scholar] [CrossRef]
- Qiu, Z.; Jin, J.; Lam, H.K.; Zhang, Y.; Wang, X.; Cichocki, A. Improved SFFS method for channel selection in motor imagery based BCI. Neurocomputing 2016, 207, 519–527. [Google Scholar] [CrossRef]
- Wang, Z.M.; Hu, S.Y.; Song, H. Channel Selection Method for EEG Emotion Recognition Using Normalized Mutual Information. IEEE Access 2019, 7, 143303–143311. [Google Scholar] [CrossRef]
- Bavkar, S.; Iyer, B.; Deosarkar, S. Optimal EEG channels selection for alcoholism screening using EMD domain statistical features and harmony search algorithm. Biocybern. Biomed. Eng. 2021, 41, 83–96. [Google Scholar] [CrossRef]
- Jin, J.; Miao, Y.; Daly, I.; Zuo, C.; Hu, D.; Cichocki, A. Correlation-based channel selection and regularized feature optimization for MI-based BCI. Neural Netw. 2019, 118, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Y.; Chen, K.; Wang, L.; Li, Z.; Ai, Q.; Ma, L. Research on Channel Selection and Multi-Feature Fusion of EEG Signals for Mental Fatigue Detection. Entropy 2021, 23, 457. [Google Scholar] [CrossRef]
- Oh, S.H.; Lee, Y.R.; Kim, H.N. A Novel EEG Feature Extraction Method Using Hjorth Parameter. Int. J. Electr. Electron. Eng. 2014, 2, 106–110. [Google Scholar] [CrossRef]
- Hjorth, B. EEG analysis based on time domain properties. Electroencephalogr. Clin. Neurophysiol. 1970, 29, 306–310. [Google Scholar] [CrossRef]
- Leite, J.P.R. Heartbeat classification with low computational cost using Hjorth parameters. IET Signal Process. 2018, 12, 431–438. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sarkar, A.K.; Hossain, M.A.; Hossain, M.S.; Islam, M.R.; Hossain, M.B.; Quinn, J.M.; Moni, M.A. Recognition of human emotions using EEG signals: A review. Comput. Biol. Med. 2021, 136, 104696. [Google Scholar] [CrossRef]
- Safi, M.S.; Safi, S.M.M. Early detection of Alzheimer’s disease from EEG signals using Hjorth parameters. Biomed. Signal Process. Control 2021, 65, 102338. [Google Scholar] [CrossRef]
- Jin, L.; Qu, H.; Pang, L.; Zhang, Z. Sensitive Channel Selection for Mental Workload Classification. Mathematics 2022, 10, 2266. [Google Scholar] [CrossRef]
- Moctezuma, L.A.; Molinas, M. EEG channel-selection method for epileptic-seizure classification based on multi-objective optimization. Front. Neurosci. 2020, 14, 593. [Google Scholar] [CrossRef] [PubMed]
- Malviya, L.; Mal, S. A novel technique for stress detection from EEG signal using hybrid deep learning model. Neural Comput. Appl. 2022, 34, 19819–19830. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Koike, Y. Sparse Logistic Regression-Based EEG Channel Optimization Algorithm for Improved Universality across Participants. Bioengineering 2023, 10, 664. [Google Scholar] [CrossRef]
- Hasan, M.A.; Khan, M.U.; Mishra, D. A computationally efficient method for hybrid EEG-fNIRS BCI based on the Pearson correlation. BioMed Res. Int. 2020, 2020, 1838140. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, R. Electroencephalogram channel selection based on pearson correlation coefficient for motor imagery-brain-computer interface. Meas. Sens. 2023, 25, 100616. [Google Scholar]
- Yu, J.; Yu, Z.L. Cross-correlation based discriminant criterion for channel selection in motor imagery BCI systems. J. Neural Eng. 2021, 18, 046083. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xiao, R.; Daly, I.; Miao, Y.; Wang, X.; Cichocki, A. Internal Feature Selection Method of CSP Based on L1-Norm and Dempster-Shafer Theory. IEEE Trans. Neural Netw. Learn. Syst. 2020, 32, 4814–4825. [Google Scholar] [CrossRef]
- Koelstra, S.; Mühl, C.; Soleymani, M.; Lee, J.S.; Yazdani, A.; Ebrahimi, T.; Pun, T.; Nijholt, A.; Patras, I. DEAP: A database for emotion analysis; Using physiological signals. IEEE Trans. Affect. Comput. 2012, 3, 18–31. [Google Scholar] [CrossRef]
- Morris, J.D. Observations: SAM: The Self-Assessment Manikin—An Efficient Cross-Cultural Measurement of Emotional Response. J. Advert. Res. 1995, 35, 63–68. [Google Scholar]
- Hasan, M.J.; Kim, J.M. A hybrid feature pool-based emotional stress state detection algorithm using EEG signals. Brain Sci. 2019, 9, 376. [Google Scholar] [CrossRef]
- Shon, D.; Im, K.; Park, J.H.; Lim, D.S.; Jang, B.; Kim, J.M. Emotional Stress State Detection Using Genetic Algorithm-Based Feature Selection on EEG Signals. Int. J. Environ. Res. Public Health 2018, 15, 2461. [Google Scholar] [CrossRef] [PubMed]
- Esteller, R.; Vachtsevanos, G.; Echauz, J.; Litt, B. A Comparison of waveform fractal dimension algorithms. IEEE Trans. Circuits Syst. I Fundam. Theory Appl. 2001, 48, 177–183. [Google Scholar] [CrossRef]
- Boonyakitanont, P.; Lek-uthai, A.; Chomtho, K.; Songsiri, J. A review of feature extraction and performance evaluation in epileptic seizure detection using EEG. Biomed. Signal Process. Control 2020, 57, 101702. [Google Scholar] [CrossRef]
- Cai, H.; Han, J.; Chen, Y.; Sha, X.; Wang, Z.; Hu, B.; Yang, J.; Feng, L.; Ding, Z.; Chen, Y.; et al. A pervasive approach to EEG-based depression detection. Complexity 2018, 2018, 5238028. [Google Scholar] [CrossRef]
- Subhani, A.R.; Mumtaz, W.; Naufal, M.; Mohamed, B.I.N.; Kamel, N.; Malik, A.S. Machine Learning Framework for the Detection of Mental Stress at Multiple Levels. IEEE Access 2017, 5, 13545–13556. [Google Scholar] [CrossRef]
- Al-shargie, F.; Tang, T.B.; Badruddin, N.; Dass, S.C.; Kiguchi, M. Mental stress assessment based on feature level fusion of fNIRS and EEG signals. In Proceedings of the 2016 6th International Conference on Intelligent and Advanced Systems (ICIAS), Kuala Lumpur, Malaysia, 15–17 August 2016; pp. 1–5. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Xu, W.; Zhang, H.; Yang, L.; Zheng, S.; Shi, Y. Spectral entropy can predict changes of working memory performance reduced by short-time training in the delayed-match-to-sample task. Front. Hum. Neurosci. 2017, 11, 437. [Google Scholar] [CrossRef]
- Yang, Y.; Kyrgyzov, O.; Wiart, J.; Bloch, I. Subject-specific channel selection for classification of motor imagery electroencephalographic data. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Vancouver, BC, Canada, 26–31 May 2013; pp. 1277–1280. [Google Scholar] [CrossRef]
- Candra, H.; Yuwono, M.; Chai, R.; Handojoseno, A.; Elamvazuthi, I.; Nguyen, H.T.; Su, S. Investigation of window size in classification of EEG-emotion signal with wavelet entropy and support vector machine. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS), Milan, Italy, 25–29 August 2015; pp. 7250–7253. [Google Scholar] [CrossRef]
- Jebelli, H.; Hwang, S.; Lee, S.H. EEG-based workers’ stress recognition at construction sites. Autom. Constr. 2018, 93, 315–324. [Google Scholar] [CrossRef]
- Fraschini, M.; Demuru, M.; Crobe, A.; Marrosu, F.; Stam, C.J.; Hillebrand, A. The effect of epoch length on estimated EEG functional connectivity and brain network organisation. J. Neural Eng. 2016, 13, 036015. [Google Scholar] [CrossRef]
- Al-Shargie, F.; Tariq, U.; Alex, M.; Mir, H.; Al-Nashash, H. Emotion Recognition Based on Fusion of Local Cortical Activations and Dynamic Functional Networks Connectivity: An EEG Study. IEEE Access 2019, 7, 143550–143562. [Google Scholar] [CrossRef]
- Alex, M.; Tariq, U.; Al-Shargie, F.; Mir, H.S.; Al Nashash, H. Discrimination of genuine and acted emotional expressions using EEG signal and machine learning. IEEE Access 2020, 8, 191080–191089. [Google Scholar] [CrossRef]
- Hag, A.; Handayani, D.; Pillai, T.; Mantoro, T.; Kit, M.H.; Al-Shargie, F. EEG Mental Stress Assessment Using Hybrid Multi-Domain Feature Sets of Functional Connectivity Network and Time-Frequency Features. Sensors 2021, 21, 6300. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Nariani, D.; Rai, A. Mental Stress Detection using EEG and Recurrent Deep Learning. In Proceedings of the 2023 IEEE Applied Sensing Conference (APSCON), Bengaluru, India, 23–25 January 2023; pp. 1–3. [Google Scholar] [CrossRef]
- Katmah, R.; Al-Shargie, F.; Tariq, U.; Babiloni, F.; Al-Mughairbi, F.; Al-Nashash, H. A Review on Mental Stress Assessment Methods Using EEG Signals. Sensors 2021, 21, 5043. [Google Scholar] [CrossRef] [PubMed]
- Al-Shargie, F.; Tang, T.B.; Badruddin, N.; Kiguchi, M. Simultaneous measurement of EEG-fNIRS in classifying and localizing brain activation to mental stress. In Proceedings of the IEEE 2015 International Conference on Signal and Image Processing Applications (ICSIPA 2015), Kuala Lumpur, Malaysia, 19–21 October 2015; pp. 282–286. [Google Scholar] [CrossRef]
- Al-shargie, F.; Tang, T.B.; Kiguchi, M. Mental stress grading based on fNIRS signals. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 October 2016; pp. 5140–5143. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, L.; Li, R.; He, Z.; Xiao, J.; Liang, Y.; Wang, F.; Pan, J. Enhancing BCI-based emotion recognition using an improved particle swarm optimization for feature selection. Sensors 2020, 20, 3028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Z.; Wang, W. Emotion recognition based on framework of BADEBA-SVM. Math. Probl. Eng. 2019, 2019, 9875250. [Google Scholar] [CrossRef]
- Hussien, A.G.; Oliva, D.; Houssein, E.H.; Juan, A.A.; Yu, X. Binary whale optimization algorithm for dimensionality reduction. Mathematics 2020, 8, 1821. [Google Scholar] [CrossRef]
- Liu, J.; Wu, G.; Luo, Y.; Qiu, S.; Yang, S.; Li, W.; Bi, Y. EEG-Based Emotion Classification Using a Deep Neural Network and Sparse Autoencoder. Front. Syst. Neurosci. 2020, 14, 43. [Google Scholar] [CrossRef]
- Al-Shargie, F.M.; Hassanin, O.; Tariq, U.; Al-Nashash, H. EEG-Based Semantic Vigilance Level Classification Using Directed Connectivity Patterns and Graph Theory Analysis. IEEE Access 2020, 8, 115941–115956. [Google Scholar] [CrossRef]

| Domain | Features | Equations | Description | No. Features |
|---|---|---|---|---|
| Time | Line Length [43,44] | Called curve length, is the total vertical length of the signal | 1 | |
| Kurtosis [45] | Shows the sharpness of EEG signals’ peaks | 1 | ||
| Peak-to-Peak Amplitude | Time of EEG signal peaks between the various windows | 1 | ||
| Skewness [45] | An asymmetry of an EEG signal | 1 | ||
| Hjorth Parameters [42,45] | A variance of the time function; | 1 | ||
| A mean frequency or the proportion of standard deviation of the power spectrum | 1 | |||
| Indicates how the shape of a signal is similar to a pure sine wave | 1 | |||
| Frequency | Relative Power [46] of: | Average absolute power of the given band interval | 5 | |
| theta (4–8 Hz) | ||||
| alpha (8–12 Hz) | ||||
| sigma (12–15 Hz) | ||||
| low beta (15–20 Hz) | ||||
| high beta (20–30 Hz) | ||||
| Time–Frequency | Energy of Wavelet Decomposition Coefficients (db4, 6 level) [11,47] | Measure the square sum of wavelet coefficients of each db level | 6 | |
| Spectral Entropy (PSD, Welch) [48] | Measure the distribution of signal power over frequency | 1 | ||
| Katz’s Fractal Dimension [43] | Compute the maximum distance between the first point and any other point of the signal time window | 1 |
| No. | Classifier | Default Value |
|---|---|---|
| 1 | SVM | C = 1.0, Kernal = Radial Basis Function (RBF), |
| 2 | KNN | K = 10, distance function = euclidean distance |
| 3 | RLDA | solver = eigen, shrinkage = none |
| Subject ID | All Channels | Proposed Channels | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KNN | SVM | RLDA | KNN | SVM | RLDA | |||||||||||||
| Precision | Recall | Accuracy | Precision | Recall | Accuracy | Precision | Recall | Accuracy | Precision | Recall | Accuracy | Precision | Recall | Accuracy | Precision | Recall | Accuracy | |
| 1 | 0.92 | 0.7 | 0.85 | 0.91 | 0.69 | 0.84 | 0.83 | 0.73 | 0.79 | 0.71 | 0.62 | 0.78 | 0.86 | 0.65 | 0.82 | 0.82 | 0.72 | 0.78 |
| 2 | 0.82 | 0.8 | 0.79 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 | 0.84 | 0.8 | 0.78 | 0.91 | 0.91 | 0.91 | 0.89 | 0.89 | 0.89 |
| 4 | 1 | 1 | 1 | 0.99 | 0.99 | 0.99 | 1 | 1 | 1 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.95 | 0.95 | 0.95 |
| 5 | 0.94 | 0.93 | 0.93 | 0.98 | 0.98 | 0.98 | 0.84 | 0.84 | 0.85 | 0.8 | 0.79 | 0.79 | 0.9 | 0.9 | 0.9 | 0.84 | 0.82 | 0.82 |
| 8 | 0.91 | 0.92 | 0.91 | 0.98 | 0.97 | 0.98 | 0.92 | 0.92 | 0.92 | 0.83 | 0.84 | 0.84 | 0.92 | 0.86 | 0.89 | 0.92 | 0.86 | 0.89 |
| 10 | 0.79 | 0.77 | 0.77 | 0.83 | 0.83 | 0.83 | 0.63 | 0.63 | 0.63 | 0.6 | 0.6 | 0.6 | 0.62 | 0.62 | 0.62 | 0.62 | 0.62 | 0.62 |
| 11 | 0.69 | 0.71 | 0.69 | 0.75 | 0.71 | 0.76 | 0.89 | 0.8 | 0.84 | 0.67 | 0.68 | 0.66 | 0.76 | 0.71 | 0.77 | 0.76 | 0.71 | 0.77 |
| 12 | 0.67 | 0.65 | 0.74 | 0.81 | 0.64 | 0.8 | 0.75 | 0.71 | 0.76 | 0.74 | 0.7 | 0.79 | 0.76 | 0.68 | 0.8 | 0.76 | 0.7 | 0.76 |
| 13 | 0.55 | 0.55 | 0.67 | 0.78 | 0.58 | 0.8 | 0.84 | 0.82 | 0.82 | 0.55 | 0.54 | 0.69 | 0.76 | 0.57 | 0.79 | 0.55 | 0.54 | 0.69 |
| 14 | 0.79 | 0.73 | 0.88 | 0.95 | 0.67 | 0.9 | 0.86 | 0.71 | 0.8 | 0.74 | 0.76 | 0.86 | 0.83 | 0.65 | 0.88 | 0.83 | 0.65 | 0.88 |
| 15 | 0.84 | 0.82 | 0.82 | 0.9 | 0.9 | 0.9 | 1 | 1 | 1 | 0.88 | 0.86 | 0.87 | 0.88 | 0.86 | 0.87 | 0.88 | 0.86 | 0.87 |
| 17 | 0.78 | 0.79 | 0.79 | 0.9 | 0.9 | 0.9 | 0.93 | 0.88 | 0.92 | 0.81 | 0.81 | 0.81 | 0.9 | 0.89 | 0.89 | 0.9 | 0.88 | 0.89 |
| 18 | 0.68 | 0.65 | 0.78 | 0.86 | 0.78 | 0.85 | 0.74 | 0.71 | 0.79 | 0.68 | 0.65 | 0.77 | 0.73 | 0.67 | 0.77 | 0.69 | 0.66 | 0.78 |
| 19 | 0.87 | 0.86 | 0.87 | 0.89 | 0.86 | 0.89 | 0.88 | 0.88 | 0.88 | 0.89 | 0.86 | 0.88 | 0.89 | 0.86 | 0.88 | 0.89 | 0.86 | 0.88 |
| 20 | 0.71 | 0.67 | 0.77 | 0.81 | 0.69 | 0.8 | 0.82 | 0.69 | 0.78 | 0.75 | 0.67 | 0.74 | 0.77 | 0.71 | 0.8 | 0.77 | 0.69 | 0.77 |
| 21 | 0.79 | 0.77 | 0.82 | 0.92 | 0.88 | 0.91 | 0.88 | 0.88 | 0.88 | 0.79 | 0.77 | 0.82 | 0.82 | 0.8 | 0.83 | 0.83 | 0.8 | 0.83 |
| 22 | 0.78 | 0.76 | 0.83 | 0.89 | 0.88 | 0.89 | 0.89 | 0.88 | 0.89 | 0.82 | 0.8 | 0.82 | 0.84 | 0.83 | 0.84 | 0.83 | 0.83 | 0.83 |
| 23 | 0.87 | 0.86 | 0.87 | 0.93 | 0.88 | 0.92 | 0.88 | 0.87 | 0.88 | 0.87 | 0.86 | 0.87 | 0.87 | 0.86 | 0.87 | 0.87 | 0.86 | 0.87 |
| 24 | 0.78 | 0.73 | 0.83 | 0.82 | 0.73 | 0.82 | 0.75 | 0.72 | 0.76 | 0.75 | 0.71 | 0.75 | 0.74 | 0.72 | 0.74 | 0.74 | 0.72 | 0.74 |
| 25 | 0.91 | 0.89 | 0.91 | 0.96 | 0.93 | 0.94 | 0.89 | 0.89 | 0.89 | 0.89 | 0.89 | 0.89 | 0.89 | 0.89 | 0.89 | 0.89 | 0.89 | 0.89 |
| 26 | 0.89 | 0.89 | 0.89 | 0.97 | 0.93 | 0.95 | 0.91 | 0.91 | 0.91 | 0.89 | 0.89 | 0.89 | 0.92 | 0.91 | 0.92 | 0.89 | 0.89 | 0.89 |
| 27 | 0.74 | 0.75 | 0.76 | 0.85 | 0.83 | 0.85 | 0.85 | 0.84 | 0.85 | 0.72 | 0.72 | 0.75 | 0.74 | 0.74 | 0.75 | 0.75 | 0.75 | 0.75 |
| 28 | 0.76 | 0.75 | 0.81 | 0.9 | 0.83 | 0.88 | 0.89 | 0.85 | 0.88 | 0.73 | 0.72 | 0.78 | 0.76 | 0.76 | 0.81 | 0.76 | 0.76 | 0.81 |
| 29 | 0.87 | 0.85 | 0.88 | 0.92 | 0.92 | 0.92 | 0.89 | 0.89 | 0.89 | 0.85 | 0.85 | 0.85 | 0.89 | 0.89 | 0.89 | 0.89 | 0.89 | 0.89 |
| 30 | 0.72 | 0.72 | 0.72 | 0.77 | 0.77 | 0.77 | 0.76 | 0.76 | 0.76 | 0.72 | 0.72 | 0.72 | 0.73 | 0.72 | 0.73 | 0.73 | 0.72 | 0.73 |
| 31 | 0.72 | 0.7 | 0.77 | 0.84 | 0.77 | 0.83 | 0.84 | 0.8 | 0.82 | 0.74 | 0.73 | 0.74 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 |
| 32 | 0.85 | 0.71 | 0.78 | 0.89 | 0.8 | 0.84 | 0.84 | 0.82 | 0.82 | 0.57 | 0.54 | 0.62 | 0.6 | 0.58 | 0.69 | 0.67 | 0.67 | 0.63 |
| Average | 0.76 | 0.73 | 0.79 | 0.85 | 0.79 | 0.85 | 0.83 | 0.80 | 0.82 | 0.71 | 0.70 | 0.75 | 0.80 | 0.76 | 0.81 | 0.77 | 0.74 | 0.79 |
| Method | No. Channels | Channel Subsets | Classifier | Accuracy | Execution Time |
|---|---|---|---|---|---|
| mRMR | 11 | ‘C4’, ‘FC2’, ‘CP6’, ‘Cz’, ‘T8’, ‘F4’, ‘F8’, ‘P4’, ‘Fz’, ‘FC6’, ‘Pz’ | SVM | 0.80 ± 0.12 | 1.42 s |
| KNN | 0.74 ± 0.12 | ||||
| RLDA | 0.79 ± 0.13 | ||||
| STFT + MI | 15 | ‘AF3’, ‘F7’, ‘FC5’, ‘P3’, ‘P7’, ‘Pz’, ‘O2’, ‘P4’, ‘FC6’, ‘Fp2’, ‘FC1’, ‘CP2’, ’C4’, ‘F4’, ‘Fz’ | SVM | 0.82 ± 0.11 | 4.46 s |
| KNN | 0.74 ± 0.12 | ||||
| RLDA | 0.80 ± 0.14 | ||||
| GA | 13 | ‘O2’, ‘O1’, ‘PO3’, ‘AF3’, ‘P4’, ‘P8’, ‘F8’, ‘P7’, ‘C4’, ‘CP5’, ‘Pz’, ‘FC5’, ‘Fp2’ | SVM | 0.82 ± 0.12 | 1 h 3 min 34 s |
| KNN | 0.76 ± 0.13 | ||||
| RLDA | 0.81 ± 0.13 | ||||
| Proposed | 8 | ‘AF3’, ‘FC5’, ‘F8’, ‘Fp1’, ‘AF4’, ‘P7’, ‘Fp2’, ‘F7’ | SVM | 0.81 ± 0.11 | 0.34 s |
| KNN | 0.75 ± 0.12 | ||||
| RLDA | 0.79 ± 0.12 |
| Author | Method | EEG Channels | Accuracy/Class |
|---|---|---|---|
| Shon [42] | Genetic Algorithm-Based Feature Selection | 32 | 71.76% (Stress/Calm) |
| Hasan [41] | Boruta-based k-NN feature selection | 32 | 73.38% (Stress/Calm) |
| Patel [56] | CONV1D + BiLSTM | 14 | 88.03% (Valance–Arousal Score Level) |
| CONV1D + BiGRU | 14 | 75% (Stress/Calm) | |
| Proposed | Full Channels SET + SVM | 32 | 85.68% (Stress/Calm) |
| CCHP + SVM | 8 | 81.56% (Stress/Calm) |
| Dataset | Channels | No. Channels | Accuracy |
|---|---|---|---|
| EDMSS | Total | 7 | 77.31% |
| Selected | 5 | 75.23% | |
| DEAP | Total | 32 | 85.68% |
| Selected | 8 | 81.56% | |
| SEED | Total | 62 | 83.21% |
| Selected | 8 | 80.31% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hag, A.; Al-Shargie, F.; Handayani, D.; Asadi, H. Mental Stress Classification Based on Selected Electroencephalography Channels Using Correlation Coefficient of Hjorth Parameters. Brain Sci. 2023, 13, 1340. https://doi.org/10.3390/brainsci13091340
Hag A, Al-Shargie F, Handayani D, Asadi H. Mental Stress Classification Based on Selected Electroencephalography Channels Using Correlation Coefficient of Hjorth Parameters. Brain Sciences. 2023; 13(9):1340. https://doi.org/10.3390/brainsci13091340
Chicago/Turabian StyleHag, Ala, Fares Al-Shargie, Dini Handayani, and Houshyar Asadi. 2023. "Mental Stress Classification Based on Selected Electroencephalography Channels Using Correlation Coefficient of Hjorth Parameters" Brain Sciences 13, no. 9: 1340. https://doi.org/10.3390/brainsci13091340
APA StyleHag, A., Al-Shargie, F., Handayani, D., & Asadi, H. (2023). Mental Stress Classification Based on Selected Electroencephalography Channels Using Correlation Coefficient of Hjorth Parameters. Brain Sciences, 13(9), 1340. https://doi.org/10.3390/brainsci13091340








