Effects of Damage to the Integrity of the Left Dual-Stream Frontotemporal Network Mediated by the Arcuate Fasciculus and Uncinate Fasciculus on Acute/Subacute Post-Stroke Aphasia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Language Assessment
2.3. Image Data Acquisition and Pre-Processing
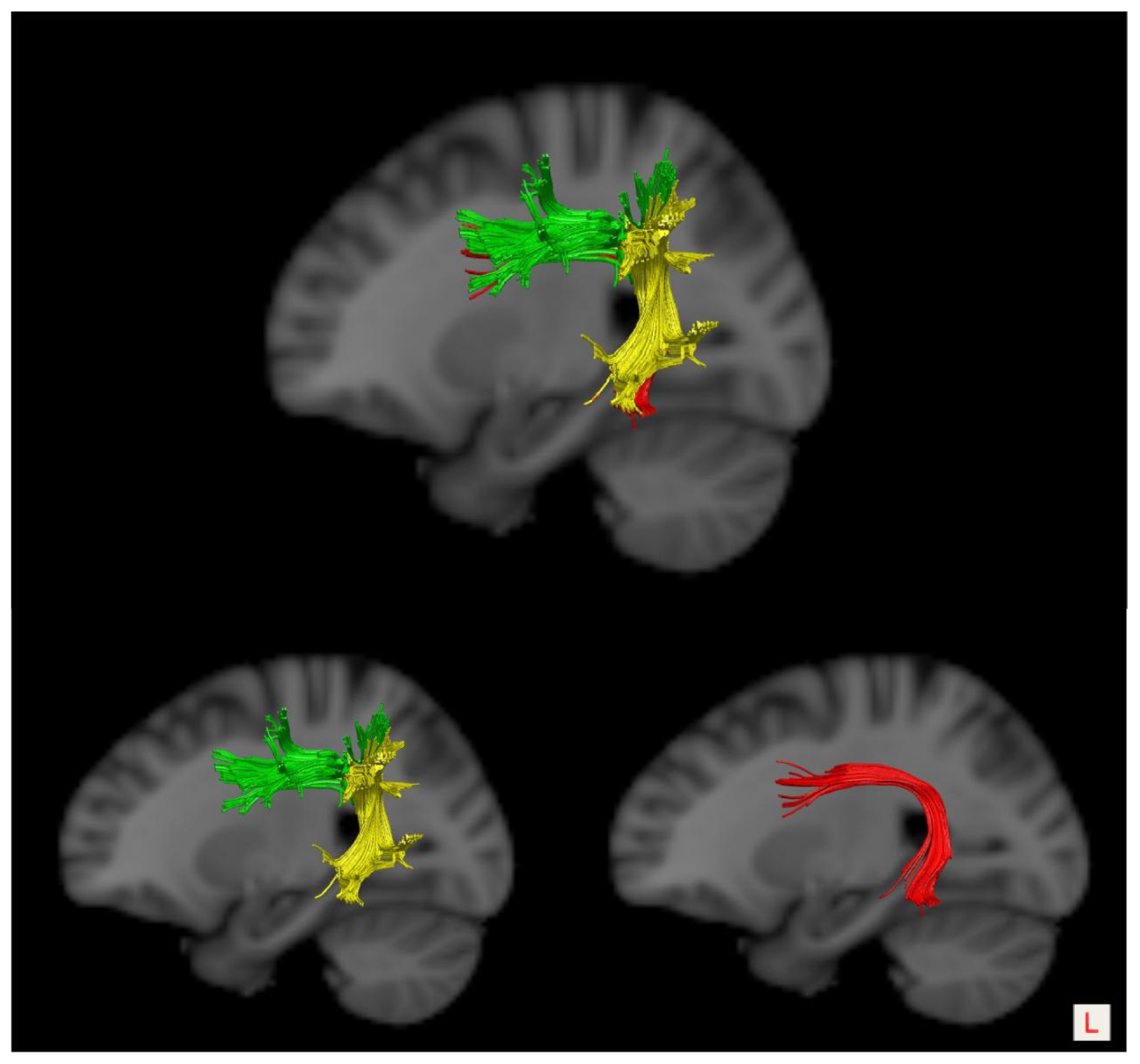
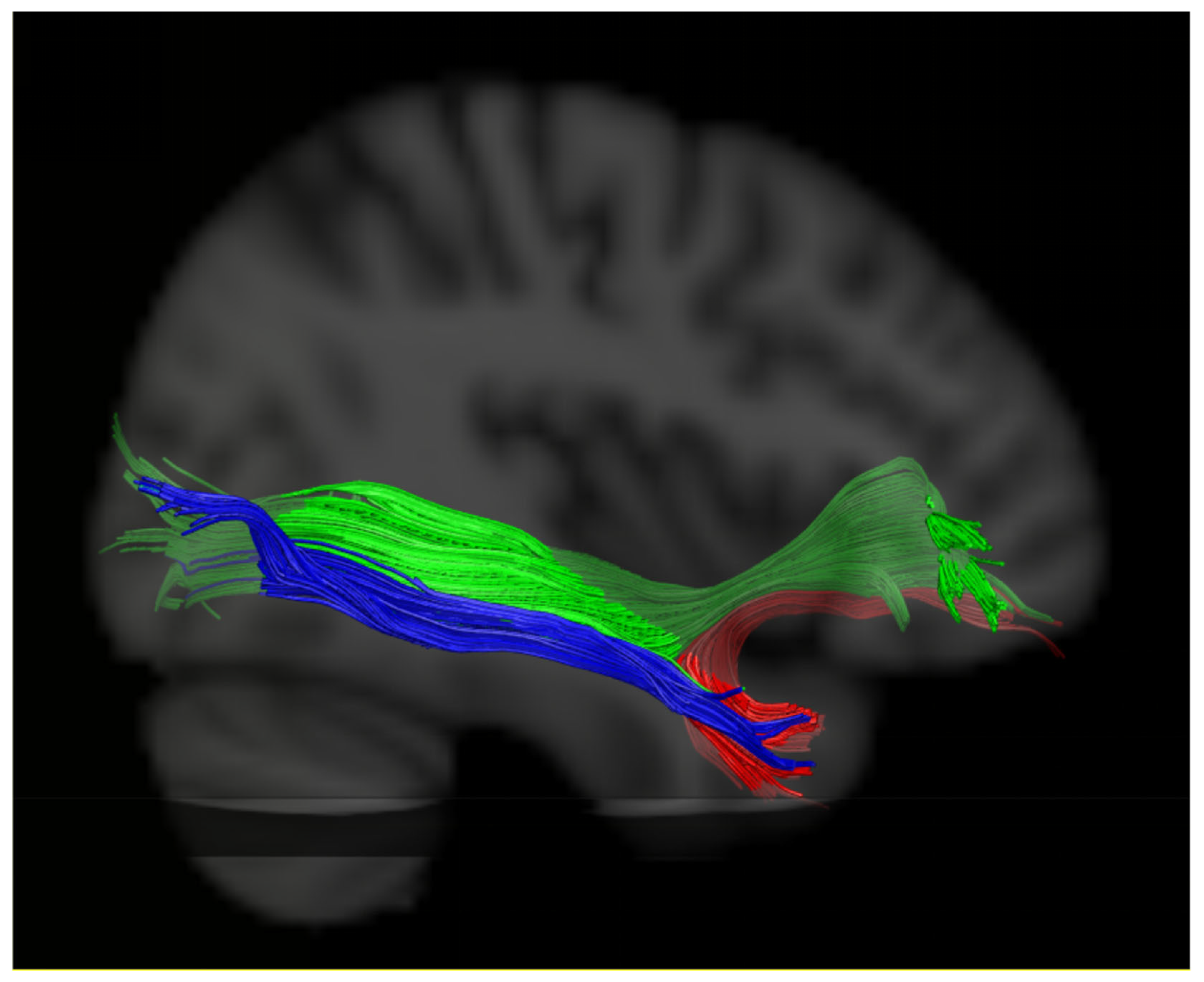
2.4. DTI Tractography
2.5. Evaluation of the White Matter Pathways
2.6. Statistical Analyses
3. Results
3.1. General Demographic and Clinical Characteristics
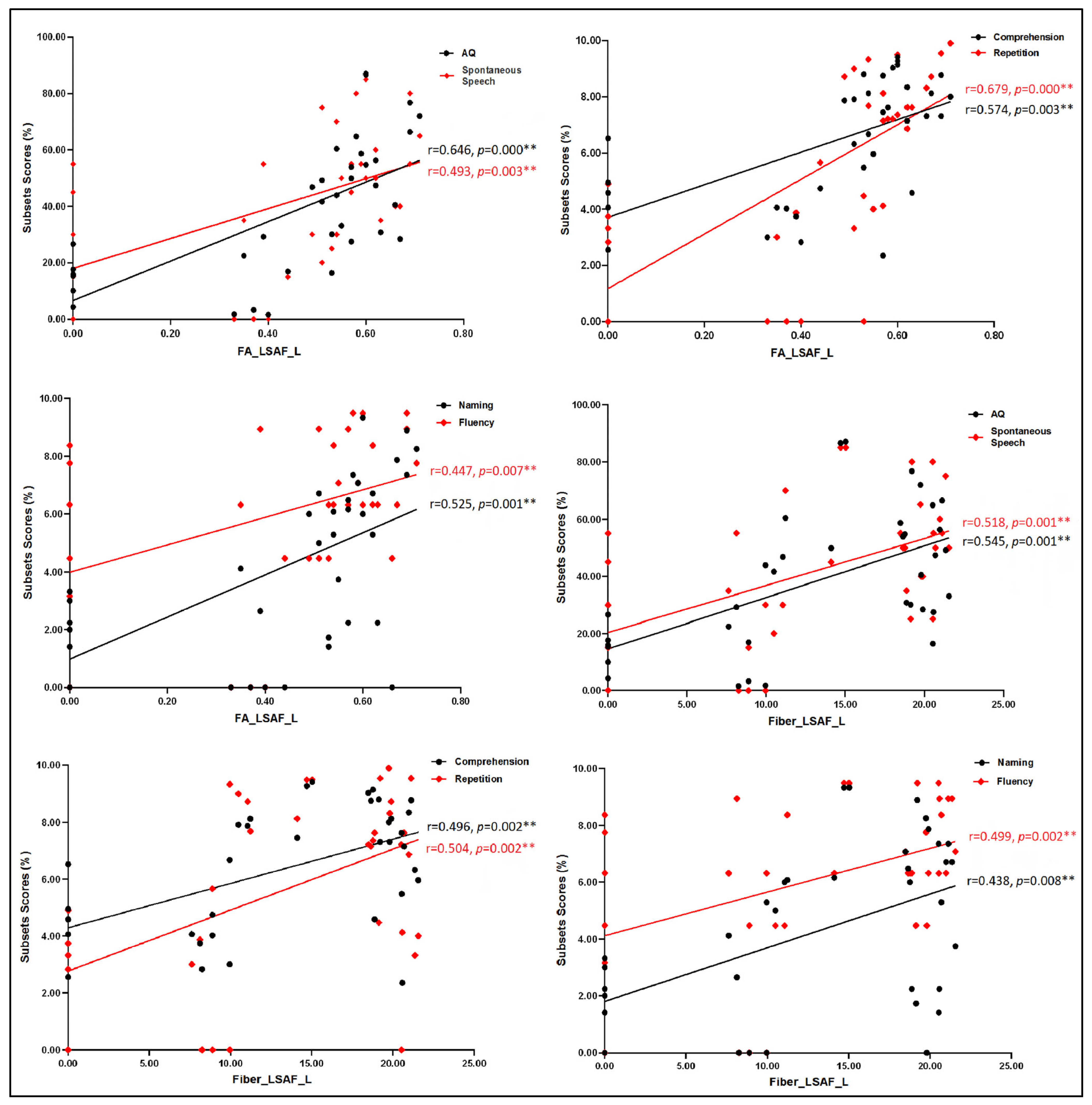
3.2. Correlation Analyses between Language Assessment and MRI Measures
3.3. Multiple Linear Regression Analysis
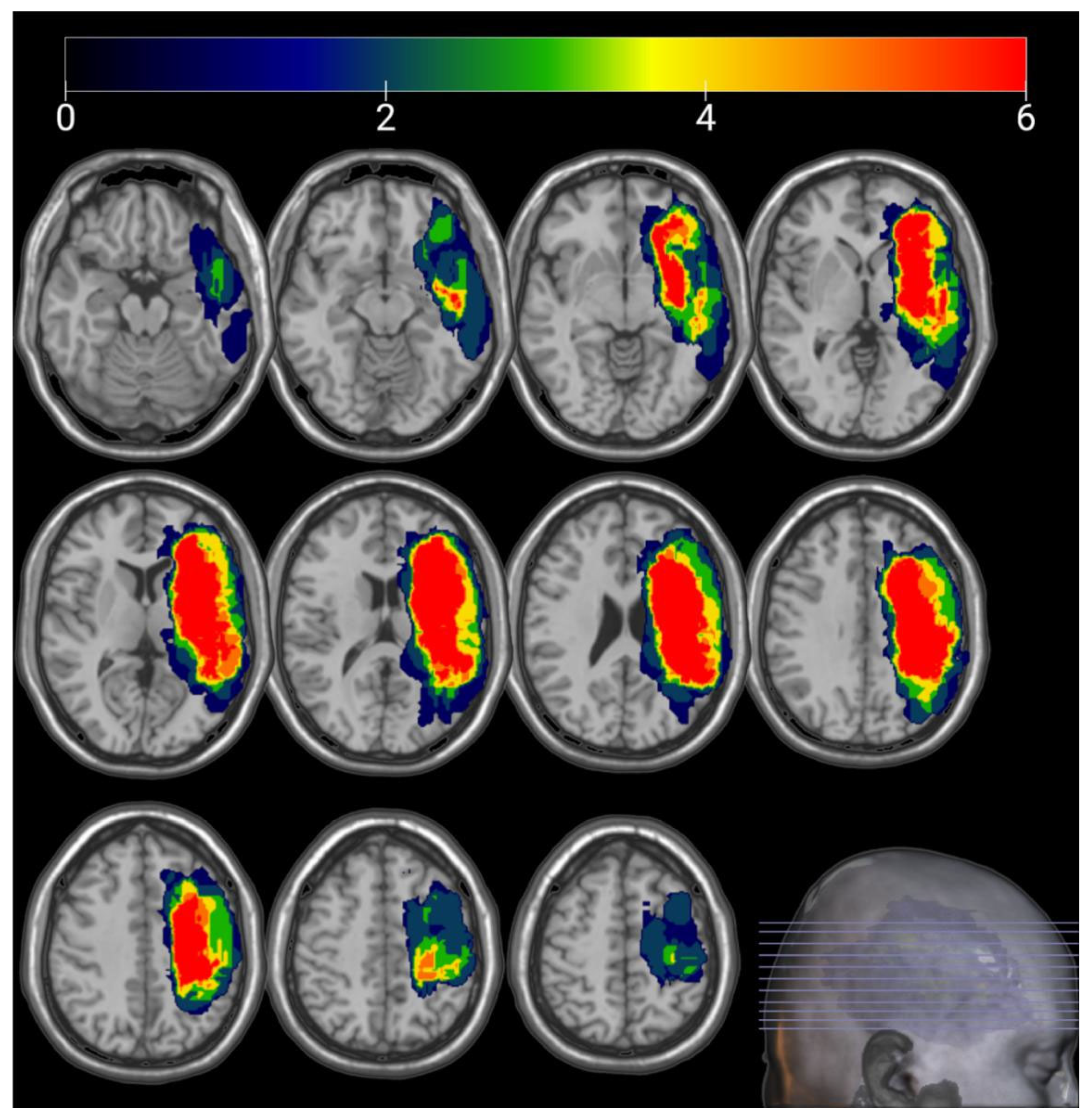
3.4. Lesion Overlay Map
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stockert, A.; Wawrzyniak, M.; Klingbeil, J.; Wrede, K.; Kümmerer, D.; Hartwigsen, G.; Kaller, C.P.; Weiller, C.; Saur, D. Dynamics of language reorganization after left temporo-parietal and frontal stroke. Brain 2020, 143, 844–861. [Google Scholar] [CrossRef] [PubMed]
- Cocquyt, E.M.; Lanckmans, E.; van Mierlo, P.; Duyck, W.; Szmalec, A.; Santens, P.; De Letter, M. The white matter architecture underlying semantic processing: A systematic review. Neuropsychologia 2020, 136, 107182. [Google Scholar] [CrossRef] [PubMed]
- Cocquyt, E.M.; Coffe, C.; van Mierlo, P.; Duyck, W.; Marien, P.; Szmalec, A.; Santens, P.; De Letter, M. The involvement of subcortical grey matter in verbal semantic comprehension: A systematic review and meta-analysis of fmri and pet studies. J. Neurolinguist. 2019, 51, 278–296. [Google Scholar] [CrossRef]
- Dick, A.S.; Bernal, B.; Tremblay, P. The language connectome: New pathways, new concepts. Neuroscientist 2014, 20, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Hickok, G.; Poeppel, D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition 2004, 92, 67–99. [Google Scholar] [CrossRef] [PubMed]
- Hickok, G.; Poeppel, D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007, 8, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Fridriksson, J.; den Ouden, D.B.; Hillis, A.E.; Hickok, G.; Rorden, C.; Basilakos, A.; Yourganov, G.; Bonilha, L. Anatomy of aphasia revisited. Brain 2018, 141, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Fridriksson, J.; Yourganov, G.; Bonilha, L.; Basilakos, A.; Den Ouden, D.B.; Rorden, C. Revealing the dual streams of speech processing. Proc. Natl. Acad. Sci. USA 2016, 113, 15108–15113. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, S.; Zhou, L.; Yu, Y.; Tan, X.; Wu, M.; Sun, P.; Zhang, W.; Li, J.; Cheng, R.; et al. Correlations between Dual-Pathway White Matter Alterations and Language Impairment in Patients with Aphasia: A Systematic Review and Meta-analysis. Neuropsychol. Rev. 2021, 31, 402–418. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, H.; Li, S.; Dai, Y. Predictive role of subcomponents of the left arcuate fasciculus in prognosis of aphasia after stroke: A retrospective observational study. Medicine 2019, 98, e15775. [Google Scholar] [CrossRef]
- Catani, M.; Jones, D.K.; Ffytche, D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005, 57, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.; Thiebaut de Schotten, M.T. Atlas of Human Brain Connections; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Bajada, C.J.; Lambon Ralph, M.A.; Cloutman, L.L. Transport for language south of the Sylvian fissure: The routes and history of the main tracts and stations in the ventral language network. Cortex 2015, 69, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Lambon Ralph, M.A. The roles of the “ventral” semantic and “dorsal” pathways in conduite d’approche: A neuroanatomically-constrained computational modeling investigation. Front. Hum. Neurosci. 2013, 7, 422. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Saito, S.; Rogers, T.T.; Lambon Ralph, M.A. Lichtheim 2: Synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron 2011, 72, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Bornkessel-Schlesewsky, I.; Schlesewsky, M. Reconciling time, space and function: A new dorsal-ventral stream model of sentence comprehension. Brain Lang. 2013, 125, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Cloutman, L.L.; Binney, R.J.; Morris, D.M.; Parker, G.J.; Lambon Ralph, M.A. Using in vivo probabilistic tractography to reveal two segregated dorsal ‘language-cognitive’ pathways in the human brain. Brain Lang. 2013, 127, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Weiller, C.; Bormann, T.; Saur, D.; Musso, M.; Rijntjes, M. How the ventral pathway got lost: And what its recovery might mean. Brain Lang. 2011, 118, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Rolheiser, T.; Stamatakis, E.A.; Tyler, L.K. Dynamic processing in the human language system: Synergy between the arcuate fascicle and extreme capsule. J. Neurosci. 2011, 31, 16949–16957. [Google Scholar] [CrossRef]
- Zavanone, C.; Samson, Y.; Arbizu, C.; Dupont, S.; Dormont, D.; Rosso, C. Critical brain regions related to post-stroke aphasia severity identified by early diffusion imaging are not the same when predicting short- and long-term outcome. Brain Lang. 2018, 186, 1–7. [Google Scholar] [CrossRef]
- Ivanova, M.V.; Zhong, A.; Turken, A.; Baldo, J.V.; Dronkers, N.F. Functional Contributions of the Arcuate Fasciculus to Language Processing. Front. Hum. Neurosci. 2021, 15, 672665. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, Y.; Liao, X.; Qian, W.; Ye, T. Integrity of the Left Arcuate Fasciculus Segments Significantly Affects Language Performance in Individuals with Acute/Subacute Post-Stroke Aphasia: A Cross-Sectional Diffusion Tensor Imaging Study. Brain Sci. 2022, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Forkel, S.J.; Rogalski, E.; Drossinos Sancho, N.; D’Anna, L.; Luque Laguna, P.; Sridhar, J.; Dell’Acqua, F.; Weintraub, S.; Thompson, C.; Mesulam, M.M.; et al. Anatomical evidence of an indirect pathway for word repetition. Neurology 2020, 94, e594–e606. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H. Introduction to the Western Aphasia Test (WAB) (1). Chin. J. Rehabil. Theory Pract. 1997, 3, 87–89. (In Chinese) [Google Scholar]
- Wang, Y.H. Introduction to the Western Aphasia Test (WAB) (2). Chin. J. Rehabil. Theory Pract. 1997, 3, 135–140. (In Chinese) [Google Scholar]
- Lahiri, D.; Dubey, S.; Ardila, A.; Ray, B.K. Factors affecting vascular aphasia severity. Aphasiology 2021, 35, 633–641. [Google Scholar] [CrossRef]
- Clarke, W.T.; Stagg, C.J.; Jbabdi, S. FSL-MRS: An end-to-end spectroscopy analysis package. Magn. Reson. Med. 2021, 85, 2950–2964. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Benner, T.; Sorensen, A.G.; Wedeen, V.J. Diffusion toolkit: A software package for diffusion imaging data processing and tractography. Proc. Intl. Soc. Mag. Reson. Med. 2007, 15, 3720. [Google Scholar]
- Thiebaut de Schotten, M.; Ffytche, D.H.; Bizzi, A.; Dell’Acqua, F.; Allin, M.; Walshe, M.; Murray, R.; Williams, S.C.; Murphy, D.G.; Catani, M. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage 2011, 54, 49–59. [Google Scholar] [CrossRef]
- Papagno, C.; Casarotti, A.; Comi, A.; Pisoni, A.; Lucchelli, F.; Bizzi, A.; Riva, M.; Bello, L. Long-term proper name anomia after removal of the uncinate fasciculus. Brain Struct. Funct. 2016, 221, 687–694. [Google Scholar] [CrossRef]
- López-Barroso, D.; Catani, M.; Ripollés, P.; Dell’Acqua, F.; Rodríguez-Fornells, A.; de Diego-Balaguer, R. Word learning is mediated by the left arcuate fasciculus. Proc. Natl. Acad. Sci. USA 2013, 110, 13168–13173. [Google Scholar] [CrossRef]
- Gullick, M.M.; Booth, J.R. The direct segment of the arcuate fasciculus is predictive of longitudinal reading change. Dev. Cogn. Neurosci. 2015, 13, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.; Bambini, V. A model for Social Communication and Language Evolution and Development (SCALED). Curr. Opin. Neurobiol. 2014, 28, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Tak, H.J.; Kim, J.H.; Son, S.M. Developmental process of the arcuate fasciculus from infancy to adolescence: A diffusion tensor imaging study. Neural Regen. Res. 2016, 11, 937–943. [Google Scholar] [PubMed]
- Friederici, A.D. The cortical language circuit: From auditory perception to sentence comprehension. Trends Cogn. Sci. 2012, 16, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.M.; Galantucci, S.; Tartaglia, M.C.; Rising, K.; Patterson, D.K.; Henry, M.L.; Ogar, J.M.; DeLeon, J.; Miller, B.L.; Gorno-Tempini, M.L. Syntactic Processing Depends on Dorsal Language Tracts. Neuron 2011, 72, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Magnusdottir, S.; Fillmore, P.; den Ouden, D.B.; Hjaltason, H.; Rorden, C.; Kjartansson, O.; Bonilha, L.; Fridriksson, J. Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: A lesion-symptom mapping study. Hum. Brain Mapp. 2013, 34, 2715–2723. [Google Scholar] [CrossRef] [PubMed]
- Gajardo-Vidal, A.; Lorca-Puls, D.L.; Team, P.; Warner, H.; Pshdary, B.; Crinion, J.T.; Leff, A.P.; Hope, T.M.H.; Geva, S.; Seghier, M.L.; et al. Damage to Broca’s area does not contribute to long-term speech production outcome after stroke. Brain 2021, 144, 817–832. [Google Scholar] [CrossRef]
- Kernbach, J.M.; Hartwigsen, G.; Lim, J.S.; Bae, H.J.; Yu, K.H.; Schlaug, G.; Bonkhoff, A.; Rost, N.S.; Bzdok, D. Bayesian stroke modeling details sex biases in the white matter substrates of aphasia. Commun. Biol. Mar. 2023, 31, 354. [Google Scholar] [CrossRef]
- Fridriksson, J.; Guo, D.; Fillmore, P.; Holland, A.; Rorden, C. Damage to the anterior arcuate fasciculus predicts non-fluent speech production in aphasia. Brain 2013, 136, 3451–3460. [Google Scholar] [CrossRef]
- Basilakos, A.; Fillmore, P.T.; Rorden, C.; Guo, D.; Bonilha, L.; Fridriksson, J. Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Front. Hum. Neurosci. 2014, 17, 845. [Google Scholar] [CrossRef]
- Ivanova, M.V.; Isaev, D.Y.; Dragoy, O.V.; Akinina, Y.S.; Petrushevskiy, A.G.; Fedina, O.N. Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex 2016, 85, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Fridriksson, J.; Kjartansson, O.; Morgan, P.S.; Hjaltason, H.; Magnusdottir, S.; Bonilha, L.; Rorden, C. Impaired speech repetition and left parietal lobe damage. J. Neurosci. 2010, 30, 11057–11061. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, D.; Hartwigsen, G.; Kellmeyer, P.; Glauche, V.; Mader, I.; Klöppel, S.; Suchan, J.; Karnath, H.O.; Weiller, C.; Saur, D. Damage to ventral and dorsal language pathways in acute aphasia. Brain 2013, 136, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Von Der Heide, R.J.; Skipper, L.M.; Klobusicky, E.; Olson, I.R. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain 2013, 136, 1692–1707. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, L.; Mesulam, M.M.; Thiebaut de Schotten, M.; Dell’Acqua, F.; Murphy, D.; Wieneke, C.; Martersteck, A.; Cobia, D.; Rogalski, E.; Catani, M. Frontotemporal networks and behavioral symptoms in primary progressive aphasia. Neurology 2016, 86, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Y.; Li, J.; Yao, D.; Liao, W.; Chen, H. Beyond the Arcuate Fasciculus: Damage to Ventral and Dorsal Language Pathways in Aphasia. Brain Topogr. 2017, 30, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Papagno, C.; Miracapillo, C.; Casarotti, A.; Romero Lauro, L.J.; Castellano, A.; Falini, A.; Casaceli, G.; Fava, E.; Bello, L. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain 2011, 134, 405–414. [Google Scholar] [CrossRef]
- Dick, A.S.; Tremblay, P. Beyond the arcuate fasciculus: Consensus and controversy in the connectional anatomy of language. Brain 2012, 135, 3529–3550. [Google Scholar] [CrossRef]
- Bonilha, L.; Hillis, A.E.; Hickok, G.; den Ouden, D.B.; Rorden, C.; Fridriksson, J. Temporal lobe networks supporting the comprehension of spoken words. Brain 2017, 140, 2370–2380. [Google Scholar] [CrossRef]
- Grossman, M.; McMillan, C.; Moore, P.; Ding, L.; Glosser, G.; Work, M.; Gee, J. What’s in a name: Voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain 2004, 127, 628–649. [Google Scholar] [CrossRef]
- Catani, M.; Mesulam, M. The arcuate fasciculus and the disconnection theme in language and aphasia: History and current state. Cortex 2008, 44, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Song, L.; Huang, R.; Ding, J.; Fang, Y.; Xu, Y.; Han, Z. Structural connectivity subserving verbal fluency revealed by lesion-behavior mapping in stroke patients. Neuropsychologia 2017, 101, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.M.H.; Seghier, M.L.; Prejawa, S.; Leff, A.P.; Price, C.J. Distinguishing the effect of lesion load from tract disconnection in the arcuate and uncinate fasciculi. NeuroImage 2016, 125, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.; Thye, M.; Mirman, D. Estimating effects of graded white matter damage and binary tract disconnection on post-stroke language impairment. NeuroImage 2019, 189, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Friederici, A.D.; Gierhan, S.M. The language network. Curr. Opin. Neurobiol. 2013, 23, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Breier, J.I.; Hasan, K.M.; Zhang, W.; Men, D.; Papanicolaou, A.C. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am. J. Neuroradiol. 2008, 29, 483–487. [Google Scholar] [CrossRef] [PubMed]
- van Hees, S.; McMahon, K.; Angwin, A.; de Zubicaray, G.; Read, S.; Copland, D.A. Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabil. Neural. Repair. 2014, 28, 325–334. [Google Scholar] [CrossRef]
- Marchina, S.; Zhu, L.L.; Norton, A.; Zipse, L.; Wan, C.Y.; Schlaug, G. Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke 2011, 42, 2251–2256. [Google Scholar] [CrossRef]
- Rosso, C.; Vargas, P.; Valabregue, R.; Arbizu, C.; Henry-Amar, F.; Leger, A.; Lehéricy, S.; Samson, Y. Aphasia severity in chronic stroke patients: A combined disconnection in the dorsal and ventral language pathways. Neurorehabil. Neural. Repair. 2015, 29, 287–295. [Google Scholar] [CrossRef]
- Ebeling, U.; von Cramon, D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir. 1992, 115, 143–148. [Google Scholar] [CrossRef]
- Hau, J.; Sarubbo, S.; Houde, J.C.; Corsini, F.; Girard, G.; Deledalle, C.; Crivello, F.; Zago, L.; Mellet, E.; Jobard, G.; et al. Revisiting the human uncinate fasciculus, its subcomponents and asymmetries with stem-based tractography and microdissection validation. Brain Struct. Funct. 2017, 222, 1645–1662. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Gatignol, P.; Moritz-Gasser, S.; Mandonnet, E. Is the left uncinate fasciculus essential for language? A cerebral stimulation study. J. Neurol. 2009, 256, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.; Allin, M.P.; Husain, M.; Pugliese, L.; Mesulam, M.M.; Murray, R.M.; Jones, D.K. Symmetries in human brain language pathways correlate with verbal recall. Proc. Natl. Acad. Sci. USA 2007, 104, 17163–17168. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Gee, M.; Camicioli, R.; Wieler, M.; Martin, W.; Beaulieu, C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage 2012, 60, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Turken, A.U.; Dronkers, N.F. The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Front. Syst. Neurosci. 2011, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- de Zubicaray, G.I.; Rose, S.E.; McMahon, K.L. The structure and connectivity of semantic memory in the healthy older adult brain. NeuroImage 2011, 54, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Moritz-Gasser, S.; Mandonnet, E. A re-examination of neural basis of language processing: Proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain Lang. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Harvey, D.Y.; Wei, T.; Ellmore, T.M.; Hamilton, A.C.; Schnur, T.T. Neuropsychological evidence for the functional role of the uncinate fasciculus in semantic control. Neuropsychologia 2013, 51, 789–801. [Google Scholar] [CrossRef]
- Saur, D.; Kreher, B.W.; Schnell, S.; Kümmerer, D.; Kellmeyer, P.; Vry, M.S.; Umarova, R.; Musso, M.; Glauche, V.; Abel, S.; et al. Ventral and dorsal pathways for language. Proc. Natl. Acad. Sci. USA 2008, 105, 18035–18040. [Google Scholar] [CrossRef]
- Makris, N.; Pandya, D.N. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct. Funct. 2009, 213, 343–358. [Google Scholar] [CrossRef]
- Kourtidou, E.; Kasselimis, D.; Angelopoulou, G.; Karavasilis, E.; Velonakis, G.; Kelekis, N.; Zalonis, I.; Evdokimidis, I.; Potagas, C.; Petrides, M. The Role of the Right Hemisphere White Matter Tracts in Chronic Aphasic Patients after Damage of the Language Tracts in the Left Hemisphere. Front. Hum. Neurosci. 2021, 15, 635750. [Google Scholar] [CrossRef]

| Patient ID | Sex/Age | Education (Years) | Time Post Onset (Days) | Stroke Type | Aphasia Type | Aphasia Severity | Lesion Site | Lesion Volume (cm3) |
|---|---|---|---|---|---|---|---|---|
| 01 | M/48 | 6 | 27 | ICH | TMA | severe | Temporal lobe, basal ganglia, and corona radiate | 22.18 |
| 02 | F/59 | 6 | 12 | Infarction | TSA | moderate | Basal ganglia, corona radiate | 8.19 |
| 03 | M/52 | 9 | 8 | Infarction | TSA | mild | Basal ganglia, corona radiate, and centrum semiovale | 7.94 |
| 04 | F/56 | 12 | 18 | ICH | Wernicke | severe | Temporal parietal lobe | 68.07 |
| 05 | M/64 | 9 | 8 | Infarction | Global | very severe | Temporal and parietal lobe, insula | 11.51 |
| 06 | M/72 | 16 | 23 | Infarction | Broca | severe | Basal ganglia, corona radiate | 12.50 |
| 07 | F/33 | 6 | 5 | Infarction | Global | severe | Temporal and parietal lobe, insula | 40.26 |
| 08 | M/61 | 12 | 45 | Infarction | Conduction | severe | Frontotemporal parietal lobe | 21.02 |
| 09 | M/59 | 12 | 17 | ICH | Wernicke | severe | Temporal lobe, insula | 75.34 |
| 10 | M/49 | 15 | 10 | Infarction | Broca | moderate | Basal ganglia, frontotemporal parietal lobe, and corona radiata | 43.43 |
| 11 | M/36 | 9 | 16 | Infarction | Broca | moderate | Basal ganglia, temporal lobe, corona radiata, and centrum semiovale | 27.79 |
| 12 | F/56 | 9 | 81 | ICH | Conduction | moderate | Basal ganglia, corona radiata | 17.15 |
| 13 | M/48 | 9 | 88 | ICH | Broca | severe | Basal ganglia, frontotemporal lobe | 29.30 |
| 14 | M/38 | 15 | 83 | ICH | Broca | moderate | Basal ganglia, frontal lobe | 27.34 |
| 15 | M/61 | 9 | 21 | Infarction | Anomic | mild | Basal ganglia, frontal lobe | 38.50 |
| 16 | F/78 | 6 | 25 | Infarction | Broca | severe | Basal ganglia, frontal lobe, corona radiate, and insula | 23.62 |
| 17 | M/42 | 16 | 64 | ICH | Global | very severe | Basal ganglia, frontal lobe, and corona radiate | 32.46 |
| 18 | M/43 | 16 | 53 | Infarction | Anomic | moderate | Frontoparietal lobe, insula | 26.95 |
| 19 | M/50 | 9 | 11 | Infarction | Wernicke | severe | Temporo-occipital junction, insula | 35.26 |
| 20 | F/50 | 12 | 15 | Infarction | Broca | severe | Temporal and parietal lobe, corona radiata, and insula | 10.84 |
| 21 | F/71 | 6 | 89 | Infarction | Global | very severe | Frontotemporal parietal and occipital lobe | 150.50 |
| 22 | M/25 | 9 | 27 | ICH | Anomic | mild | Temporo-occipital junction | 21.82 |
| 23 | M/53 | 16 | 56 | ICH | TMA | severe | Frontotemporal parietal lobe, corona radiata, and insula | 70.30 |
| 24 | M/61 | 12 | 7 | Infarction | Global | very severe | Temporoparietal-occipital junction, and insula | 54.76 |
| 25 | M/72 | 9 | 13 | Infarction | Global | very severe | Temporoparietal-occipital junction, and corona radiata | 40.78 |
| 26 | M/62 | 9 | 23 | Infarction | Global | very severe | Frontotemporal parietal lobe, basal ganglia, insula | 41.53 |
| 27 | M/64 | 6 | 13 | ICH | Global | very severe | Frontotemporal parietal and occipital lobe | 39.62 |
| 28 | M/56 | 15 | 85 | Infarction | Global | severe | Basal ganglia, frontoparietal lobe, and insula | 76.59 |
| 29 | M/34 | 12 | 77 | Infarction | Global | severe | Frontotemporal parietal lobe | 54.30 |
| 30 | M/48 | 9 | 24 | Infarction | Global | very severe | Frontotemporal parietal lobe, basal ganglia | 1.44 |
| 31 | M/48 | 15 | 10 | ICH | Conduction | very severe | Frontotemporal and occipital lobe, | 203.50 |
| 32 | M/64 | 15 | 73 | Infarction | MTA | severe | Frontoparietal lobe, insula | 151.30 |
| 33 | M/69 | 6 | 68 | ICH | TMA | severe | Basal ganglia, frontal lobe | 46.70 |
| 34 | F/37 | 12 | 34 | ICH | Anomic | mild | Basal ganglia, frontal lobe, corona radiata, and centrum semiovale | 124.00 |
| 35 | F/48 | 15 | 60 | ICH | Global | very severe | Basal ganglia, corona radiate, and centrum semiovale | 62.8 |
| 36 | M/52 | 9 | 27 | ICH | Global | very severe | Basal ganglia, corona radiate, and centrum semiovale | 69.6 |
| Lesion Volume | FA_LSAF_L | FA_LSAF_R | Fiber_LSAF_L | Fiber_LSAF_R | FA_UF_L | FA_UF_R | Fiber_UF_L | Fiber_UF_R | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | ρ | p | ρ | p | ρ | p | ρ | p | ρ | p | ρ | p | |
| Aphasia quotient | −0.359 * | 0.032 | 0.761 ** | 0.000 | 0.442 ** | 0.007 | 0.559 ** | 0.000 | × | × | × | × | 0.343 * | 0.040 | × | × | × | × |
| Spontaneous speech | × | × | 0.588 ** | 0.000 | × | × | 0.508 ** | 0.002 | × | × | × | × | × | × | × | × | ||
| Comprehension | × | × | 0.637 ** | 0.000 | 0.476 ** | 0.003 | 0.440 ** | 0.007 | × | × | × | × | 0.343 * | 0.040 | × | × | × | × |
| Repetition | × | × | 0.768 ** | 0.000 | 0.459 ** | 0.005 | 0.401 * | 0.015 | × | × | × | × | 0.426 * | 0.010 | × | × | × | × |
| Naming | −0.351 * | 0.036 | 0.639 ** | 0.000 | 0.401 ** | 0.015 | 0.450 ** | 0.006 | × | × | × | × | 0.361 * | 0.030 | × | × | × | × |
| Fluency | × | × | 0.459 ** | 0.005 | × | × | 0.430 ** | 0.009 | × | × | × | × | × | × | × | × | ||
| Y | X # | B (SE) | Beta | t | p | F Value | Adjusted R2 |
|---|---|---|---|---|---|---|---|
| Aphasia quotient | FA_UF_L | 3.646 (32.725) | 0.024 | 0.111 | 0.912 | 5.056 a | 0.481 |
| FA_LSAF_L | 150.828 (39.336) | 0.968 | 3.834 | 0.001 * | |||
| UF × LSAF | −111.755 (121.240) | −0.307 | −0.922 | 0.365 | |||
| Lesion volume | 0.162 (1.241) | 0.019 | 0.131 | 0.897 | |||
| Spontaneous speech | FA_UF_L | −14.531 (40.613) | −0.092 | −0.358 | 0.723 | 2.419 a | 0.245 |
| FA_LSAF_L | 94.882 (48.816) | 0.592 | 1.944 | 0.032 * | |||
| UF × LSAF | −0.279 (150.461) | −0.001 | −0.002 | 0.999 | |||
| Lesion volume | 0.941 (1.541) | 0.108 | 0.611 | 0.546 | |||
| Comprehension # | FA_UF_L | −1.339 (3.328) | −0.096 | −0.402 | 0.691 | 3.413 a | 0.355 |
| FA_LSAF_L | 11.973 (4.000) | 0.842 | 2.993 | 0.006 * | |||
| UF × LSAF | −6.292 (12.329) | −0.189 | −0.510 | 0.614 | |||
| Lesion volume | −0.012 (0.126) | −0.015 | −0.094 | 0.926 | |||
| Repetition # | FA_UF_L | −2.525 (4.348) | −0.119 | −0.581 | 0.566 | 5.869 b | 0.527 |
| FA_LSAF_L | 18.203 (5.226) | 0.840 | 3.483 | 0.002 * | |||
| UF × LSAF | 4.206 (16.107) | 0.083 | 0.261 | 0.796 | |||
| Lesion volume | 0.308 (0.165) | 0.262 | 1.870 | 0.072 | |||
| Naming # | FA_UF_L | −3.333 (4.711) | −0.174 | −0.707 | 0.485 | 3.011 a | 0.315 |
| FA_LSAF_L | 12.910 (5.662) | 0.661 | 2.280 | 0.031 * | |||
| UF × LSAF | −0.280 (17.453) | −0.006 | −0.016 | 0.987 | |||
| Lesion volume | 0.003 (0.179) | 0.002 | 0.015 | 0.988 | |||
| Fluency # | FA_UF_L | −1.771 (4.894) | −0.099 | −0.362 | 0.720 | 1.777 | 0.151 |
| FA_LSAF_L | 8.279 (5.883) | 0.455 | 1.407 | 0.041 * | |||
| UF × LSAF | 6.085 (18.132) | 0.143 | 0.336 | 0.740 | |||
| Lesion volume | 0.285 (0.186) | 0.288 | 1.534 | 0.137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Jiang, Y.; Sun, Y.; Ju, X.; Ye, T.; Liu, N.; Qian, S.; Liu, K. Effects of Damage to the Integrity of the Left Dual-Stream Frontotemporal Network Mediated by the Arcuate Fasciculus and Uncinate Fasciculus on Acute/Subacute Post-Stroke Aphasia. Brain Sci. 2023, 13, 1324. https://doi.org/10.3390/brainsci13091324
Yu Q, Jiang Y, Sun Y, Ju X, Ye T, Liu N, Qian S, Liu K. Effects of Damage to the Integrity of the Left Dual-Stream Frontotemporal Network Mediated by the Arcuate Fasciculus and Uncinate Fasciculus on Acute/Subacute Post-Stroke Aphasia. Brain Sciences. 2023; 13(9):1324. https://doi.org/10.3390/brainsci13091324
Chicago/Turabian StyleYu, Qiwei, Yuer Jiang, Yan Sun, Xiaowen Ju, Tianfen Ye, Na Liu, Surong Qian, and Kefu Liu. 2023. "Effects of Damage to the Integrity of the Left Dual-Stream Frontotemporal Network Mediated by the Arcuate Fasciculus and Uncinate Fasciculus on Acute/Subacute Post-Stroke Aphasia" Brain Sciences 13, no. 9: 1324. https://doi.org/10.3390/brainsci13091324
APA StyleYu, Q., Jiang, Y., Sun, Y., Ju, X., Ye, T., Liu, N., Qian, S., & Liu, K. (2023). Effects of Damage to the Integrity of the Left Dual-Stream Frontotemporal Network Mediated by the Arcuate Fasciculus and Uncinate Fasciculus on Acute/Subacute Post-Stroke Aphasia. Brain Sciences, 13(9), 1324. https://doi.org/10.3390/brainsci13091324






