Abstract
Kernicterus is a serious complication of hyperbilirubinemia, caused by neuronal injury due to excessive unconjugated bilirubin (UCB) in specific brain areas. This injury induced by this accumulation in the globus pallidus can induce severe motor dysfunction. Repetitive transcranial magnetic stimulation (rTMS) has shown neuroprotective effects in various neurological diseases. This study aimed to investigate the effects of rTMS on pallidal nerve damage and motor dysfunction in a rat model of kernicterus. Rats were divided into a sham group (n = 16), a model group (bilirubin with sham rTMS; n = 16) and an rTMS group (bilirubin with rTMS; n = 16). High-frequency rTMS (10 Hz) was applied starting from 24 h postmodeling for 7 days. The rotarod test, western blotting and immunohistochemical staining were performed to measure motor function and protein expression levels. The rTMS mitigated the negative effects of UCB on the general health of kernicterus-model rats and improved their growth and development. Furthermore, the rTMS alleviated UCB-induced motor dysfunction and increased the expression of GABAergic neuronal marker GAD67 in the globus pallidus. Notably, it also inhibited apoptosis-related protein caspase-3 activation. In conclusion, rTMS could alleviate motor dysfunction by inhibiting apoptosis and increasing globus pallidus GAD67 in kernicterus rat models, indicating that it may be a promising treatment for kernicterus.
1. Introduction
Hyperbilirubinemia is a common and mostly benign condition, manifesting with yellow discoloration caused by bilirubin deposition in the skin, the sclera and other organs throughout the body, and it occurs in approximately 60% of term and 80% of preterm infants [1]. Conditions that increase bilirubin production or impede its elimination, like polycythemia, hemolysis caused by Rh alloimmunization or deficiencies in bilirubin metabolism enzymes, can induce hyperbilirubinemia [2,3]. During severe neonatal hyperbilirubinemia, unconjugated bilirubin (UCB) crosses the blood–brain barrier to penetrate and damage specific brain regions, resulting in irreversible brain injury, termed kernicterus [4]. Due to its lipid solubility, UCB is capable of crossing the immature blood–brain barrier and entering the brain tissue. Excessive UCB induces oxidative stress damage, impacting mitochondrial oxidative metabolism, suppressing cellular oxygen utilization, leading to cellular energy depletion and triggering intracellular calcium overload, which is the main cause of neuron damage [3]. Phototherapy stands as a widely accepted and effective primary treatment for neonatal jaundice [5]. Although phototherapy and exchange transfusion have therapeutic effects, kernicterus still causes disability and even death [6]. It remains a persistent issue in the contemporary world. It not only persists in developing countries with underdeveloped healthcare systems and health organizations disrupted by the impacts of war but also maintains its prevalence in industrialized nations [7]. In some developing nations, the incidence of severe kernicterus is roughly 100 times as high as it is in the developed world [3]. Severe neurological sequelae have serious impacts on patients’ quality of life and impose heavy burdens on society and families.
Kernicterus presents as abnormal motor movements and muscle tone, auditory disorder, oculomotor impairments and deciduous tooth enamel dysplasia [8]. The corresponding dystonia is mainly caused by globus pallidus lesions in the basal ganglia [9]. Gamma-aminobutyric acid-ergic (GABAergic) neurons are the main neurons in the globus pallidus [10,11]. Due to its neurotoxic selectivity, UCB induces neuronal apoptotic death in the globus pallidus via the intrinsic mitochondrial pathway through caspase-3 activation [12,13]. Several studies have reported the efficacy of minocycline in preventing neurological impairment and death in mouse models of severe neonatal hyperbilirubinemia [14]. Nevertheless, the prophylactic use of minocycline for the treatment of hyperbilirubinemia to prevent acute bilirubin encephalopathy or kernicterus is improbable due to its adverse effects on dental and dermal health [15]. In addition, few therapeutic options exist for patients with kernicterus who suffer from moderate to severe motor dysfunction. The current therapies for these patients encompass oral medications, particularly benzodiazepines, such as diazepam or clonazepam, alongside baclofen, which activates GABA-B receptors. The application of selective botulinum toxin aims to enhance function and alleviate painful muscle hypertonia [16]. Thus, our study aimed to explore a novel potential therapeutic approach for mitigating UCB-induced neuronal demise in the globus pallidus.
Repetitive transcranial magnetic stimulation (rTMS) is a safe and non-invasive therapeutic technique. It utilizes magnetic pulses to induce an electrical field within the brain via electromagnetic induction, thereby eliciting or modulating neural activity. Furthermore, the magnetic fields generated by conventional rTMS devices can penetrate the cerebral cortex at a depth of up to 1–2 cm [17,18,19]. Previous research has reported that rTMS decreased the quantity of caspase-3 positive cells in a rat model of transient cerebral ischemia [20]. Recent findings have further indicated that rTMS exhibits a neuroprotective effect through the downregulation of proapoptotic caspase-3 cleavage, thereby diminishing both infarct volume and cell death in the identical model [21]. Therefore, the objective of this study was to investigate whether rTMS can protect GABAergic neurons by mitigating UCB-induced cell death in the globus pallidus, ultimately improving the motor ability of kernicterus-model rats.
2. Materials and Methods
2.1. Animals
Pregnant Sprague Dawley (SD) rats were procured from the Experimental Animal Center of Chongqing Medical University (license No. SCXK [Yu] 2022-0010) and subsequently housed in a specific pathogen-free (SPF) animal laboratory. The rats were maintained under a 12 h light/dark cycle at a temperature of 23 ± 2 °C, with ad libitum access to food and water until natural birth. These experimental procedures received approval from the Ethics Committee of the Children’s Hospital of Chongqing Medical University (CHCMU-IACUC20230529003). All efforts were undertaken to minimize both the number of animals utilized and their distress.
2.2. Kernicterus Model
Bilirubin (Sigma-Aldrich, St Louis, MO, USA) was dissolved in 0.5 M NaOH solution (100 mg/mL), further diluted to a concentration of 10 mg/mL with ddH2O and subsequently adjusted to a pH of 8.5 with 0.5 M HCl. The solution was then stored in darkness at −20 °C [22].
On postnatal day 5, the rat pups (weighing 9–12 g) were randomly allocated into three groups: the sham group (n = 16), the model group (bilirubin with sham rTMS; n = 16) and the rTMS group (bilirubin with rTMS, n = 16). The pups were anesthetized with diethyl ether before the model establishment. The rats in the model group and the rTMS group each received an intracisternal injection of a 10 μg/g (body weight) bilirubin solution into the cisterna magna using a microinjector. The sham group was injected with equal volumes of ddH2O (pH = 8.5). Before the bilirubin or ddH2O was applied, an equal volume of the cerebrospinal fluid specimen was drained to prevent intracranial hypertension [23]. The complete experimental process is shown in Figure 1.
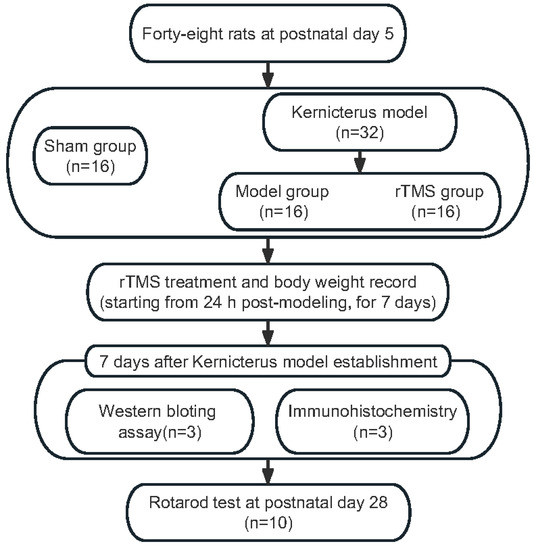
Figure 1.
Experimental flow chart.
2.3. rTMS
A magnetic stimulator (CCY-II; Yiruide, Wuhan, China) with a circular coil (Y064; Yiruide, Wuhan, China) was applied to administer stimulation to conscious rats 24 h after model establishment. The coil was placed horizontally above each head, in close proximity to the scalp. The rats in the rTMS group received a total of 960 stimuli per day for 7 consecutive days at an intensity of 13% of the maximum stimulator output, including 48 trains at 10 Hz for 2 s, with 5 s intervals between the trains. The rats in the model group and the sham group underwent a similar procedure as those in the rTMS group, whereby the coil was vertically positioned 30 cm above the head. This elicited comparable auditory and tactile sensations but did not generate a magnetic field. The rats showed no signs of discomfort during the rTMS. They were sacrificed at seven days after the bilirubin injection for western blotting and immunohistochemistry. At postnatal day 28, the rats were subjected to the rotarod test to detect their motor ability.
2.4. Western Blotting (WB)
According to the instructions, the total protein was extracted from the globus pallidus with a total protein extraction kit (KGP250; KeyGen Biotech, Nanjing, China), and a bicinchoninic assay (BCA) protein detection kit (KGPBCA; KeyGen Biotech, Nanjing, China) was used to quantify the protein concentrations. Target proteins were isolated using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and subsequently transferred to a polyvinylidene difluoride (PVDF) membrane. After cleaning with Tris-buffered saline with a Tween 20 (TBST) solution, the PVDF membranes were sealed in a blocking solution for 15 min. They were incubated overnight at 4 °C with anti-GAD67 (1:1000; Abcam, Cambridge, UK), anti-caspase-3 (1:1000; Abcam, Cambridge, UK) and anti-β-actin (1:2000; ABclonal, Wuhan, China) antibodies. The next day, the PVDF membranes were washed with TBST and incubated with the respective secondary antibodies for 1 h. Subsequently, each PVDF membrane was treated with an enhanced chemiluminescence (ECL) solution and exposed using the Bio-Rad ChemiDoc Touch System (Bio-Rad Laboratories, Hercules, CA, USA). Image analysis was performed using ImageJ software (Version 1.53; NIH, Bethesda, MD, USA).
2.5. Immunohistochemistry
The rats were sacrificed and then perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde solution (PFA) 7 days after modeling. Whole brains were removed and fixed with 4% paraformaldehyde for 48 h after sacrifice. Subsequently, 4 μm-thick paraffin sections of the globus pallidus were cut following paraffin embedding, with reference to the rat brain atlas [24]. Immunohistochemistry assays were performed using the universal two-step detection kit (ZSGB-BIO, Beijing, China). To evaluate the immunopositivity for GAD67 in the globi pallidi, the paraffin sections designated for immunohistochemistry were subjected to 1 h of baking at 60 °C. After deparaffinization and rehydration, heat-mediated antigen retrieval was performed with a Tris/EDTA buffer at pH 9.0, and then the sections were incubated overnight at 4 °C with the anti-GAD67 (1:500; Abcam, Cambridge, UK) antibody. Subsequently, the sections were incubated with the HRP-conjugated secondary antibody at room temperature for 30 min. Following rinsing with phosphate-buffered saline (PBS), the sections were treated with diaminobenzidine (DAB substrate kit; Abcam, Cambridge, UK) for 10 min and counterstained with hematoxylin for 1 min 30 s. Immunopositivity was examined with a slide scanning image system (Teksqray, Shenzhen, China), and representative images were displayed. For a clearer presentation of the results, Image-Pro Plus 6.0 (Media Cybernetics; Washington, D.C., USA) was used to calculate the mean optical density of the positive areas.
2.6. Body Weight
The body weights of the rats were recorded daily from day 5 to day 13 after birth to assess their general health during the rTMS intervention.
2.7. Rotarod Test
The rotarod test was performed at 28 days of age to evaluate motor ability. The rats were allowed to acclimate to the environment and instrument 1 day before this experiment. In the formal test, the rats were placed on a rotarod, which gradually increased in speed from 10 to 80 rpm over 3 min. This test concluded if the rat fell off the rotating rod, completed a full revolution while clinging to the rod or successfully remained on the rod for the entire 3 min. The equipment was cleaned with 70% ethanol before and after the testing of each rat. The duration of time that each rat stayed on the rotating rod was recorded, and this experiment was repeated three times to calculate the mean duration.
2.8. Statistical Analysis
Statistical analysis and graphing were performed using GraphPad Prism 9.0 (GraphPad Software, La Jolla, CA, USA). The data were expressed as means ± standard deviations (SDs). Group comparisons were assessed using one-way ANOVA followed by Tukey’s test for multiple comparisons. For the body weight data, two-way repeated-measures ANOVA, followed by Tukey’s multiple comparison test, was employed. Statistical significance was defined as p < 0.05.
3. Results
3.1. rTMS Improved the General Health and Motor Ability of Rats with Kernicterus
On the first day after modeling, the body weights of the model group and the rTMS group significantly decreased compared to the sham group (p < 0.001). Subsequently, although the body weights of the rats in the model group and the rTMS group gradually increased, they remained lower than those in the sham group (p < 0.001). The rTMS group, which underwent rTMS intervention, exhibited significant increases in body weight and faster recovery (p < 0.05) (Figure 2A). In the present experiment, the injection of UCB through the cisterna magna had a detrimental effect on the general health of the rats while the rTMS mitigated the damage caused by the UCB and promoted their recovery.
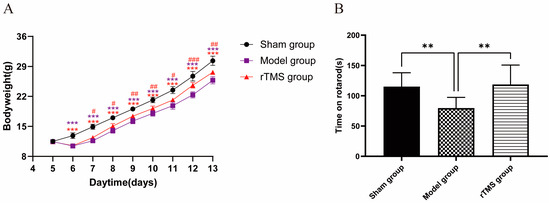
Figure 2.
rTMS improves the general health and motor ability of rats with kernicterus. (A) The effects of rTMS on the body weights of rats with kernicterus. Data are expressed as means ± SDs; *** p < 0.001 vs. the sham group; # p < 0.05, ## p < 0.01, ### p < 0.001 vs. the model group; n = 5. (B) Time on rotarod in the rotarod test. Data are expressed as means ± SDs; ** p < 0.01; n = 10.
The ultimate goal of this treatment was to improve motor ability, which was assessed using the rotarod test. The results revealed a significant increase in the residence times of the rats in the rTMS group compared to the model group (p < 0.05). Furthermore, there were no significant differences in the time spent on the rotarod between the rTMS group and the sham group (Figure 2B). These results suggest that the injection of UCB through the cisterna magna resulted in impaired motor ability, which could be mitigated by rTMS.
3.2. rTMS Increased the Expression of GAD67 in the Globi Pallidi of Rats with Kernicterus
The effects of the rTMS on the pallidal GABAergic neurons were assessed using western blotting and immunohistochemistry to detect the expression of GAD67 (GABAergic neuronal marker). The western blotting revealed a significant decrease in the GAD67 expression at 7 days after modeling (p < 0.001), while the rTMS treatment demonstrated a significant increase in the GAD67 expression (p < 0.05). Immunohistochemistry further supported these results by showing an increased number of GABAergic neurons in the globi pallidi in the rTMS group compared to the model group. The rTMS group showed stronger immunohistochemical staining for GAD67 in the globus pallidus than that in the model group. Quantitative analysis corroborated the western blotting results, confirming a significant upregulation of GAD67 in the rTMS group compared to the model group (p < 0.05) (Figure 3). The present results demonstrate that the UCB impaired the GABAergic neurons in the globus pallidus, while the rTMS were able to ameliorate the UCB-induced GABAergic neuronal lesions in the same area.
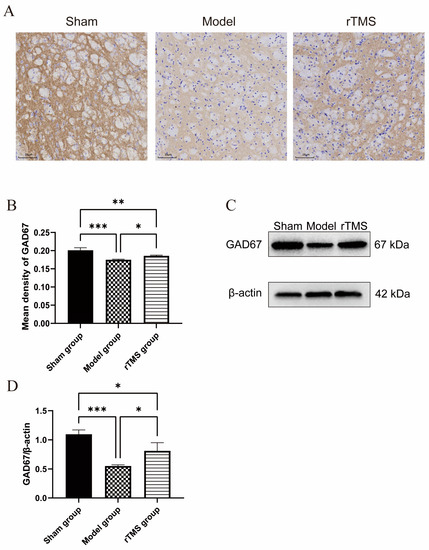
Figure 3.
rTMS increases GABAergic neuronal marker GAD67’s expression level in the globi pallidi of rats with kernicterus. (A) Results of immunohistochemistry staining in the globus pallidus. Scale bars: 25 μm. (B) Quantitative analysis of GAD67 in the globi pallidi. Data are expressed as means ± SDs; n = 3. * p < 0.05, ** p < 0.01, *** p < 0.001. (C) Results of western blotting analysis of GAD67 expression in the globus pallidus. (D) Relative protein expression of GAD67, normalized with β-actin. Data are expressed as means ± SDs; n = 3. * p < 0.05, *** p < 0.001.
3.3. rTMS Inhibited the Activation of Caspase-3
To investigate the potential mechanisms underlying the protective effects of rTMS on GABAergic neurons in the globus pallidus, we assessed caspase-3, a protein associated with apoptotic cell death. At 7 days after modeling, the western blotting demonstrated a significant increase in cleaved Caspase-3 expression in the model group compared to the sham group (p < 0.001). Conversely, rats treated with rTMS exhibited downregulation of cleaved Caspase-3 expression compared to the model group (p < 0.05) (Figure 4).
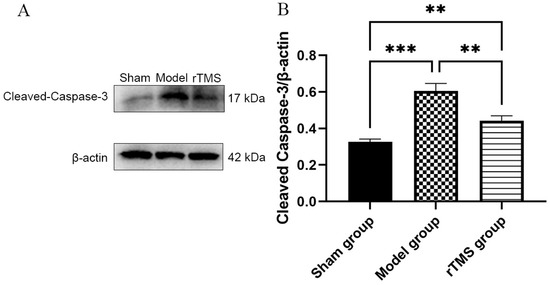
Figure 4.
rTMS inhibits activation of caspase-3 in the globi pallidi of rats with kernicterus. (A) Results of western blotting analysis of cleaved Caspase-3 expression in the globus pallidus. (B) Relative protein expression of cleaved Caspase-3, normalized with β-actin. Data are expressed as means ± SDs; n = 3. ** p < 0.01, *** p < 0.001.
4. Discussion
To our knowledge, this study is the first to have confirmed the protective effects of rTMS on pallidal GABAergic neurons in rats with kernicterus: improved general health and motor ability. The findings of this study revealed that the rTMS increased the protein expression levels of GAD67 while simultaneously inhibiting caspase-3 activation. The mechanism described in this study is shown in diagram form in Figure 5. In summary, rTMS could be a promising treatment for kernicterus, with the potential to inhibit apoptosis and exhibit protective effects on GABAergic neurons in the globus pallidus, thereby alleviating UCB-induced motor dysfunction.
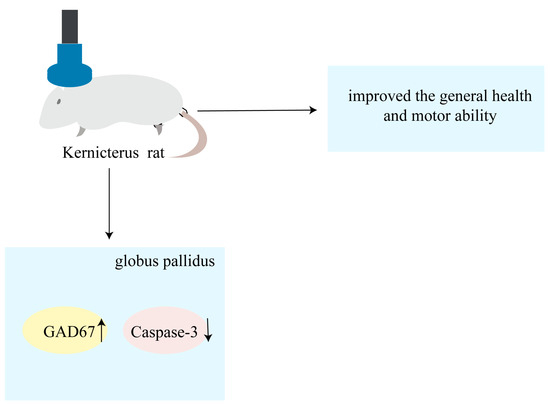
Figure 5.
Schematic representation showing that rTMS alleviates motor dysfunction and improves general health by inhibiting apoptosis and increasing globus pallidus GAD67 in rat models of kernicterus.
Kernicterus is caused by the accumulation of excessive UCB in the brain during severe neonatal hyperbilirubinemia [8]. It manifests as a preference for bilirubin neurotoxicity toward neurons and exhibits a regional pattern of UCB-induced neuronal injury, prominently affecting the globus pallidus, subthalamic nucleus, hippocampus, oculomotor nuclei, ventral cochlear nuclei and cerebellum [25]. Clinical manifestations of kernicterus include dystonia, choreoathetosis, auditory processing disturbances (with or without hearing loss), oculomotor impairments and dysplasia of the enamel of deciduous teeth [26]. It is believed that kernicteric dystonia arises from neurotoxic damage caused by UCB in the globus pallidus. Human magnetic resonance imaging (MRI) and autopsies have revealed the presence of bilateral lesions in the globus pallidus [27,28,29,30]. Furthermore, there has been a suggestion that kernicteric dystonia results from decreased output of the globus pallidus, which subsequently affects the input to downstream motor nuclei, causing abnormal movements and muscle tension [31]. UCB-induced neurological dysfunction encompasses various factors, including oxidative stress, neuroexcitotoxicity, neuroinflammation, mitochondrial energy failure, etc. [32,33,34,35]. UCB increases and prolongs the presence of the excitatory neurotransmitter glutamate in the synaptic cleft. Excessive glutamate overstimulates neuronal N-methyl-D-aspartate (NMDA) receptors, leading to increased influxes of sodium, calcium, chloride and water. This process generates free radicals and activates caspase-3, resulting in apoptosis [36]. UCB also interacts with neuronal cell membranes, inducing oxidative damage to these membranes and increased permeability. This interaction compromises the integrity of membrane-associated lipoprotein structures, disrupts cellular homeostasis and ultimately triggers neuronal apoptosis [37]. This study aimed to investigate the hypothesis that rTMS could ameliorate the motor dysfunction induced by UCB and exert a neuroprotective effect on the GABAergic neurons in the globi pallidi of rats with kernicterus. Our findings demonstrated that rTMS mitigated UCB-induced motor impairments and led to improved general health in rats with kernicterus. Additionally, our histological investigation revealed a higher preservation of GABAergic neurons in the globi pallidi in the rTMS group compared to the model group, indicating the neuroprotective effect of rTMS. Moreover, we have presented evidence showing that the rTMS significantly attenuated caspase-3 activation in the globus pallidus. These data demonstrate that rTMS may reduce UCB-induced loss of GABAergic neurons in the globus pallidus, partly by regulating apoptotic pathways.
rTMS is a safe and non-invasive therapeutic technique that employs an electromagnetic field generated by coils to stimulate induced currents within the brain, causing ions to move across cell membranes. The induced current generated by rTMS causes membrane potential depolarization and hyperpolarization, thereby modulating neuronal activity in damaged areas of the brain and ultimately ameliorating neurological dysfunction [38,39,40]. In recent decades, rTMS has been extensively utilized in the study of neuropsychiatric disorders. The neuroprotective effect of rTMS involves the following mechanisms: the regulation of neurotransmitters, such as increased dopamine in various brain regions as well as glutamate and GABA in the striatum and hippocampus, respectively [41,42]; the modulation of gene expression, such as upregulating brain-derived neurotrophic factor (BDNF) gene expression [43,44]; the alleviation of oxidative stress [45,46]; and the reduction of neuroinflammation and apoptosis [47,48]. rTMS was reported to inhibit the neurotoxic transformations of astrocytes, leading to reduced neuronal apoptosis and infarct volumes in ischemic rats [49]. A previous study has shown that rTMS attenuated oxidative stress, causing decreased cell loss in the striatum in a 3-nitropropionic model of Huntington’s disease [47]. Animal studies of Parkinson’s disease have revealed that rTMS decreased the levels of cyclooxygenase-2 and tumor necrosis factor-α in rat substantia nigra [48], improved motor functions and preserved the function of dopamine neurons [50]. Additionally, rTMS has been found to reduce glutamate-driven excitotoxicity and motor neuron death in amyotrophic lateral sclerosis (ALS) [51]. The neuronal damage caused by UCB is known to be associated with oxidative stress, neuroexcitotoxicity and neuroinflammation in kernicterus. In this study, the neuroprotective effects of rTMS on pallidal GABAergic neurons may involve oxidative stress, neuroexcitotoxicity and neuroinflammation.
Motor dysfunction is one of the primary reasons for physical disability in kernicterus. In this study, we evaluated the motor ability of the rats by the rotarod test. It was reported that the kernicterus-model rats had significantly worse performances on the rotarod test than the controls, indicating that the kernicterus-model rats experienced motor function deficits [52]. The UCB-treated rats showed significant motor dysfunction in this test. After rTMS intervention, we found that the rats in the rTMS group performed better in the rotarod test than those in the model group, which indicates that the rTMS alleviated the UCB-induced motor dysfunction. A previous study has revealed that the body weights of rats with kernicterus were lower than those of a sham group [23], which is consistent with our results. Our data have shown that rTMS can reduce UCB-induced adverse effects on kernicterus-model rats’ body weights, suggesting that it could improve the growth and development of rats with kernicterus.
Irreversible damage becomes apparent in brain tissue 24 h after bilirubin injection, and the symptoms will become noticeably pronounced after seven days of modeling [53]. In this study, rTMS was conducted during the acute phase. Western blotting and immunohistochemistry were employed to evaluate the expression levels of GAD67 and apoptosis-related protein caspase-3 after the rTMS interventions. Early exposure to bilirubin would result in long-term motor function abnormalities; thus, behavioral tests were performed to evaluate the effects of rTMS on the long-term motor function of the model rats. Our data demonstrated a higher expression of GABAergic neuronal marker GAD67 in the rTMS group compared to the model group. The results of our study have suggested that rTMS may not only improve motor functions but also exert neuroprotective effects on GABAergic neurons in the globi pallidi of rats with kernicterus. The neuroprotective effects of rTMS on GABAergic neurons may be attributed to reductions in neuroexcitotoxicity, the attenuation of oxidative stress and neuroinflammation; the upregulation of neurotrophic factors such as the brain-derived neurotrophic factor (BDNF); and the mitigation of astrogliosis [35,36,54]. The globus pallidus is a primary target of UCB-induced injury in a rat model of kernicterus, primarily consisting of GABAergic and cholinergic neurons, with the GABAergic neurons accounting for 95% of the population. The neurotoxic impacts of unconjugated bilirubin can result in the damage or death of pallidal neurons, ultimately resulting in irreversible motor function impairment [55,56]. GABA serves as the principal inhibitory neurotransmitter in the basal ganglia, and abnormalities in GABAergic circuitry within these ganglia have been implicated in various neurological disorders and degenerative diseases, such as Huntington’s disease, Parkinson’s disease and hyperkinesia [57,58,59]. In the present study, the improvement of motor ability may be associated with the increased expression of GABAergic neuronal marker GAD67. Deep brain stimulation (DBS) has been established as an effective treatment technology for movement disorders [60,61]. Moreover, DBS leads are usually placed in the globus pallidus interna in the treatment of dystonia, which partly explains the increased expression of the pallidal neuron marker GAD67 and the improvement in motor function in our study. Previous studies have revealed that bilirubin injection could trigger the activation of caspase-3, particularly in the globus pallidus [53,62]. Therefore, in this study, pallidal GABAergic neurons were selected as the subjects of investigation for examination of the effects of rTMS on these neurons and their subsequent impacts on motor function. We found that rTMS suppressed the activation of caspase-3, suggesting that the neuroprotective effect of rTMS may involve the inhibition of the apoptotic program mediated by caspase-3. Despite this progress, the precise underlying mechanism remains unclear, necessitating further research to elucidate the detailed neuroprotective mechanism of rTMS in kernicterus.
There were several limitations to our study. First, it was conducted as a preliminary investigation, wherein we provided initial evidence of the neuroprotective effect of rTMS in rats with kernicterus. Thus, further research and verification are required to elucidate the specific mechanism involved. Second, despite our use of the smallest available animal-specific coil, rTMS in rodents has limitations that may result in whole-brain stimulation. Third, the sample size was relatively small; thus, our findings should be confirmed in future large-scale studies. Fourth, we omitted the inclusion of a sham + rTMS group, since GAD67 regulation is among the therapeutic effects of rTMS in rat models of kernicterus, which were our primary focus. The effects of rTMS in isolation have yet to be elucidated and warrant further investigation. Finally, we relied solely on the rotarod test to evaluate the motor ability of the rats. Considering the limitations of our study, further investigation is needed.
5. Conclusions
The present study has provided evidence that rTMS could alleviate motor dysfunction in rats with kernicterus. It may also exert neuroprotective effects on pallidum damage induced by UCB by inhibiting apoptosis and improving the function of the GABAergic neurons in the globus pallidus. Our findings suggest that rTMS is a promising treatment for kernicterus.
Author Contributions
Conceptualization, N.X. and N.W.; methodology, N.X. and N.W.; software, H.Z.; validation, N.X., Y.J. and N.W.; formal analysis, X.W. and Y.J.; investigation, N.W.; resources, N.W.; data curation, Y.J.; writing—original draft preparation, N.W.; writing—review and editing, N.W. and X.Z.; visualization, N.W.; supervision, N.X.; project administration, N.X.; funding acquisition, N.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Children’s Hospital of Chongqing Medical University (CHCMU-IACUC20230529003).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding authors upon reasonable request.
Acknowledgments
Thanks go to the experts and colleagues for their help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mitra, S.; Rennie, J. Neonatal jaundice: Aetiology, diagnosis and treatment. Br. J. Hosp. Med. 2017, 78, 699–704. [Google Scholar] [CrossRef]
- Kuzniewicz, M.W.; Escobar, G.J.; Wi, S.; Liljestrand, P.; McCulloch, C.; Newman, T.B. Risk factors for severe hyperbilirubinemia among infants with borderline bilirubin levels: A nested case-control study. J. Pediatr. 2008, 153, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Watchko, J.F.; Tiribelli, C. Bilirubin-induced neurologic damage-mechanisms and management approaches. N. Engl. J. Med. 2013, 369, 2021–2030. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Hammerman, C. Understanding severe hyperbilirubinemia and preventing kernicterus: Adjuncts in the interpretation of neonatal serum bilirubin. Clin. Chim. Acta 2005, 356, 9–21. [Google Scholar] [CrossRef]
- Gottimukkala, S.B.; Lobo, L.; Gautham, K.S.; Bolisetty, S.; Fiander, M.; Schindler, T. Intermittent phototherapy versus continuous phototherapy for neonatal jaundice. Cochrane Database Syst. Rev. 2023, 3, Cd008168. [Google Scholar] [CrossRef]
- Olusanya, B.O.; Ogunlesi, T.A.; Slusher, T.M. Why is kernicterus still a major cause of death and disability in low-income and middle-income countries? Arch. Dis. Child. 2014, 99, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Kasirer, Y.; Kaplan, M.; Hammerman, C. Kernicterus on the Spectrum. NeoReviews 2023, 24, e329–e342. [Google Scholar] [CrossRef]
- Shapiro, S.M. Chronic bilirubin encephalopathy: Diagnosis and outcome. Semin. Fetal Neonatal Med. 2010, 15, 157–163. [Google Scholar] [CrossRef]
- Riordan, S.M.; Shapiro, S.M. Review of bilirubin neurotoxicity I: Molecular biology and neuropathology of disease. Pediatr. Res. 2020, 87, 327–331. [Google Scholar] [CrossRef]
- Hegeman, D.J.; Hong, E.S.; Hernandez, V.M.; Chan, C.S. The external globus pallidus: Progress and perspectives. Eur. J. Neurosci. 2016, 43, 1239–1265. [Google Scholar] [CrossRef]
- Mallet, N.; Micklem, B.R.; Henny, P.; Brown, M.T.; Williams, C.; Bolam, J.P.; Nakamura, K.C.; Magill, P.J. Dichotomous organization of the external globus pallidus. Neuron 2012, 74, 1075–1086. [Google Scholar] [CrossRef]
- Gazzin, S.; Zelenka, J.; Zdrahalova, L.; Konickova, R.; Zabetta, C.C.; Giraudi, P.J.; Berengeno, A.L.; Raseni, A.; Robert, M.C.; Vitek, L.; et al. Bilirubin accumulation and Cyp mRNA expression in selected brain regions of jaundiced Gunn rat pups. Pediatr. Res. 2012, 71, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.; Sola, S.; Brites, D. Bilirubin induces apoptosis via the mitochondrial pathway in developing rat brain neurons. Hepatology 2002, 35, 1186–1195. [Google Scholar] [CrossRef]
- Vodret, S.; Bortolussi, G.; Iaconcig, A.; Martinelli, E.; Tiribelli, C.; Muro, A.F. Attenuation of neuro-inflammation improves survival and neurodegeneration in a mouse model of severe neonatal hyperbilirubinemia. Brain Behav. Immun. 2018, 70, 166–178. [Google Scholar] [CrossRef]
- Smith, K.; Leyden, J.J. Safety of doxycycline and minocycline: A systematic review. Clin. Ther. 2005, 27, 1329–1342. [Google Scholar] [CrossRef]
- Shapiro, S.M.; Riordan, S.M. Review of bilirubin neurotoxicity II: Preventing and treating acute bilirubin encephalopathy and kernicterus spectrum disorders. Pediatr. Res. 2020, 87, 332–337. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A.; Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.J.; Tunez, I. Mechanisms and pathways underlying the therapeutic effect of transcranial magnetic stimulation. Rev. Neurosci. 2013, 24, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Aguera, E.; Caballero-Villarraso, J.; Feijoo, M.; Escribano, B.M.; Conde, C.; Bahamonde, M.C.; Giraldo, A.I.; Paz-Rojas, E.; Tunez, I. Clinical and Neurochemical Effects of Transcranial Magnetic Stimulation (TMS) in Multiple Sclerosis: A Study Protocol for a Randomized Clinical Trial. Front. Neurol. 2020, 11, 750. [Google Scholar] [CrossRef]
- Gao, F.; Wang, S.; Guo, Y.; Wang, J.; Lou, M.; Wu, J.; Ding, M.; Tian, M.; Zhang, H. Protective effects of repetitive transcranial magnetic stimulation in a rat model of transient cerebral ischaemia: A microPET study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 954–961. [Google Scholar] [CrossRef]
- Buetefisch, C.M.; Wei, L.; Gu, X.; Epstein, C.M.; Yu, S.P. Neuroprotection of Low-Frequency Repetitive Transcranial Magnetic Stimulation after Ischemic Stroke in Rats. Ann. Neurol. 2023, 93, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, R.P.; Hance, A.J. Experimental bilirubin encephalopathy: Importance of total bilirubin, protein binding, and blood-brain barrier. Pediatr. Res. 1986, 20, 789–792. [Google Scholar] [CrossRef]
- Song, S.; Hu, Y.; Gu, X.; Si, F.; Hua, Z. A novel newborn rat kernicterus model created by injecting a bilirubin solution into the cisterna magna. PLoS ONE 2014, 9, e96171. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Watchko, J.F. Kernicterus and the molecular mechanisms of bilirubin-induced CNS injury in newborns. Neuromolecular Med. 2006, 8, 513–529. [Google Scholar] [CrossRef]
- Das, S.; van Landeghem, F.K.H. Clinicopathological Spectrum of Bilirubin Encephalopathy/Kernicterus. Diagnostics 2019, 9, 24. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Alper, G.; Kilicoglu, G.; Celik, L.; Karadeniz, L.; Yilmaz-Degirmenci, S. Magnetic resonance imaging findings in patients with severe neonatal indirect hyperbilirubinemia. J. Child Neurol. 2001, 16, 452–455. [Google Scholar] [CrossRef]
- Sugama, S.; Soeda, A.; Eto, Y. Magnetic resonance imaging in three children with kernicterus. Pediatr. Neurol. 2001, 25, 328–331. [Google Scholar] [CrossRef]
- Govaert, P.; Lequin, M.; Swarte, R.; Robben, S.; De Coo, R.; Weisglas-Kuperus, N.; De Rijke, Y.; Sinaasappel, M.; Barkovich, J. Changes in globus pallidus with (pre)term kernicterus. Pediatrics 2003, 112, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, Y.; Hayashi, M. Bilirubin encephalopathy: A study of neuronal subpopulations and neurodegenerative mechanisms in 12 autopsy cases. Brain Dev. 2008, 30, 269–278. [Google Scholar] [CrossRef]
- Johnston, M.V.; Hoon, A.H., Jr. Possible mechanisms in infants for selective basal ganglia damage from asphyxia, kernicterus, or mitochondrial encephalopathies. J. Child Neurol. 2000, 15, 588–591. [Google Scholar] [CrossRef]
- Rawat, V.; Bortolussi, G.; Gazzin, S.; Tiribelli, C.; Muro, A.F. Bilirubin-Induced Oxidative Stress Leads to DNA Damage in the Cerebellum of Hyperbilirubinemic Neonatal Mice and Activates DNA Double-Strand Break Repair Pathways in Human Cells. Oxidative Med. Cell. Longev. 2018, 2018, 1801243. [Google Scholar] [CrossRef]
- Brito, M.A.; Vaz, A.R.; Silva, S.L.; Falcao, A.S.; Fernandes, A.; Silva, R.F.; Brites, D. N-methyl-aspartate receptor and neuronal nitric oxide synthase activation mediate bilirubin-induced neurotoxicity. Mol. Med. 2010, 16, 372–380. [Google Scholar] [CrossRef]
- Ostrow, J.D.; Pascolo, L.; Brites, D.; Tiribelli, C. Molecular basis of bilirubin-induced neurotoxicity. Trends Mol. Med. 2004, 10, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Watchko, J.F. Bilirubin-Induced Neurotoxicity in the Preterm Neonate. Clin. Perinatol. 2016, 43, 297–311. [Google Scholar] [CrossRef]
- Qian, S.; Kumar, P.; Testai, F.D. Bilirubin Encephalopathy. Curr. Neurol. Neurosci. Rep. 2022, 22, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.P.; Solá, S.; Castro, R.E.; Laires, P.A.; Brites, D.; Moura, J.J.G. Perturbation of membrane dynamics in nerve cells as an early event during bilirubin-induced apoptosis. J. Lipid Res. 2002, 43, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Zorzo, C.; Higarza, S.G.; Mendez, M.; Martinez, J.A.; Pernia, A.M.; Arias, J.L. High frequency repetitive transcranial magnetic stimulation improves neuronal activity without affecting astrocytes and microglia density. Brain Res. Bull. 2019, 150, 13–20. [Google Scholar] [CrossRef]
- Hong, Y.; Lyu, J.; Zhu, L.; Wang, X.; Peng, M.; Chen, X.; Deng, Q.; Gao, J.; Yuan, Z.; Wang, D.; et al. High-frequency repetitive transcranial magnetic stimulation (rTMS) protects against ischemic stroke by inhibiting M1 microglia polarization through let-7b-5p/HMGA2/NF-kappaB signaling pathway. BMC Neurosci. 2022, 23, 49. [Google Scholar] [CrossRef]
- Lefaucheur, J.P. Principles of therapeutic use of transcranial and epidural cortical stimulation. Clin. Neurophysiol. 2008, 119, 2179–2184. [Google Scholar] [CrossRef]
- Cho, S.S.; Strafella, A.P. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS ONE 2009, 4, e6725. [Google Scholar] [CrossRef]
- Yue, L.; Xiao-lin, H.; Tao, S. The effects of chronic repetitive transcranial magnetic stimulation on glutamate and gamma-aminobutyric acid in rat brain. Brain Res. 2009, 1260, 94–99. [Google Scholar] [CrossRef]
- Müller, M.B.; Toschi, N.; Kresse, A.E.; Post, A.; Keck, M.E. Long-term repetitive transcranial magnetic stimulation increases the expression of brain-derived neurotrophic factor and cholecystokinin mRNA, but not neuropeptide tyrosine mRNA in specific areas of rat brain. Neuropsychopharmacology 2000, 23, 205–215. [Google Scholar] [CrossRef]
- Lang, U.E.; Hellweg, R.; Gallinat, J.; Bajbouj, M. Acute prefrontal cortex transcranial magnetic stimulation in healthy volunteers: No effects on brain-derived neurotrophic factor (BDNF) concentrations in serum. J. Affect. Disord. 2008, 107, 255–258. [Google Scholar] [CrossRef]
- Tunez, I.; Montilla, P.; del Carmen Munoz, M.; Medina, F.J.; Drucker-Colin, R. Effect of transcranial magnetic stimulation on oxidative stress induced by 3-nitropropionic acid in cortical synaptosomes. Neurosci. Res. 2006, 56, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Aguera, E.; Caballero-Villarraso, J.; Feijoo, M.; Escribano, B.M.; Bahamonde, M.C.; Conde, C.; Galvan, A.; Tunez, I. Impact of Repetitive Transcranial Magnetic Stimulation on Neurocognition and Oxidative Stress in Relapsing-Remitting Multiple Sclerosis: A Case Report. Front. Neurol. 2020, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Tunez, I.; Drucker-Colin, R.; Jimena, I.; Medina, F.J.; Munoz Mdel, C.; Pena, J.; Montilla, P. Transcranial magnetic stimulation attenuates cell loss and oxidative damage in the striatum induced in the 3-nitropropionic model of Huntington’s disease. J. Neurochem. 2006, 97, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, L.; Liu, Z. The effect of repetitive transcranial magnetic stimulation on a model rat of Parkinson’s disease. Neuroreport 2010, 21, 268–272. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, Q.; Peng, M.; Bai, M.; Li, J.; Sun, R.; Guo, H.; Xu, P.; Xie, Y.; Li, Y.; et al. High-frequency repetitive transcranial magnetic stimulation improves functional recovery by inhibiting neurotoxic polarization of astrocytes in ischemic rats. J. Neuroinflammation 2020, 17, 150. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; He, X.-K.; Liu, H.-H.; Chen, J.-J.J.; Peng, C.-W.; Liu, H.-L.; Rotenberg, A.; Chen, K.-T.; Chang, M.-Y.; Chiang, Y.-H.; et al. Early Repetitive Transcranial Magnetic Stimulation Exerts Neuroprotective Effects and Improves Motor Functions in Hemiparkinsonian Rats. Neural Plast. 2021, 2021, 1763533. [Google Scholar] [CrossRef]
- Dileone, M.; Profice, P.; Pilato, F.; Ranieri, F.; Capone, F.; Musumeci, G.; Florio, L.; Di Iorio, R.; Di Lazzaro, V. Repetitive transcranial magnetic stimulation for ALS. CNS Neurol. Disord. Drug Targets 2010, 9, 331–334. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Wei, Q.; He, C.; Feng, J.; Wang, Y.; Li, M.; Zhang, Q.; Xia, X.; Hua, Z. Depression of Pyroptosis by Inhibiting Caspase-1 Activation Improves Neurological Outcomes of Kernicterus Model Rats. ACS Chem. Neurosci. 2021, 12, 2929–2939. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.H.; Fan, P.; Zhang, L.R.; Chen, C.Y.; Xu, J.; Huang, J.; Lu, W.T.; Zhu, S.J.; Qiu, G.P.; Xu, S.Y.; et al. Neuroglobin expression and function in the temporal cortex of bilirubin encephalopathy rats. Anat. Rec. 2022, 305, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Villarraso, J.; Medina, F.J.; Escribano, B.M.; Agüera, E.; Santamaría, A.; Pascual-Leone, A.; Túnez, I. Mechanisms Involved in Neuroprotective Effects of Transcranial Magnetic Stimulation. CNS Neurol. Disord. Drug Targets 2022, 21, 557–573. [Google Scholar] [CrossRef]
- Hernandez, V.M.; Hegeman, D.J.; Cui, Q.; Kelver, D.A.; Fiske, M.P.; Glajch, K.E.; Pitt, J.E.; Huang, T.Y.; Justice, N.J.; Chan, C.S. Parvalbumin+ Neurons and Npas1+ Neurons Are Distinct Neuron Classes in the Mouse External Globus Pallidus. J. Neurosci. 2015, 35, 11830–11847. [Google Scholar] [CrossRef] [PubMed]
- Abdi, A.; Mallet, N.; Mohamed, F.Y.; Sharott, A.; Dodson, P.D.; Nakamura, K.C.; Suri, S.; Avery, S.V.; Larvin, J.T.; Garas, F.N.; et al. Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus. J. Neurosci. 2015, 35, 6667–6688. [Google Scholar] [CrossRef]
- Kleppner, S.R.; Tobin, A.J. GABA signalling: Therapeutic targets for epilepsy, Parkinson’s disease and Huntington’s disease. Expert Opin. Ther. Targets 2001, 5, 219–239. [Google Scholar] [CrossRef]
- Gajcy, K.; Lochyński, S.; Librowski, T. A role of GABA analogues in the treatment of neurological diseases. Curr. Med. Chem. 2010, 17, 2338–2347. [Google Scholar] [CrossRef]
- Galvan, A.; Wichmann, T. GABAergic circuits in the basal ganglia and movement disorders. Prog. Brain Res. 2007, 160, 287–312. [Google Scholar] [CrossRef]
- Haridas, A.; Tagliati, M.; Osborn, I.; Isaias, I.; Gologorsky, Y.; Bressman, S.B.; Weisz, D.; Alterman, R.L. Pallidal deep brain stimulation for primary dystonia in children. Neurosurgery 2011, 68, 738–743. [Google Scholar] [CrossRef]
- Sanger, T.D.; Liker, M.; Arguelles, E.; Deshpande, R.; Maskooki, A.; Ferman, D.; Tongol, A.; Robison, A. Pediatric Deep Brain Stimulation Using Awake Recording and Stimulation for Target Selection in an Inpatient Neuromodulation Monitoring Unit. Brain Sci. 2018, 8, 135. [Google Scholar] [CrossRef]
- Baysal, B.; Tüzün, F.; Yücesoy, E.; Özbal, S.; Uğur, B.E.; Sonmez, A.; Olgun, Y.; Kirkim, G.; Evin, H.; Duman, N.; et al. Topiramate and darbepoetin alpha ameliorate bilirubin-induced neuronal cell damage. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3585–3593. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).