Early Measures of TBI Severity Poorly Predict Later Individual Impairment in a Rat Fluid Percussion Model
Abstract
1. Introduction
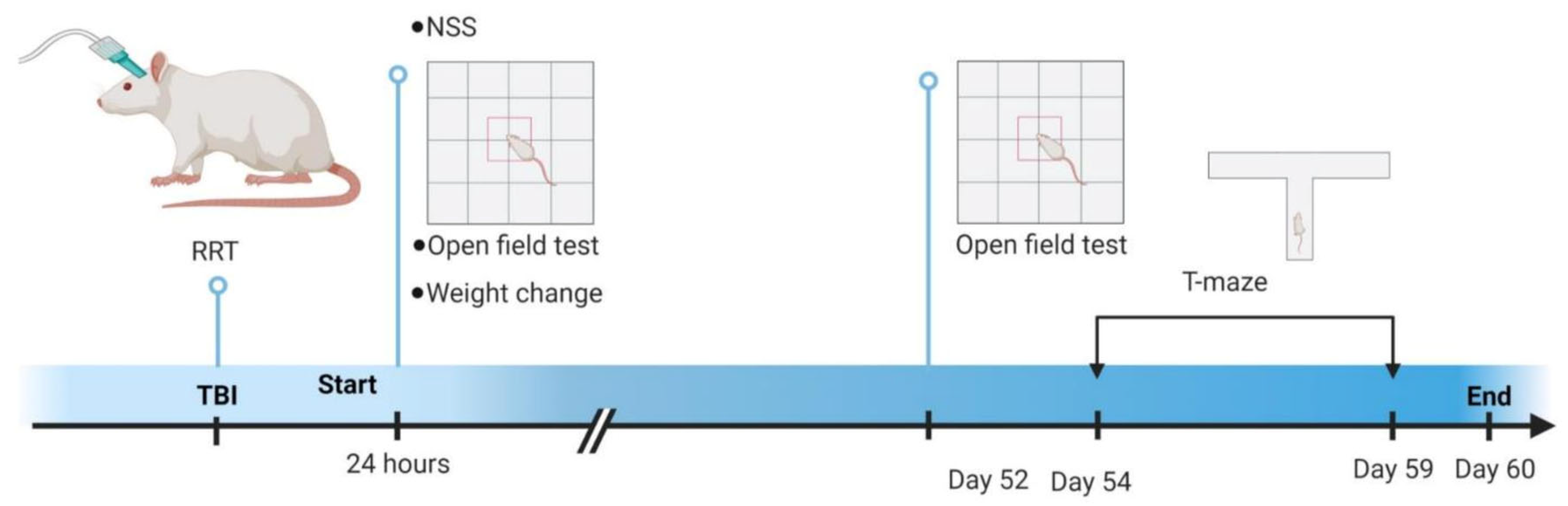
2. Materials and Methods
2.1. Subjects
2.2. Fluid Percussion
2.3. Injury Appraisal
2.4. Neurological Severity Score and Locomotor Activity
2.5. T-Maze Alternation
2.6. Statistical Analysis
3. Results
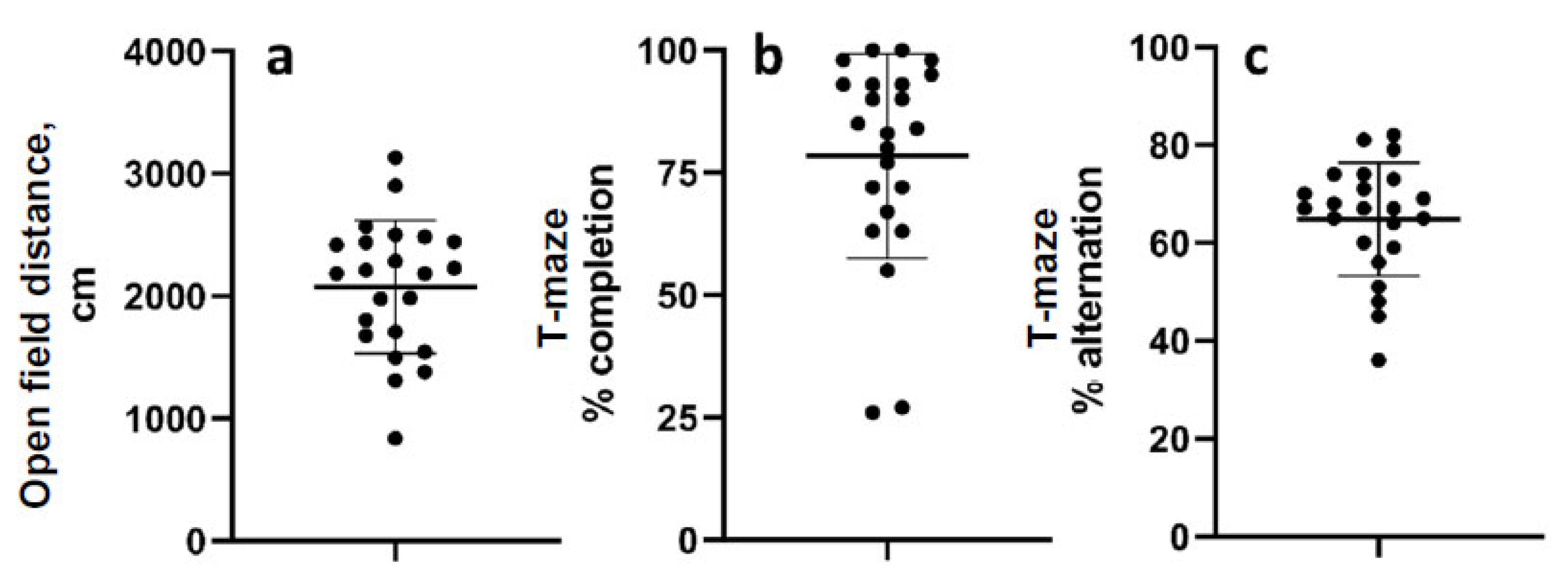
3.1. Acute Injury Evaluation
3.2. Chronic Behavioral Measurements
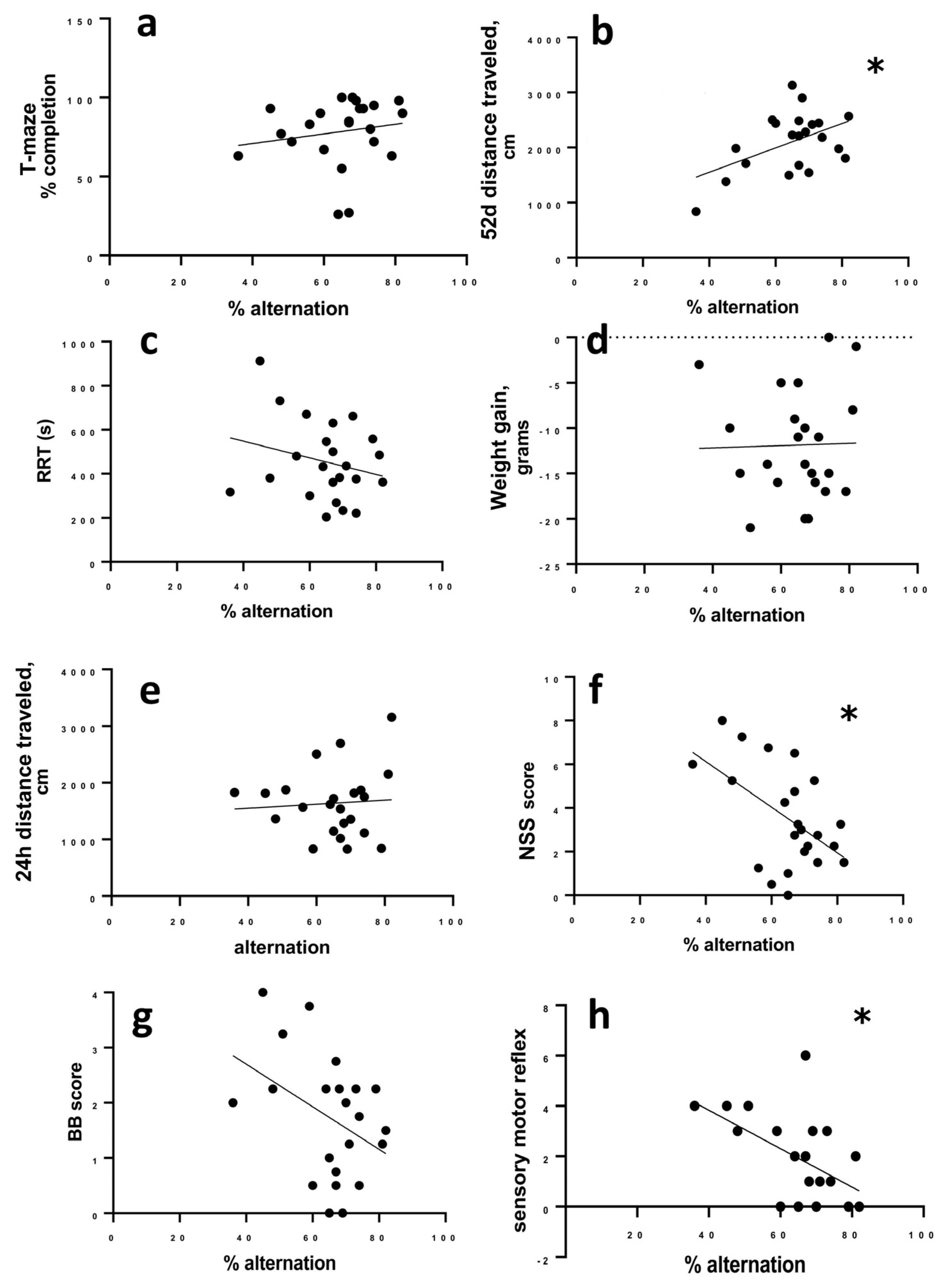
3.3. Correlation of Behavioral and Injury Severity Measurements to Percent Alternation
4. Discussion
4.1. Reflex Righting Time
4.2. Change in Weight after TBI
4.3. Neurological Severity Score
4.4. Open Field Test
4.5. Spontaneous Alternation Task
4.6. Clinical Prediction Tools
4.7. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The epidemiology and impact of traumatic brain injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Roozenbeek, B.; Maas, A.I.; Menon, D.K. Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 2013, 9, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Van der Naalt, J.; Timmerman, M.E.; de Koning, M.E.; van der Horn, H.J.; Scheenen, M.E.; Jacobs, B.; Hageman, G.; Yilmaz, T.; Roks, G.; Spikman, J.M. Early predictors of outcome after mild traumatic brain injury (UPFRONT): An observational cohort study. Lancet Neurol. 2017, 16, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Seagly, K.S.; O’Neil, R.L.; Hanks, R.A. Pre-injury psychosocial and demographic predictors of long-term functional outcomes post-TBI. Brain Inj. 2018, 32, 78–83. [Google Scholar] [CrossRef]
- Fay, T.B.; Yeates, K.O.; Taylor, H.G.; Bangert, B.; Dietrich, A.; Nuss, K.E.; Rusin, J.; Wright, M. Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2010, 16, 94–105. [Google Scholar] [CrossRef]
- Hukkelhoven, C.W.; Steyerberg, E.W.; Rampen, A.J.; Farace, E.; Habbema, J.D.; Marshall, L.F.; Murray, G.D.; Maas, A.I. Patient age and outcome following severe traumatic brain injury: An analysis of 5600 patients. J. Neurosurg. 2003, 99, 666–673. [Google Scholar] [CrossRef]
- Hukkelhoven, C.W.; Steyerberg, E.W.; Habbema, J.D.; Farace, E.; Marmarou, A.; Murray, G.D.; Marshall, L.F.; Maas, A.I. Predicting outcome after traumatic brain injury: Development and validation of a prognostic score based on admission characteristics. J. Neurotrauma 2005, 22, 1025–1039. [Google Scholar] [CrossRef]
- Hukkelhoven, C.W.; Rampen, A.J.; Maas, A.I.; Farace, E.; Habbema, J.D.; Marmarou, A.; Marshall, L.F.; Murray, G.D.; Steyerberg, E.W. Some prognostic models for traumatic brain injury were not valid. J. Clin. Epidemiol. 2006, 59, 132–143. [Google Scholar] [CrossRef]
- Smith, D.H.; Hicks, R.R.; Johnson, V.E.; Bergstrom, D.A.; Cummings, D.M.; Noble, L.J.; Hovda, D.; Whalen, M.; Ahlers, S.T.; LaPlaca, M.; et al. Pre-Clinical Traumatic Brain Injury Common Data Elements: Toward a Common Language Across Laboratories. J. Neurotrauma 2015, 32, 1725–1735. [Google Scholar] [CrossRef]
- Osier, N.D.; Carlson, S.W.; DeSana, A.; Dixon, C.E. Chronic Histopathological and Behavioral Outcomes of Experimental Traumatic Brain Injury in Adult Male Animals. J. Neurotrauma 2015, 32, 1861–1882. [Google Scholar] [CrossRef]
- Perrin, P.B.; Niemeier, J.P.; Mougeot, J.L.; Vannoy, C.H.; Hirsch, M.A.; Watts, J.A.; Rossman, W.; Grafton, L.M.; Guerrier, T.D.; Pershad, R.; et al. Measures of injury severity and prediction of acute traumatic brain injury outcomes. J. Head Trauma Rehabil. 2015, 30, 136–142. [Google Scholar] [CrossRef]
- Ouyang, W.; Wu, W.; Fan, Z.; Wang, J.; Pan, H.; Yang, W. Modified device for fluid percussion injury in rodents. J. Neurosci. Res. 2018, 96, 1412–1429. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.L.; Correll, E.A.; Lowery, A.C.; Rhame, K.; Anwar, F.N.; McCullumsmith, R.E.; Ngwenya, L.B. Pioglitazone improves working memory performance when administered in chronic TBI. Neurobiol. Dis. 2019, 132, 104611. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.L.; DePasquale, E.A.K.; Watanabe, M.; Anwar, F.; Ngwenya, L.B.; Atluri, G.; Romick-Rosendale, L.E.; McCullumsmith, R.E.; Evanson, N.K. Chronic Dysregulation of Cortical and Subcortical Metabolism After Experimental Traumatic Brain Injury. Mol. Neurobiol. 2019, 56, 2908–2921. [Google Scholar] [CrossRef] [PubMed]
- Yarnell, A.M.; Barry, E.S.; Mountney, A.; Shear, D.; Tortella, F.; Grunberg, N.E. The Revised Neurobehavioral Severity Scale (NSS-R) for Rodents. Curr. Protoc. Neurosci. 2016, 75, 9.52.51–59.52.16. [Google Scholar] [CrossRef]
- Deacon, R.M.; Rawlins, J.N. T-maze alternation in the rodent. Nat. Protoc. 2006, 1, 7–12. [Google Scholar] [CrossRef]
- McCrory, P.R.; Berkovic, S.F. Video analysis of acute motor and convulsive manifestations in sport-related concussion. Neurology 2000, 54, 1488–1491. [Google Scholar] [CrossRef]
- Whyte, J.; Cifu, D.; Dikmen, S.; Temkin, N. Prediction of functional outcomes after traumatic brain injury: A comparison of 2 measures of duration of unconsciousness. Arch. Phys. Med. Rehabil. 2001, 82, 1355–1359. [Google Scholar] [CrossRef]
- Dewitt, D.S.; Perez-Polo, R.; Hulsebosch, C.E.; Dash, P.K.; Robertson, C.S. Challenges in the development of rodent models of mild traumatic brain injury. J. Neurotrauma 2013, 30, 688–701. [Google Scholar] [CrossRef]
- Prins, M.L.; Lee, S.M.; Cheng, C.L.; Becker, D.P.; Hovda, D.A. Fluid percussion brain injury in the developing and adult rat: A comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res. Dev. Brain Res. 1996, 95, 272–282. [Google Scholar] [CrossRef]
- Dixon, C.E.; Lyeth, B.G.; Povlishock, J.T.; Findling, R.L.; Hamm, R.J.; Marmarou, A.; Young, H.F.; Hayes, R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987, 67, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Hallam, T.M.; Floyd, C.L.; Folkerts, M.M.; Lee, L.L.; Gong, Q.Z.; Lyeth, B.G.; Muizelaar, J.P.; Berman, R.F. Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. J. Neurotrauma 2004, 21, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Grin’kina, N.M.; Li, Y.; Haber, M.; Sangobowale, M.; Nikulina, E.; Le’Pre, C.; El Sehamy, A.M.; Dugue, R.; Ho, J.S.; Bergold, P.J. Righting Reflex Predicts Long-Term Histological and Behavioral Outcomes in a Closed Head Model of Traumatic Brain Injury. PLoS ONE 2016, 11, e0161053. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.K.; Harrison, J.L.; Ellis, T.W.; Adelson, P.D.; Lifshitz, J. Midline (central) fluid percussion model of traumatic brain injury in pediatric and adolescent rats. J. Neurosurg. Pediatr. 2018, 22, 22–30. [Google Scholar] [CrossRef]
- Bondi, C.O.; Cheng, J.P.; Tennant, H.M.; Monaco, C.M.; Kline, A.E. Old Dog, New Tricks: The Attentional Set-Shifting Test as a Novel Cognitive Behavioral Task after Controlled Cortical Impact Injury. J. Neurotrauma 2014, 31, 926–937. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Povlishock, J.T.; Katz, D.I. Update of neuropathology and neurological recovery after traumatic brain injury. J. Head Trauma Rehabil. 2005, 20, 76–94. [Google Scholar] [CrossRef]
- Ross, B.L.; Temkin, N.R.; Newell, D.; Dikmen, S.S. Neuropsychological outcome in relation to head injury severity. Contributions of coma length and focal abnormalities. Am. J. Phys. Med. Rehabil. 1994, 73, 341–347. [Google Scholar] [CrossRef]
- Kothari, S.; Zhang, B.E.I.; Darji, N.; Woo, J. Prognosis After Moderate to Severe Traumatic Brain Injury: A Practical, Evidence-Based Approach; Zasler, N.D., Katz, D.I., Zafonte, R.D., Arciniegas, D.B., Bullock, M.R., Hammond, F.M., Kreutzer, J.S., Nakase-Richardson, R., Watanabe, T.K., Eds.; Springer Publishing Company: New York, NY, USA, 2021; pp. 247–270. [Google Scholar]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Marmarou, A.; Foda, M.A.; van den Brink, W.; Campbell, J.; Kita, H.; Demetriadou, K. A new model of diffuse brain injury in rats. Part I: Pathophysiology and biomechanics. J. Neurosurg. 1994, 80, 291–300. [Google Scholar] [CrossRef]
- Eakin, K.; Rowe, R.K.; Lifshitz, J. Modeling Fluid Percussion Injury: Relevance to Human Traumatic Brain Injury. In Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects; Kobeissy, F.H., Ed.; Taylor & Francis Group, LLC: Abingdon, UK, 2015. [Google Scholar]
- Lapinlampi, N.; Andrade, P.; Paananen, T.; Hämäläinen, E.; Ekolle Ndode-Ekane, X.; Puhakka, N.; Pitkänen, A. Postinjury weight rather than cognitive or behavioral impairment predicts development of posttraumatic epilepsy after lateral fluid-percussion injury in rats. Epilepsia 2020, 61, 2035–2052. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wang, H.; Xu, J.; Lu, X.; Zhang, L.; Zhu, L. Melatonin reduced microglial activation and alleviated neuroinflammation induced neuron degeneration in experimental traumatic brain injury: Possible involvement of mTOR pathway. Neurochem. Int. 2014, 76, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Yatsiv, I.; Grigoriadis, N.; Simeonidou, C.; Stahel, P.F.; Schmidt, O.I.; Alexandrovich, A.G.; Tsenter, J.; Shohami, E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005, 19, 1701–1703. [Google Scholar] [CrossRef] [PubMed]
- Huynh, L.M.; Burns, M.P.; Taub, D.D.; Blackman, M.R.; Zhou, J. Chronic Neurobehavioral Impairments and Decreased Hippocampal Expression of Genes Important for Brain Glucose Utilization in a Mouse Model of Mild TBI. Front. Endocrinol. 2020, 11, 556380. [Google Scholar] [CrossRef]
- Smith, D.; Rau, T.; Poulsen, A.; MacWilliams, Z.; Patterson, D.; Kelly, W.; Poulsen, D. Convulsive seizures and EEG spikes after lateral fluid-percussion injury in the rat. Epilepsy Res. 2018, 147, 87–94. [Google Scholar] [CrossRef]
- Tsenter, J.; Beni-Adani, L.; Assaf, Y.; Alexandrovich, A.G.; Trembovler, V.; Shohami, E. Dynamic changes in the recovery after traumatic brain injury in mice: Effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions. J. Neurotrauma 2008, 25, 324–333. [Google Scholar] [CrossRef]
- Israel, I.; Ohsiek, A.; Al-Momani, E.; Albert-Weissenberger, C.; Stetter, C.; Mencl, S.; Buck, A.K.; Kleinschnitz, C.; Samnick, S.; Sirén, A.-L. Combined [(18)F]DPA-714 micro-positron emission tomography and autoradiography imaging of microglia activation after closed head injury in mice. J. Neuroinflammation 2016, 13, 140. [Google Scholar] [CrossRef]
- Teng, S.X.; Katz, P.S.; Maxi, J.K.; Mayeux, J.P.; Gilpin, N.W.; Molina, P.E. Alcohol exposure after mild focal traumatic brain injury impairs neurological recovery and exacerbates localized neuroinflammation. Brain Behav. Immun. 2015, 45, 145–156. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, X.; Wang, L.; Hao, S.; Xu, X.; Niu, F.; He, W.; Liu, B. Dexamethasone impairs neurofunctional recovery in rats following traumatic brain injury by reducing circulating endothelial progenitor cells and angiogenesis. Brain Res. 2019, 1725, 146469. [Google Scholar] [CrossRef]
- Ohayon, S.; Boyko, M.; Saad, A.; Douvdevani, A.; Gruenbaum, B.F.; Melamed, I.; Shapira, Y.; Teichberg, V.I.; Zlotnik, A. Cell-free DNA as a marker for prediction of brain damage in traumatic brain injury in rats. J. Neurotrauma 2012, 29, 261–267. [Google Scholar] [CrossRef]
- Crawley, J.N. What’s Wrong with My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Lalonde, R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002, 26, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.; Rawlins, J.N. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav. Brain Res. 2005, 156, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, J.N.; Olton, D.S. The septo-hippocampal system and cognitive mapping. Behav. Brain Res. 1982, 5, 331–358. [Google Scholar] [CrossRef] [PubMed]
- Babikian, T.; Asarnow, R. Neurocognitive outcomes and recovery after pediatric TBI: Meta-analytic review of the literature. Neuropsychology 2009, 23, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Finnanger, T.G.; Olsen, A.; Skandsen, T.; Lydersen, S.; Vik, A.; Evensen, K.A.; Catroppa, C.; Haberg, A.K.; Andersson, S.; Indredavik, M.S. Life after Adolescent and Adult Moderate and Severe Traumatic Brain Injury: Self-Reported Executive, Emotional, and Behavioural Function 2-5 Years after Injury. Behav. Neurol. 2015, 2015, 329241. [Google Scholar] [CrossRef]
- Xiong, K.; Zhu, Y.; Zhang, Y.; Yin, Z.; Zhang, J.; Qiu, M.; Zhang, W. White matter integrity and cognition in mild traumatic brain injury following motor vehicle accident. Brain Res. 2014, 1591, 86–92. [Google Scholar] [CrossRef]
- Nyam, T.-T.E.; Ao, K.-H.; Hung, S.-Y.; Shen, M.-L.; Yu, T.-C.; Kuo, J.-R. FOUR Score Predicts Early Outcome in Patients After Traumatic Brain Injury. Neurocrit. Care 2017, 26, 225–231. [Google Scholar] [CrossRef]
- Jalali, R.; Rezaei, M. A comparison of the glasgow coma scale score with full outline of unresponsiveness scale to predict patients’ traumatic brain injury outcomes in intensive care units. Crit. Care Res. Pract. 2014, 2014, 289803. [Google Scholar] [CrossRef]
- Khanal, K.; Bhandari, S.S.; Shrestha, N.; Acharya, S.P.; Marhatta, M.N. Comparison of outcome predictions by the Glasgow coma scale and the Full Outline of UnResponsiveness score in the neurological and neurosurgical patients in the Intensive Care Unit. Indian J. Crit. Care Med. 2016, 20, 473–476. [Google Scholar] [CrossRef]
- Silverberg, N.D.; Gardner, A.J.; Brubacher, J.R.; Panenka, W.J.; Li, J.J.; Iverson, G.L. Systematic review of multivariable prognostic models for mild traumatic brain injury. J. Neurotrauma 2015, 32, 517–526. [Google Scholar] [CrossRef]
- Hiploylee, C.; Dufort, P.A.; Davis, H.S.; Wennberg, R.A.; Tartaglia, M.C.; Mikulis, D.; Hazrati, L.N.; Tator, C.H. Longitudinal Study of Postconcussion Syndrome: Not Everyone Recovers. J. Neurotrauma 2017, 34, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.L.; Eagle, S.R.; Marchetti, G.; Mucha, A.; Collins, M.W.; Kontos, A.P. Association of acute vestibular/ocular motor screening scores to prolonged recovery in collegiate athletes following sport-related concussion. Brain Inj. 2020, 34, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Putukian, M.; Riegler, K.; Amalfe, S.; Bruce, J.; Echemendia, R. Preinjury and Postinjury Factors That Predict Sports-Related Concussion and Clinical Recovery Time. Clin. J. Sport Med. 2021, 31, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, S.; Machamer, J.; Temkin, N. Mild Traumatic Brain Injury: Longitudinal Study of Cognition, Functional Status, and Post-Traumatic Symptoms. J. Neurotrauma 2017, 34, 1524–1530. [Google Scholar] [CrossRef]
- Nitta, M.E.; Savitz, J.; Nelson, L.D.; Teague, T.K.; Hoelzle, J.B.; McCrea, M.A.; Meier, T.B. Acute elevation of serum inflammatory markers predicts symptom recovery after concussion. Neurology 2019, 93, e497–e507. [Google Scholar] [CrossRef]
- Sveen, U.; Bautz-Holter, E.; Sandvik, L.; Alvsåker, K.; Røe, C. Relationship between competency in activities, injury severity, and post-concussion symptoms after traumatic brain injury. Scand. J. Occup. Ther. 2010, 17, 225–232. [Google Scholar] [CrossRef]
- Rowe, R.K.; Harrison, J.L.; Thomas, T.C.; Pauly, J.R.; Adelson, P.D.; Lifshitz, J. Using anesthetics and analgesics in experimental traumatic brain injury. Lab Anim. 2013, 42, 286–291. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetzer, S.M.; Casagrande, A.; Qu’d, D.; Dobrozsi, N.; Bohnert, J.; Biguma, V.; Evanson, N.K.; McGuire, J.L. Early Measures of TBI Severity Poorly Predict Later Individual Impairment in a Rat Fluid Percussion Model. Brain Sci. 2023, 13, 1230. https://doi.org/10.3390/brainsci13091230
Hetzer SM, Casagrande A, Qu’d D, Dobrozsi N, Bohnert J, Biguma V, Evanson NK, McGuire JL. Early Measures of TBI Severity Poorly Predict Later Individual Impairment in a Rat Fluid Percussion Model. Brain Sciences. 2023; 13(9):1230. https://doi.org/10.3390/brainsci13091230
Chicago/Turabian StyleHetzer, Shelby M., Andrew Casagrande, Dima Qu’d, Nicholas Dobrozsi, Judy Bohnert, Victor Biguma, Nathan K. Evanson, and Jennifer L. McGuire. 2023. "Early Measures of TBI Severity Poorly Predict Later Individual Impairment in a Rat Fluid Percussion Model" Brain Sciences 13, no. 9: 1230. https://doi.org/10.3390/brainsci13091230
APA StyleHetzer, S. M., Casagrande, A., Qu’d, D., Dobrozsi, N., Bohnert, J., Biguma, V., Evanson, N. K., & McGuire, J. L. (2023). Early Measures of TBI Severity Poorly Predict Later Individual Impairment in a Rat Fluid Percussion Model. Brain Sciences, 13(9), 1230. https://doi.org/10.3390/brainsci13091230





