A Comparison of the Neuromodulation Effects of Frontal and Parietal Transcranial Direct Current Stimulation on Disorders of Consciousness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. Protocol for tDCS Stimulation
2.4. Behavioral Assessment
2.5. EEG Measurement
2.6. EEG Pre-Processing and Processing
2.7. Genuine Permutation Cross-Mutual Information
2.8. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and CRS-R Scores
3.2. EEG Results
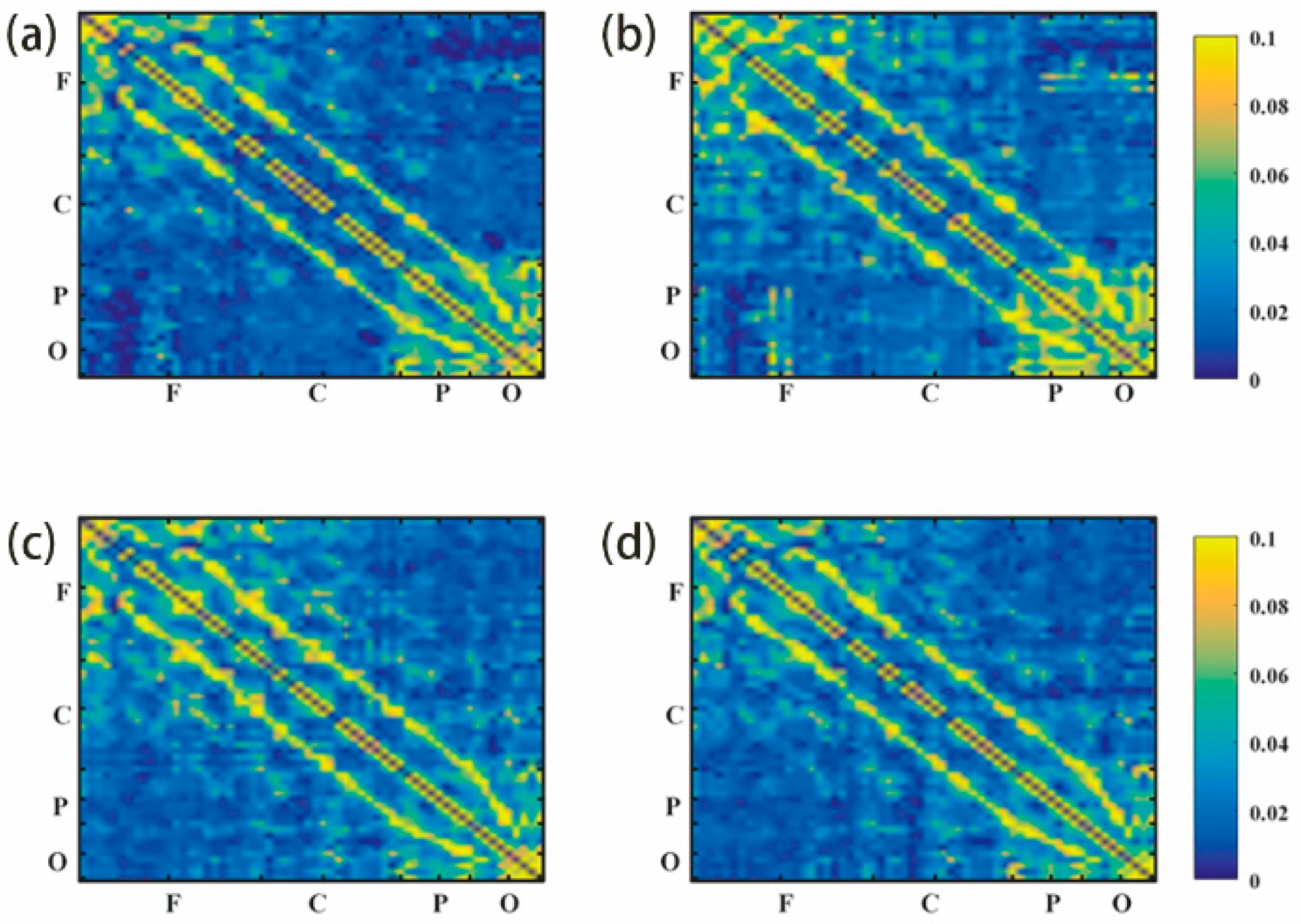
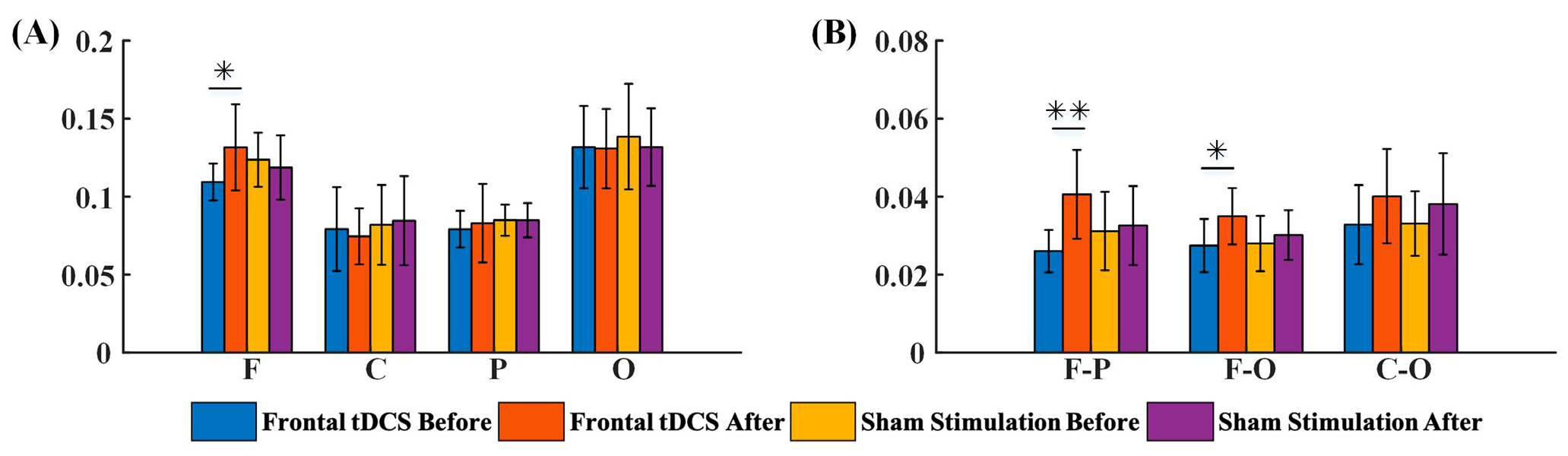
3.2.1. G_PCMI before and after Frontal tDCS
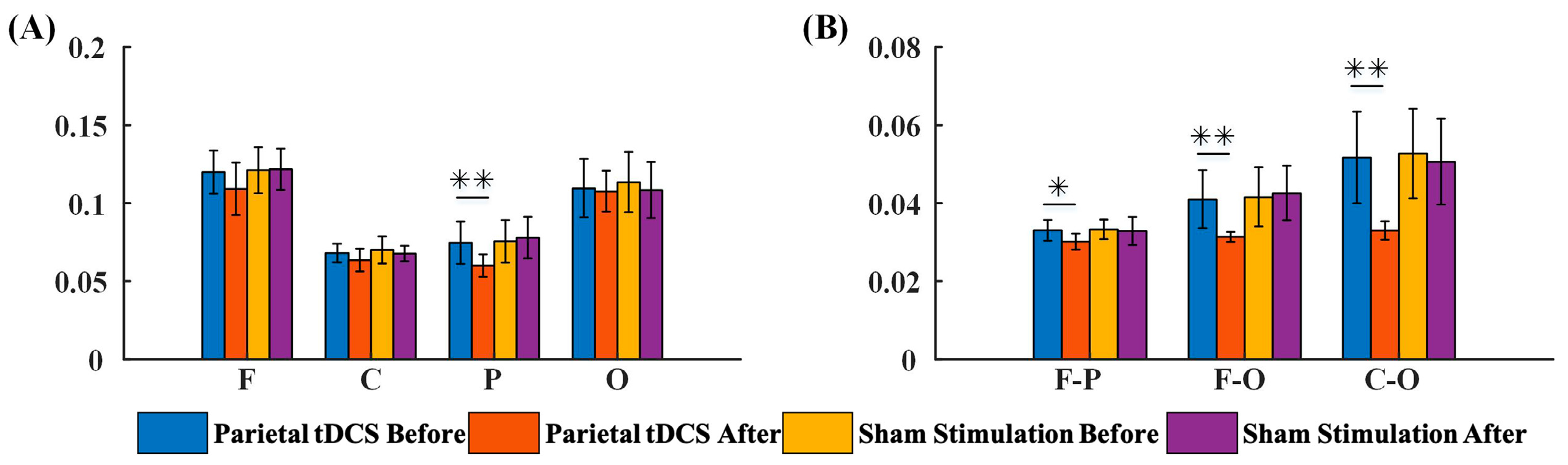
3.2.2. G_PCMI before and after Parietal tDCS
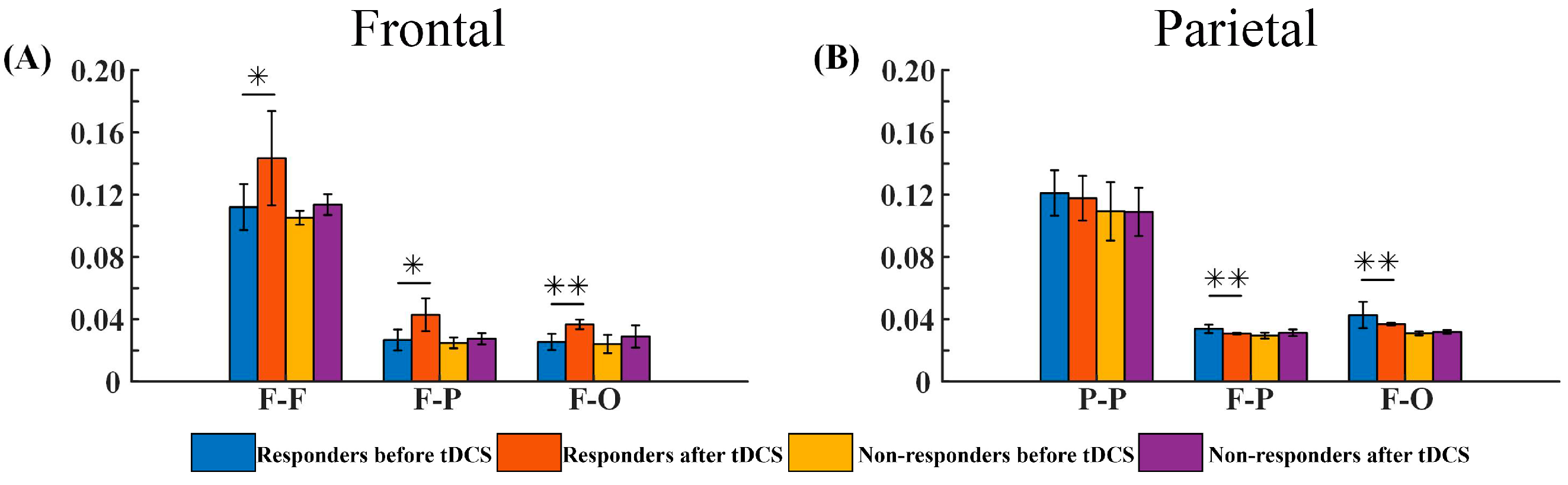
3.2.3. G_PCMI of Different Outcome Groups before and after tDCS
3.3. Correlations between G_PCMI and CRS-R Values
3.3.1. Correlations between the Changes in G_PCMI and Baseline CRS-R Values
3.3.2. Correlations between the Changes in G_PCMI Values and the Changes in CRS-R Values
4. Discussion
4.1. The Modulatory Effects of Frontal tDCS and Parietal tDCS
4.2. The Prognosis of DoC Based on Responses to Frontal and Parietal tDCS
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strauss, D.J.; Ashwal, S.; Day, S.M.; Shavelle, R.M. Life expectancy of children in vegetative and minimally conscious states. Pediatr. Neurol. 2000, 23, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Pisa, F.E.; Biasutti, E.; Drigo, D.; Barbone, F. The prevalence of vegetative and minimally conscious states: A systematic review and methodological appraisal. J. Head Trauma Rehabil. 2014, 29, E23–E30. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Guernon, A.; Chalcraft, L.; Harton, B.; Smith, B.; Pape, T.L.-B. Medical comorbidities in disorders of consciousness patients and their association with functional outcomes. Arch. Phys. Med. Rehabil. 2013, 94, 1899–1907.e3. [Google Scholar] [CrossRef]
- Leonardi, M.; Giovannetti, A.M.; Pagani, M.; Raggi, A.; Sattin, D.; Consortiu, O.B.O.T.N. Burden and needs of 487 caregivers of patients in vegetative state and in minimally conscious state: Results from a national study. Brain INJ. 2012, 26, 1201–1210. [Google Scholar] [CrossRef]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018, 91, 450–460. [Google Scholar] [CrossRef]
- Camacho-Conde, J.A.; Gonzalez-Bermudez, M.d.R.; Carretero-Rey, M.; Khan, Z.U. Brain stimulation: A therapeutic approach for the treatment of neurological disorders. CNS Neurosci. Ther. 2022, 28, 5–18. [Google Scholar] [CrossRef]
- Angelakis, E.; Liouta, E.; Andreadis, N.; Korfias, S.; Ktonas, P.; Stranjalis, G.; Sakas, D.E. Transcranial direct current stimulation effects in disorders of consciousness. Arch. Phys. Med. Rehabil. 2014, 95, 283–289. [Google Scholar] [CrossRef]
- Thibaut, A.; Bruno, M.-A.; LeDoux, D.; Demertzi, A.; Laureys, S. tDCS in patients with disorders of consciousness: Sham-controlled randomized double-blind study. Neurology 2014, 82, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Schiff, N.; Giacino, J.; Laureys, S.; Gosseries, O. Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol. 2019, 18, 600–614. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W. Transcranial direct current stimulation in disorders of consciousness: A review. Int. J. Neurosci. 2018, 128, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xia, X.; Wang, Y.; Guo, Y.; Yang, Y.; He, J.; Li, X. Fronto-parietal coherence response to tDCS modulation in patients with disorders of consciousness. Int. J. Neurosci. 2018, 128, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Barra, A.; Rosenfelder, M.; Mortaheb, S.; Carrière, M.; Martens, G.; Bodien, Y.G.; Morales-Quezada, L.; Bender, A.; Laureys, S.; Thibaut, A.; et al. Transcranial Pulsed-Current Stimulation versus Transcranial Direct Current Stimulation in Patients with Disorders of Consciousness: A Pilot, Sham-Controlled Cross-Over Double-Blind Study. Brain Sci. 2022, 12, 429. [Google Scholar] [CrossRef]
- Carrière, M.; Mortaheb, S.; Raimondo, F.; Annen, J.; Barra, A.; Fossati, M.C.B.; Chatelle, C.; Hermann, B.; Martens, G.; Di Perri, C.; et al. Neurophysiological Correlates of a Single Session of Prefrontal tDCS in Patients with Prolonged Disorders of Consciousness: A Pilot Double-Blind Randomized Controlled Study. Brain Sci. 2020, 10, 469. [Google Scholar] [CrossRef]
- Cavinato, M.; Genna, C.; Formaggio, E.; Gregorio, C.; Storti, S.F.; Manganotti, P.; Casanova, E.; Piperno, R.; Piccione, F. Behavioural and electrophysiological effects of tDCS to prefrontal cortex in patients with disorders of consciousness. Clin. Neurophysiol. 2019, 130, 231–238. [Google Scholar] [CrossRef]
- Hermann, B.; Raimondo, F.; Hirsch, L.; Huang, Y.; Denis-Valente, M.; Pérez, P.; Engemann, D.; Faugeras, F.; Weiss, N.; Demeret, S.; et al. Combined behavioral and electrophysiological evidence for a direct cortical effect of prefrontal tDCS on disorders of consciousness. Sci. Rep. 2020, 10, 4323. [Google Scholar] [CrossRef]
- Thibaut, A.; Chennu, S.; Chatelle, C.; Martens, G.; Annen, J.; Cassol, H.; Laureys, S. Theta network centrality correlates with tDCS response in disorders of consciousness. Brain Stimul. 2018, 11, 1407–1409. [Google Scholar] [CrossRef]
- Wu, M.; Yu, Y.; Luo, L.; Wu, Y.; Gao, J.; Ye, X.; Luo, B. Efficiency of Repetitive Transcranial Direct Current Stimulation of the Dorsolateral Prefrontal Cortex in Disorders of Consciousness: A Randomized Sham-Controlled Study. Neural Plast. 2019, 2019, 7089543. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Zhang, T.; Du, J.; Li, R.; Huo, R.; Song, W. P300 correlates with tDCS response in minimally conscious state patients. Neurosci. Lett. 2022, 774, 136534. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Du, J.; Huo, S.; Li, R.; Song, W. Neural correlates of different behavioral response to transcranial direct current stimulation between patients in the unresponsive wakefulness syndrome and minimally conscious state. Neurol. Sci. 2020, 41, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Straudi, S.; Bonsangue, V.; Mele, S.; Craighero, L.; Montis, A.; Fregni, F.; Lavezzi, S.; Basaglia, N. Bilateral M1 anodal transcranial direct current stimulation in post traumatic chronic minimally conscious state: A pilot EEG-tDCS study. Brain Inj. 2019, 33, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xia, X.; Kang, J.; Yang, Y.; He, J.; Li, X. TDCS modulates cortical excitability in patients with disorders of consciousness. NeuroImage Clin. 2017, 15, 702–709. [Google Scholar] [CrossRef]
- Dimitri, D.; De Filippis, D.; Galetto, V.; Zettin, M. Evaluation of the effectiveness of transcranial direct current stimulation (tDCS) and psychosensory stimulation through DOCS scale in a minimally conscious subject. Neurocase 2017, 23, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Martens, G.; Lejeune, N.; O’Brien, A.T.; Fregni, F.; Martial, C.; Wannez, S.; Laureys, S.; Thibaut, A. Randomized controlled trial of home-based 4-week tDCS in chronic minimally conscious state. Brain Stimul. 2018, 11, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, A.; Wannez, S.; Donneau, A.-F.; Chatelle, C.; Gosseries, O.; Bruno, M.-A.; Laureys, S. Controlled clinical trial of repeated prefrontal tDCS in patients with chronic minimally conscious state. Brain Inj. 2017, 31, 466–474. [Google Scholar] [CrossRef]
- Cai, T.; Xia, X.; Zhang, H.; Guo, Y.; Bai, Y. High-definition transcranial direct current stimulation modulates neural activities in patients with prolonged disorders of consciousness. Brain Stimul. 2019, 12, 1619–1621. [Google Scholar] [CrossRef]
- Guo, Y.; Bai, Y.; Xia, X.; Li, J.; Wang, X.; Dai, Y.; Dang, Y.; He, J.; Liu, C.; Zhang, H. Effects of Long-Lasting High-Definition Transcranial Direct Current Stimulation in Chronic Disorders of Consciousness: A Pilot Study. Front. Neurosci. 2019, 13, 412. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Zhang, Y.; Li, J.; Gao, Z.; Li, Y.; Zhou, T.; Zhang, H.; He, J.; Cong, F. Combined Behavioral and Mismatch Negativity Evidence for the Effects of Long-Lasting High-Definition tDCS in Disorders of Consciousness: A Pilot Study. Front. Neurosci. 2020, 14, 381. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, L.; Guo, Y.; Shi, L.; Gao, J.; Wang, X.; Hu, Y. Effects of High-Definition Transcranial Direct-Current Stimulation on Resting-State Functional Connectivity in Patients With Disorders of Consciousness. Front. Hum. Neurosci. 2020, 14, 560586. [Google Scholar] [CrossRef]
- Kondziella, D.; Friberg, C.K.; Frokjaer, V.G.; Fabricius, M.; Møller, K. Preserved consciousness in vegetative and minimal conscious states: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 485–492. [Google Scholar] [CrossRef]
- Schnakers, C.; Vanhaudenhuyse, A.; Giacino, J.; Ventura, M.; Boly, M.; Majerus, S.; Moonen, G.; Laureys, S. Diagnostic accuracy of the vegetative and minimally conscious state: Clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Wannez, S.; Heine, L.; Thonnard, M.; Gosseries, O.; Laureys, S. Coma Science Group collaborators The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann. Neurol. 2017, 81, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Bayne, T. Theories of consciousness. Nat. Rev. Neurosci. 2022, 23, 439–452. [Google Scholar] [CrossRef]
- Afrasiabi, M.; Redinbaugh, M.J.; Phillips, J.M.; Kambi, N.A.; Mohanta, S.; Raz, A.; Haun, A.M.; Saalmann, Y.B. Consciousness depends on integration between parietal cortex, striatum, and thalamus. Cell Syst. 2021, 12, 363–373.e11. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Cheng, L.; Shao, S.; Jin, X.; Yu, T.; Sleigh, J.W.; Li, X. Information Integration and Mesoscopic Cortical Connectivity during Propofol Anesthesia. Anesthesiology 2020, 132, 504–524. [Google Scholar] [CrossRef]
- Engemann, D.A.; Raimondo, F.; King, J.-R.; Rohaut, B.; Louppe, G.; Faugeras, F.; Annen, J.; Cassol, H.; Gosseries, O.; Fernandez-Slezak, D.; et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain 2018, 141, 3179–3192. [Google Scholar] [CrossRef] [PubMed]
- King, J.-R.; Sitt, J.D.; Faugeras, F.; Rohaut, B.; El Karoui, I.; Cohen, L.; Naccache, L.; Dehaene, S. Information Sharing in the Brain Indexes Consciousness in Noncommunicative Patients. Curr. Biol. 2013, 23, 1914–1919. [Google Scholar] [CrossRef]
- Sitt, J.D.; King, J.-R.; El Karoui, I.; Rohaut, B.; Faugeras, F.; Gramfort, A.; Cohen, L.; Sigman, M.; Dehaene, S.; Naccache, L. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 2014, 137, 2258–2270. [Google Scholar] [CrossRef]



| Patients | Age (Year) | Gender | Etiology | Course (Month) | CRS-R (Baseline) | CRS-R (After tDCS) | CRS-R (Follow-Up) | Diagnosis (Follow-Up) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MCS | 52 | Female | Anoxic | 36 | 8 | 8 | 8 | - |
| 2 | MCS | 31 | Female | Traumatic | 6 | 11 | 11 | 16 | improved |
| 3 | MCS | 47 | Male | Hemorrhagic | 2 | 9 | 9 | 9 | - |
| 4 | UWS | 52 | Male | Hemorrhagic | 30 | 6 | 6 | 6 | - |
| 5 | UWS | 50 | Female | Anoxic | 8 | 5 | 5 | 5 | - |
| 6 | MCS | 53 | Male | Hemorrhage | 8 | 11 | 11 | 15 | MCS+ |
| 7 | MCS | 43 | Male | Traumatic | 8 | 9 | 9 | 11 | improved |
| 8 | MCS | 29 | Female | Anoxic | 28 | 7 | 7 | 9 | improved |
| 9 | UWS | 50 | Female | Traumatic | 4 | 7 | 7 | 11 | improved |
| 10 | UWS | 19 | Male | Traumatic | 1.5 | 6 | 6 | 8 | improved |
| 1 | UWS | 25 | Male | Traumatic | 7 | 6 | 6 | 8 | MCS |
| 2 | MCS | 24 | Female | Traumatic | 3 | 9 | 9 | 10 | improved |
| 3 | MCS | 60 | Female | Anoxic | 3 | 8 | 8 | 8 | - |
| 4 | UWS | 44 | Male | Anoxic | 3 | 7 | 7 | 8 | improved |
| 5 | UWS | 20 | Male | Traumatic | 3 | 6 | 6 | 6 | - |
| 6 | UWS | 42 | Male | Ischemic | 8 | 6 | 6 | 6 | - |
| 7 | MCS | 62 | Male | Anoxic | 3 | 10 | 10 | 13 | improved |
| 8 | MCS | 24 | Male | Ischemic | 24 | 8 | 8 | 9 | improved |
| 9 | MCS | 56 | Male | Ischemic | 8 | 9 | 9 | 12 | improved |
| 10 | MCS | 57 | Female | Traumatic | 6 | 8 | 8 | 10 | MCS+ |
| Stimulation Target | Number | Age (Years) | Gender | Course of Disease (Months) | CRS-R (Baseline) | CRS-R (Follow-Up) |
|---|---|---|---|---|---|---|
| Frontal | 10 (6 MCS, 4 UWS) | 42.6 ± 11.36 | 5 males, 5 females | 13.15 ± 12.25 | 7.90 ± 1.97 | 9.80 ± 3.55 |
| Parietal | 10 (6 MCS, 4 UWS) | 41.4 ± 16.02 | 7 males, 3 females | 6.80 ± 6.10 | 7.70 ± 1.35 | 9.00 ± 2.31 |
| Location | Spearman Correlation | F–F | P–P | F–P | F–O |
|---|---|---|---|---|---|
| Frontal tDCS | r | 0.67 | - | 0.67 | 0.53 |
| p | <0.05 | - | <0.05 | >0.05 | |
| Parietal tDCS | r | - | 0.70 | 0.66 | 0.61 |
| p | - | <0.05 | <0.05 | >0.05 |
| Location | Spearman Correlation | F–F | P–P | F–P | F–O |
|---|---|---|---|---|---|
| frontal | r | 0.68 | - | 0.75 | 0.21 |
| p | <0.05 | - | <0.05 | >0.05 | |
| parietal | r | - | 0.15 | 0.72 | 0.64 |
| p | - | >0.05 | <0.05 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Wang, Y.; Zhang, Y.; Song, W. A Comparison of the Neuromodulation Effects of Frontal and Parietal Transcranial Direct Current Stimulation on Disorders of Consciousness. Brain Sci. 2023, 13, 1295. https://doi.org/10.3390/brainsci13091295
Wan X, Wang Y, Zhang Y, Song W. A Comparison of the Neuromodulation Effects of Frontal and Parietal Transcranial Direct Current Stimulation on Disorders of Consciousness. Brain Sciences. 2023; 13(9):1295. https://doi.org/10.3390/brainsci13091295
Chicago/Turabian StyleWan, Xiaoping, Yong Wang, Ye Zhang, and Weiqun Song. 2023. "A Comparison of the Neuromodulation Effects of Frontal and Parietal Transcranial Direct Current Stimulation on Disorders of Consciousness" Brain Sciences 13, no. 9: 1295. https://doi.org/10.3390/brainsci13091295
APA StyleWan, X., Wang, Y., Zhang, Y., & Song, W. (2023). A Comparison of the Neuromodulation Effects of Frontal and Parietal Transcranial Direct Current Stimulation on Disorders of Consciousness. Brain Sciences, 13(9), 1295. https://doi.org/10.3390/brainsci13091295






