Glial Glutamate Transporter Modulation Prevents Development of Complete Freund’s Adjuvant-Induced Hyperalgesia and Allodynia in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
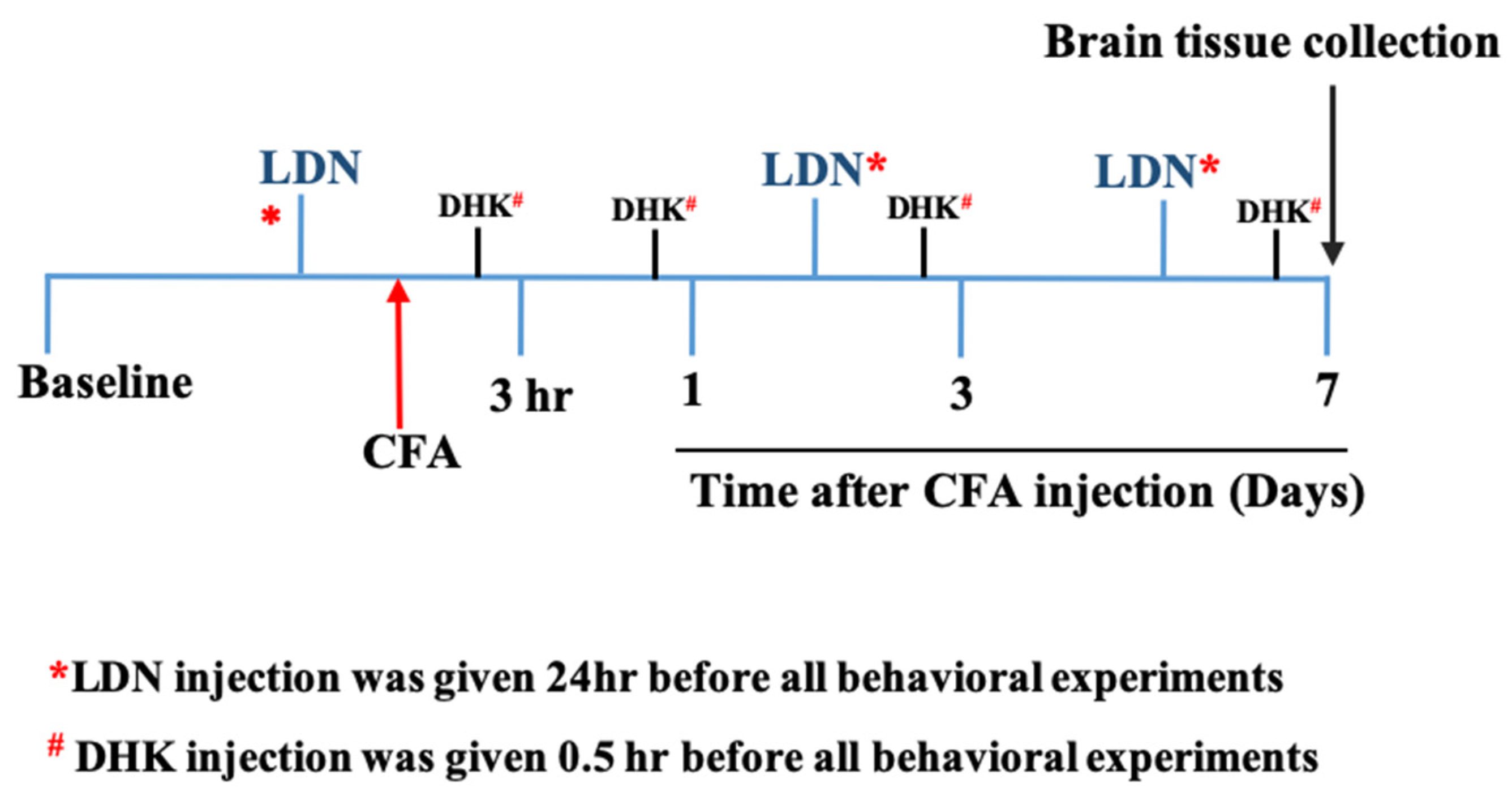
2.2. Drugs and Treatment
2.3. CFA-Induced Allodynia and Hyperalgesia
2.4. Western Blot Analysis
2.5. Immunofluorescence Assay
2.6. IL-1β Estimation by ELISA
2.7. Data Analyses
3. Results
3.1. Effects of LDN-212320 or Gabapentin on CFA-Induced Tactile Allodynia and Thermal Hyperalgesia
3.2. Effects of DHK on the Anti-Allodynic and Anti-Hyperalgesic Effects of LDN-212320
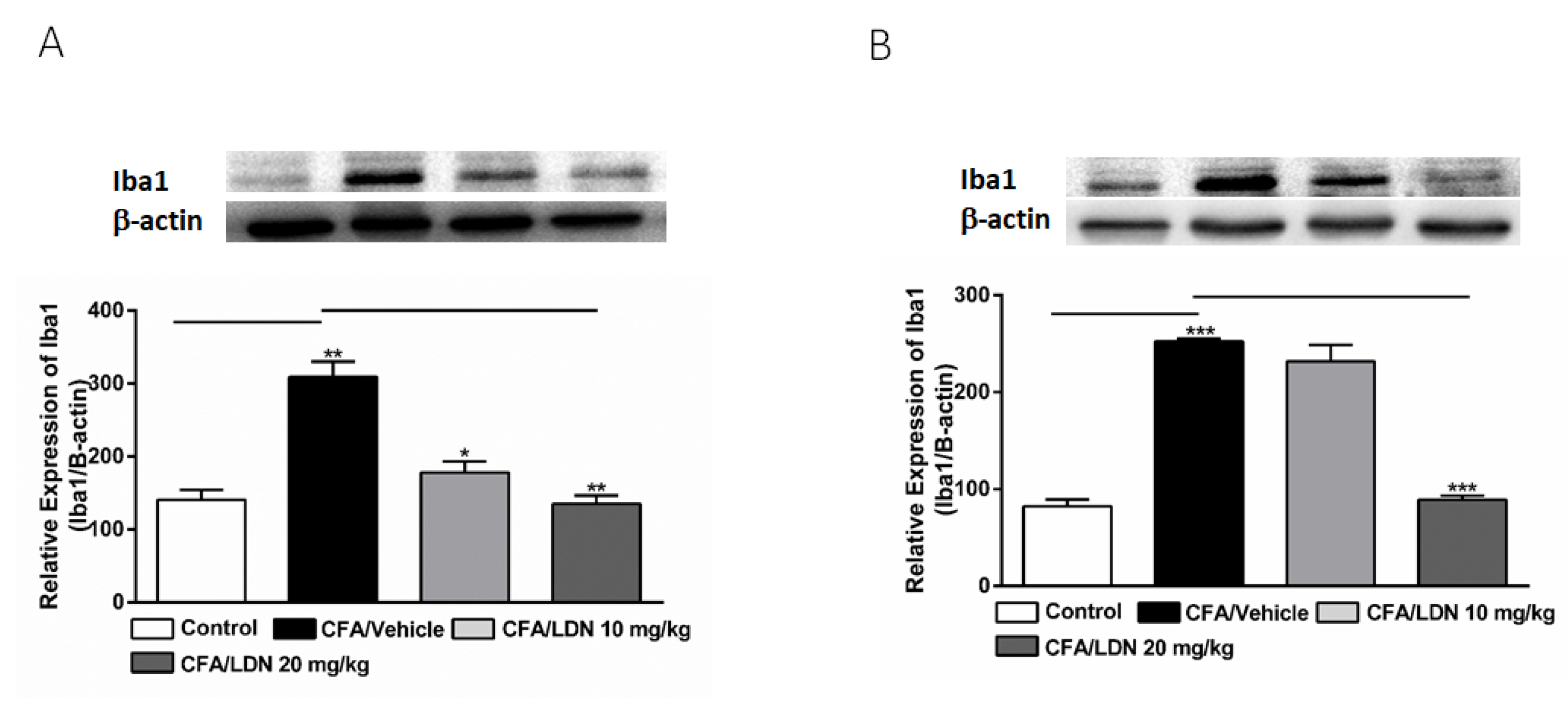
3.3. Effects of LDN-212320 on Microglial Iba1 Expression in the Hippocampus and ACC
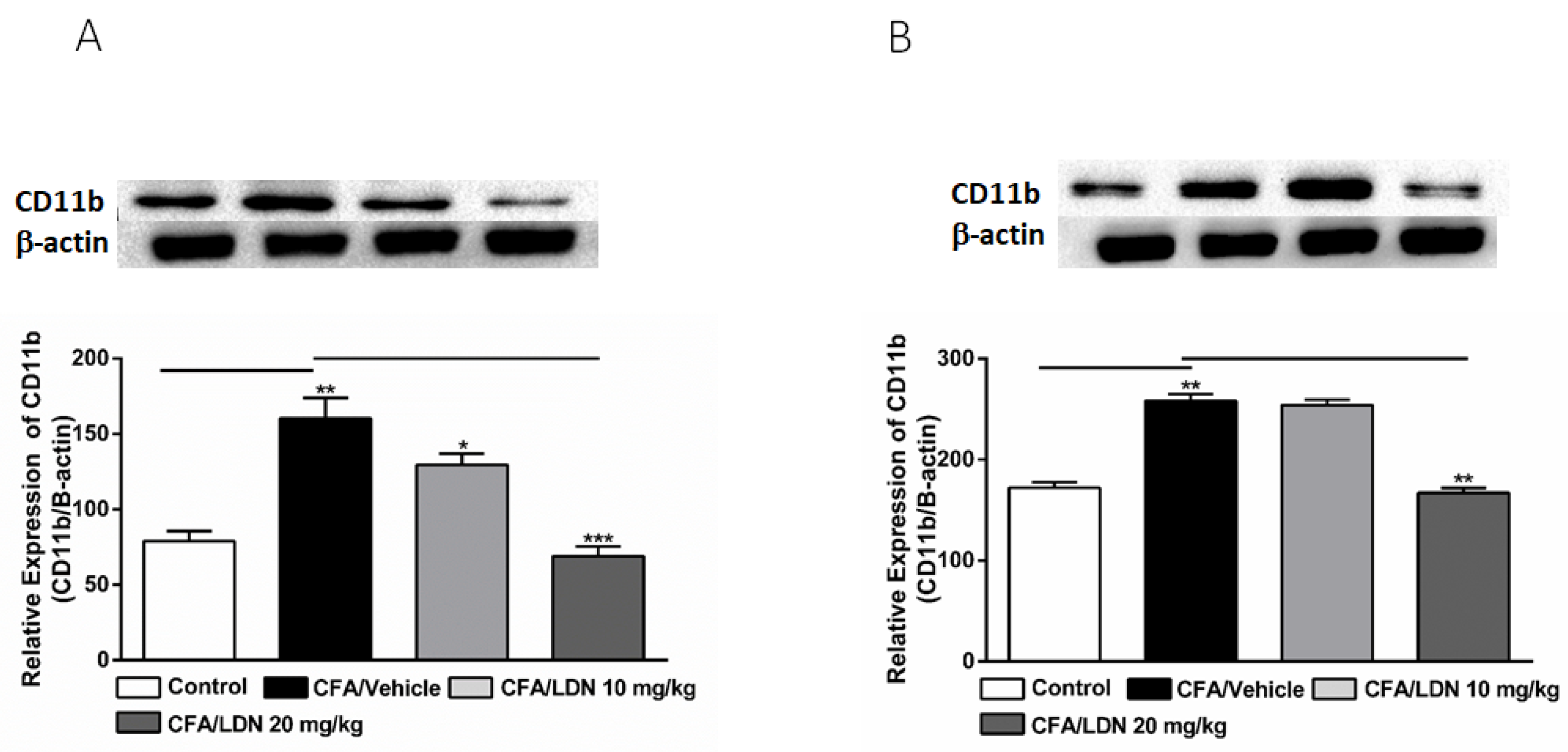
3.4. Effects of LDN-212320 on Microglial CD11b Expression in the Hippocampus and ACC
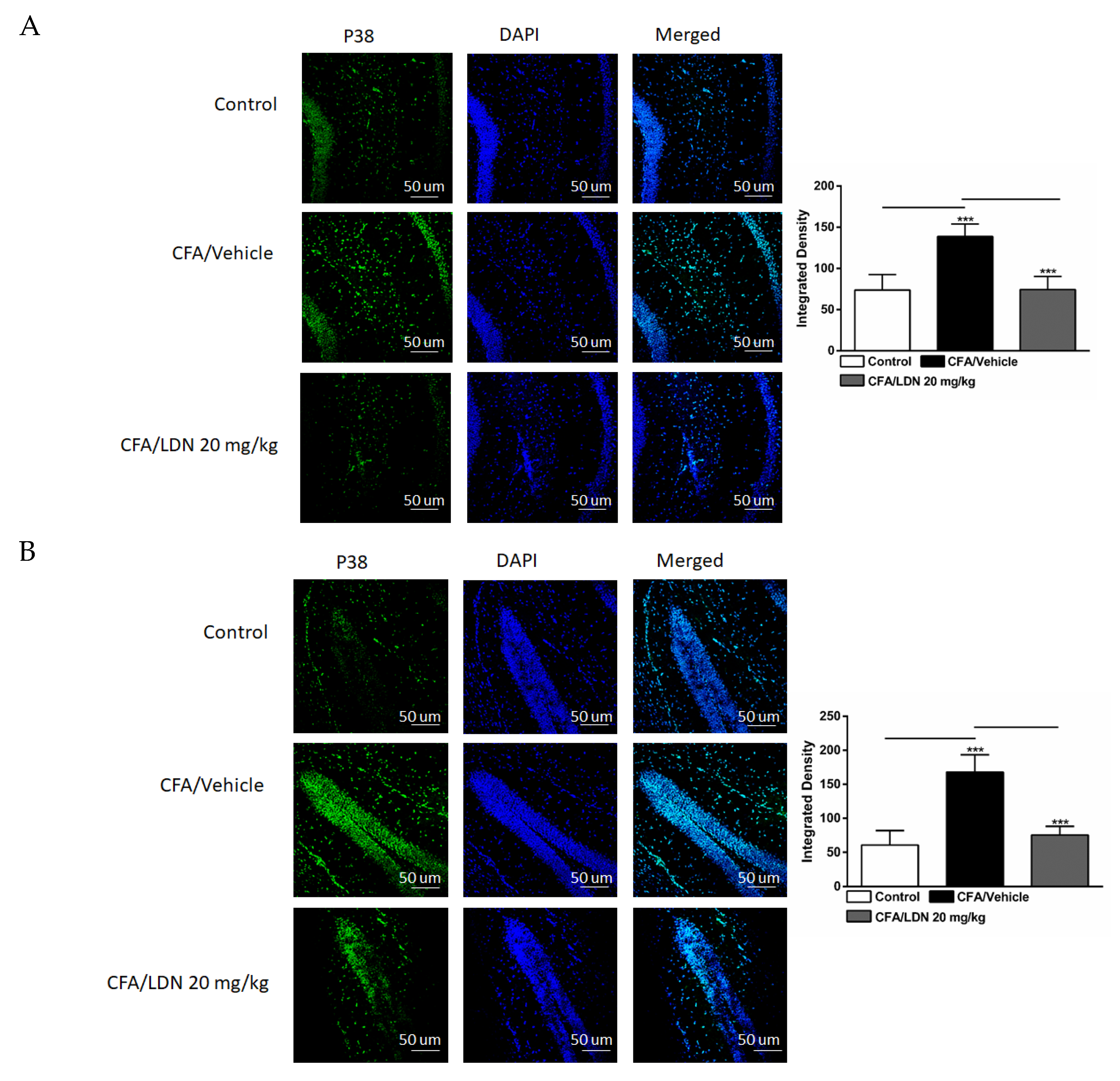
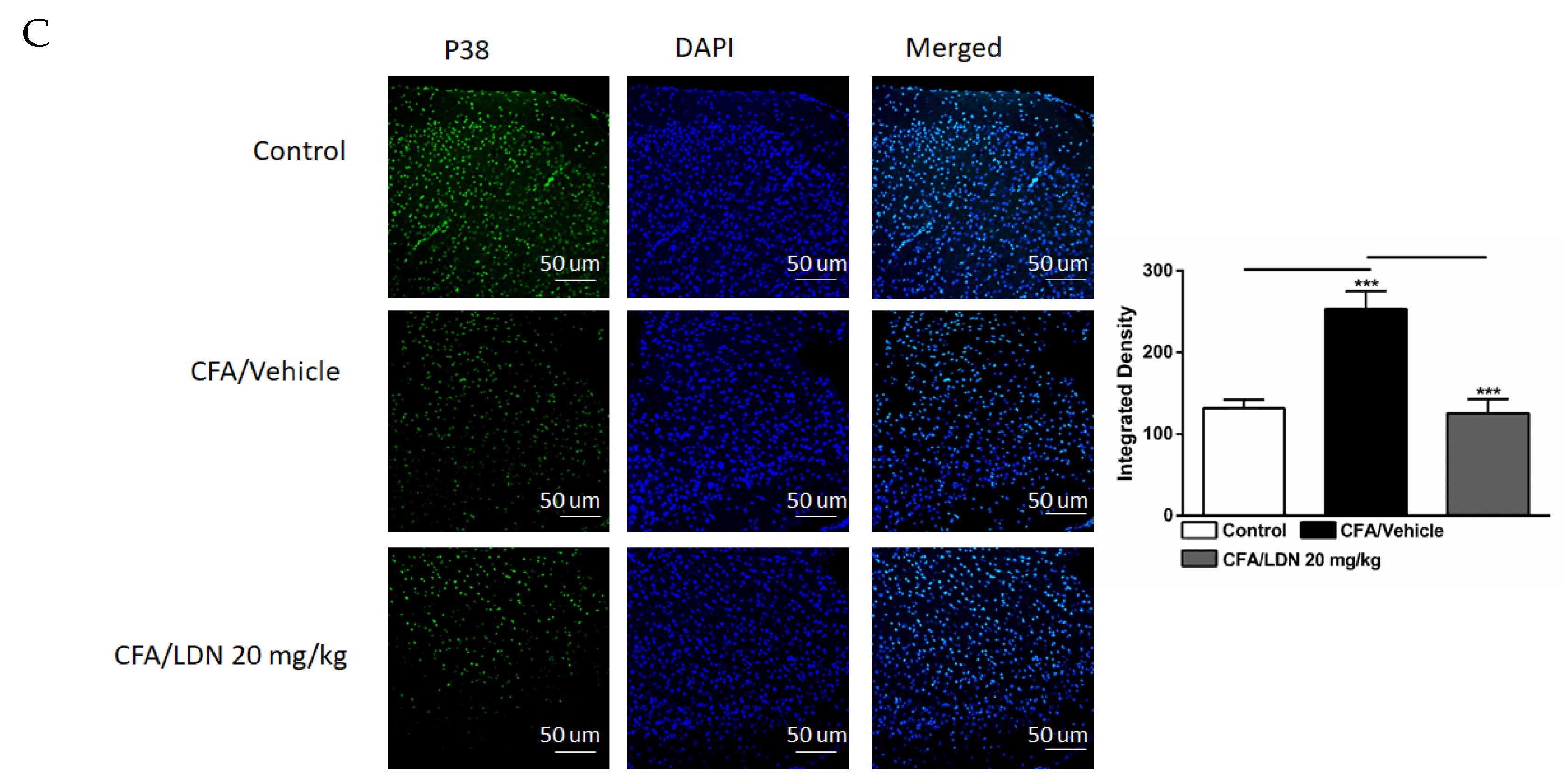
3.5. Effects of LDN-212320 on Microglial p38 Immunoreactivity in the Hippocampus and ACC
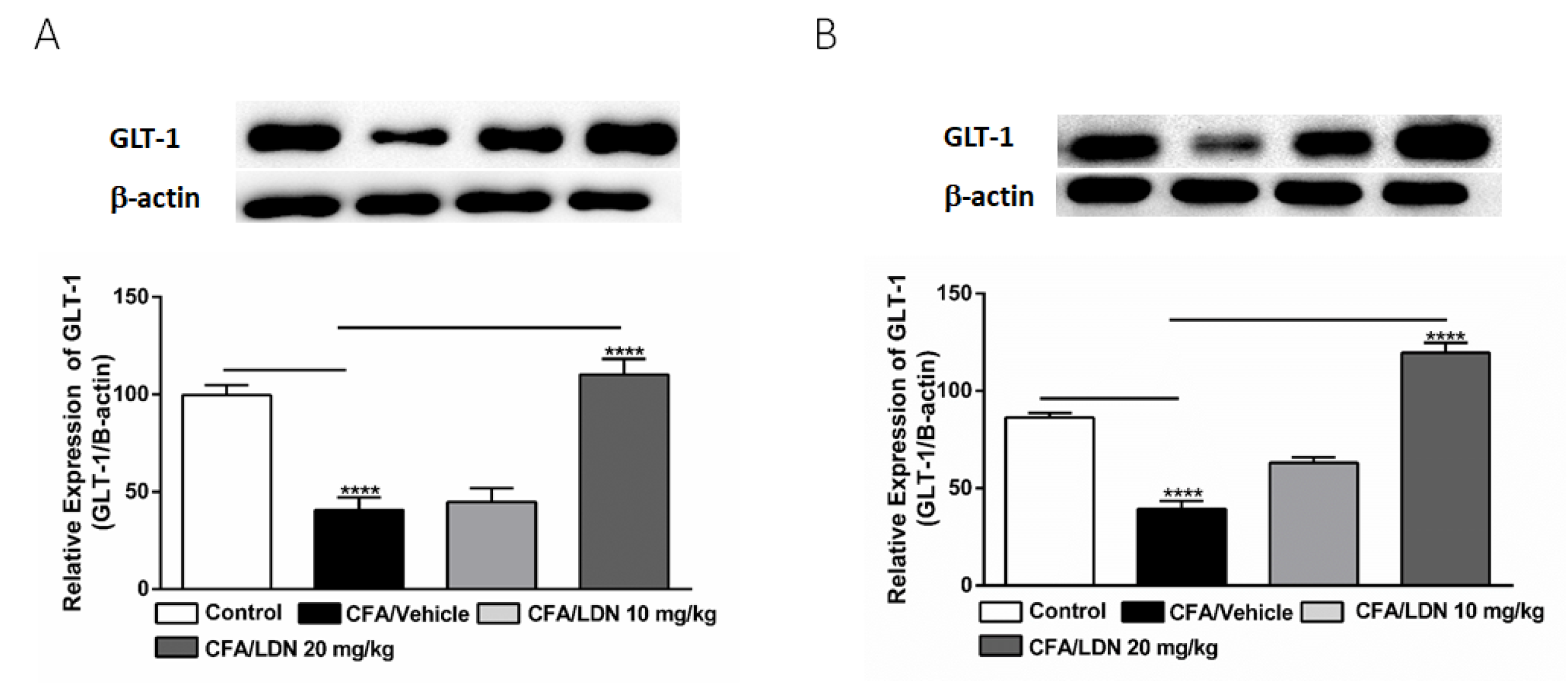
3.6. Effects of LDN-212320 on Astroglial GLT-1 Expression in the Hippocampus and ACC
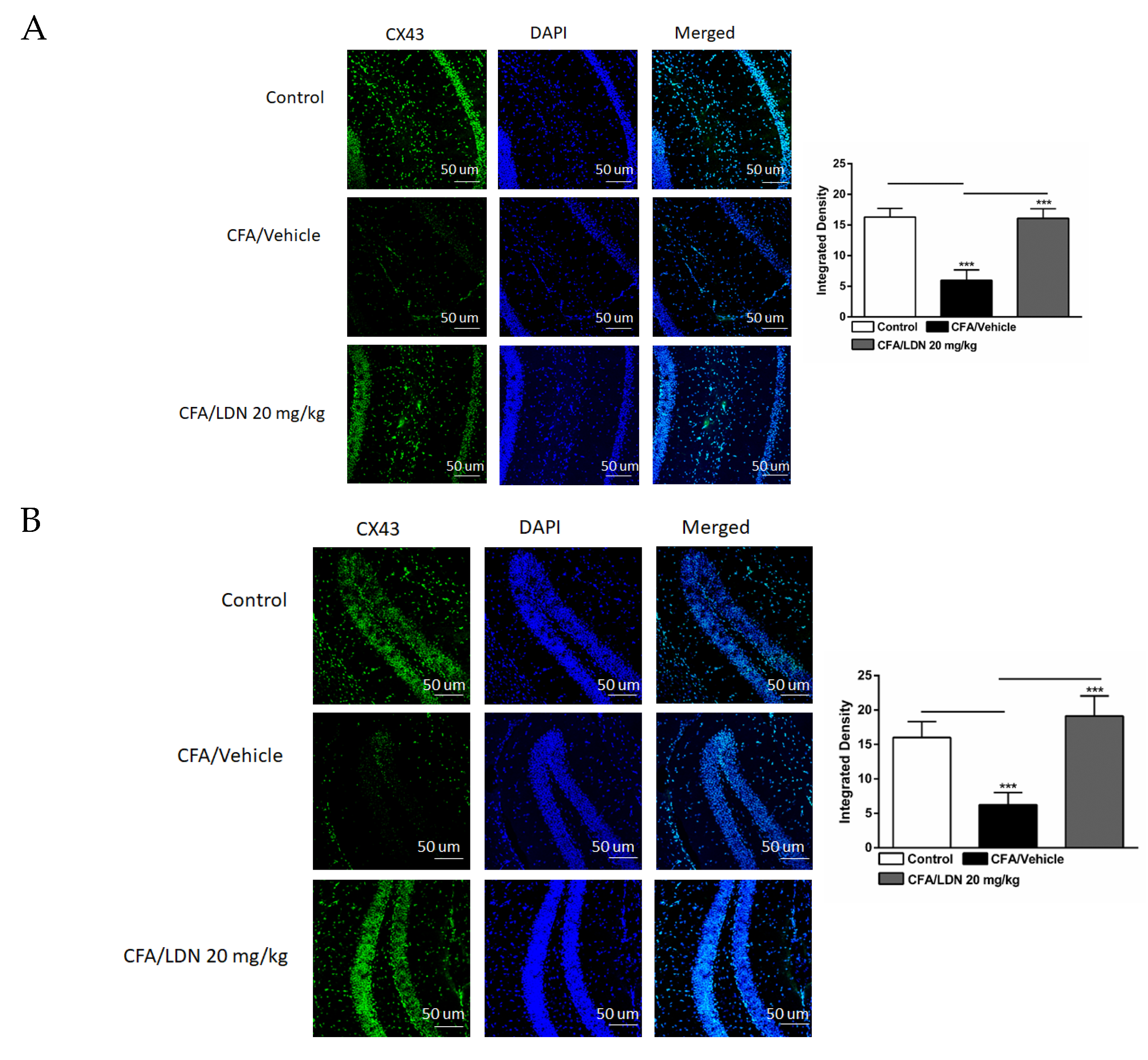
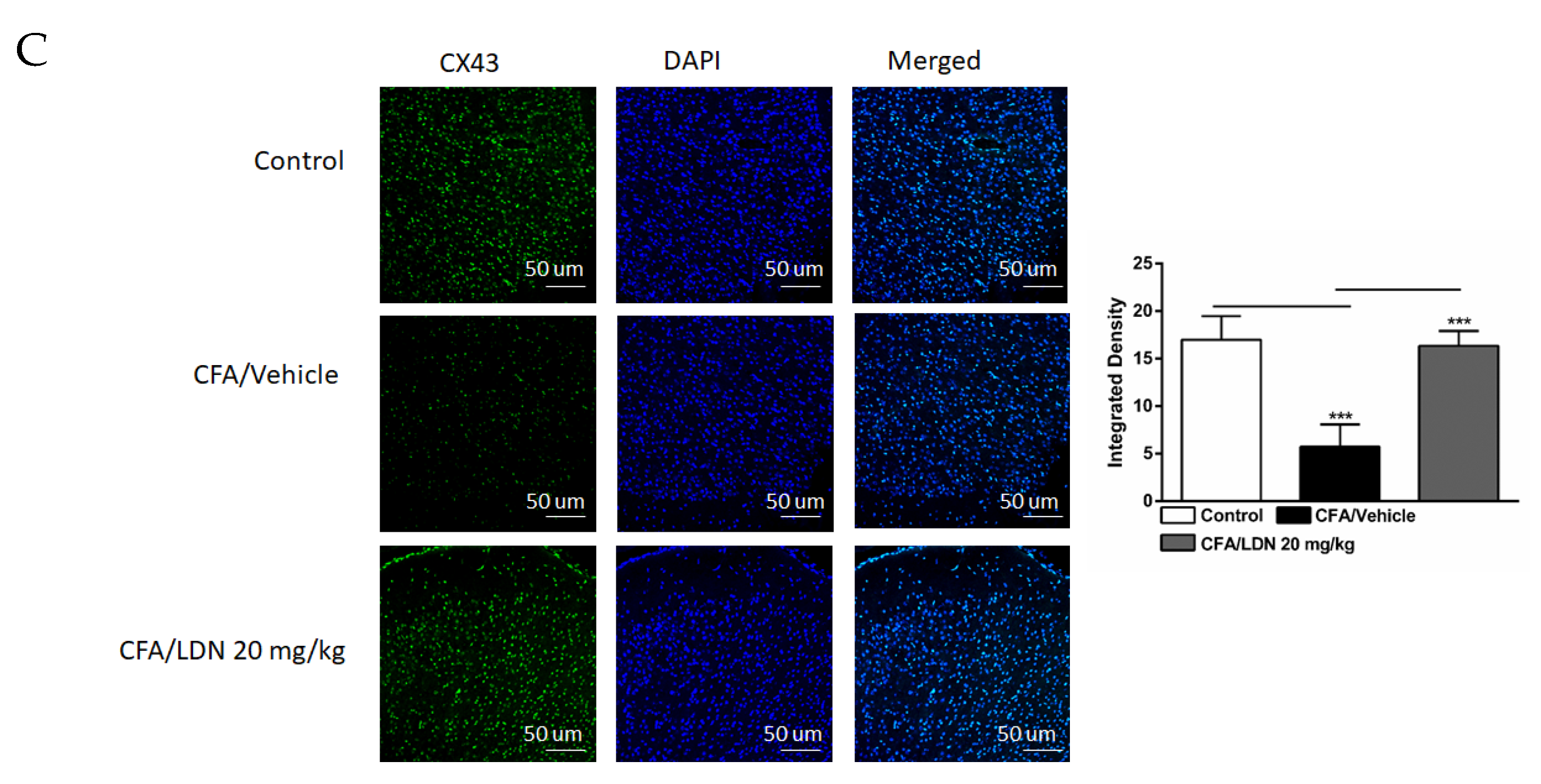
3.7. Effects of LDN-212320 on Astroglial CX43 Immunoreactivity in the Hippocampus and ACC
3.8. Effects of LDN-212320 on IL-1β Level in the Hippocampus and ACC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Rice, A.S.; Smith, B.H.; Blyth, F.M. Pain and the global burden of disease. Pain 2016, 157, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.M. NSAID-induced gastrointestinal injury: A focused update for clinicians. J. Clin. Gastroenterol. 2016, 50, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Wasan, A.; Correll, D.; Kissin, I.; O’Shea, S.; Jamison, R. Iatrogenic addiction in patients treated for acute or subacute pain: A systematic review. J. Opioid Manag. 2006, 2, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Dubner, R. Activity-triggered tetrapartite neuron–glial interactions following peripheral injury. Curr. Opin. Pharmacol. 2016, 26, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Kiritoshi, T.; Murase, K. Contribution of microglia and astrocytes to the central sensitization, inflammatory and neuropathic pain in the juvenile rat. Mol. Pain 2012, 8, 43. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Xu, Z.Z.; Wang, X.; Park, J.Y.; Zhuang, Z.Y.; Tan, P.H.; Gao, Y.J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef]
- Von Hehn, C.A.; Baron, R.; Woolf, C.J. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012, 73, 638–652. [Google Scholar] [CrossRef]
- Ji, R.-R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain 2007, 3, 33. [Google Scholar] [CrossRef]
- Clark, A.K.; Gentry, C.; Bradbury, E.J.; McMahon, S.B.; Malcangio, M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur. J. Pain 2007, 11, 223–230. [Google Scholar] [CrossRef]
- Echeverry, S.; Shi, X.Q.; Zhang, J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. PAIN® 2008, 135, 37–47. [Google Scholar] [CrossRef]
- Raghavendra, V.; Tanga, F.; DeLeo, J.A. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 2003, 306, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Schafers, M.; Svensson, C.I.; Sommer, C.; Sorkin, L.S. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Jeon, S.M.; Cho, H.J. Interleukin-6 induces microglial CX3CR1 expression in the spinal cord after peripheral nerve injury through the activation of p38 MAPK. Eur. J. Pain 2010, 14, 682.e681-612. [Google Scholar] [CrossRef] [PubMed]
- Mutso, A.A.; Radzicki, D.; Baliki, M.N.; Huang, L.; Banisadr, G.; Centeno, M.V.; Radulovic, J.; Martina, M.; Miller, R.J.; Apkarian, A.V. Abnormalities in hippocampal functioning with persistent pain. J. Neurosci. 2012, 32, 5747–5756. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.N.; Peng, Y.B.; Boyette-Davis, J.A.; Uhelski, M.L. The anterior cingulate cortex and pain processing. Front. Integr. Neurosci. 2014, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.P.; Fields, H.L. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat. Neurosci. 2004, 7, 398–403. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L.; Kaang, B.K.; Zhuo, M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci. 2016, 17, 485–496. [Google Scholar] [CrossRef]
- Tsuda, M.; Koga, K.; Chen, T.; Zhuo, M. Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex. J. Neurochem. 2017, 141, 486–498. [Google Scholar] [CrossRef]
- Zhuo, M. Glutamate receptors and persistent pain: Targeting forebrain NR2B subunits. Drug Discov. Today 2002, 7, 259–267. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zhong, X.-L.; Li, Z.-B.; Wang, H.-T.; Zhang, J.; Li, F.; Zhang, J.-Y.; Dai, R.-P.; Xin-Fu, Z.; Li, C.-Q.; et al. Differential roles of hippocampal glutamatergic receptors in neuropathic anxiety-like behavior after partial sciatic nerve ligation in rats. BMC Neurosci. 2015, 16, 14. [Google Scholar] [CrossRef]
- Urban, M.O.; Gebhart, G.F. Supraspinal contributions to hyperalgesia. Proc. Natl. Acad. Sci. USA 1999, 96, 7687. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; He, T.; Wang, Y.; Wang, J.-L.; Yan, X.-B.; Zhou, H.-C.; Wang, R.-R.; Du, R.; Wang, X.-L.; Chen, J. Ceftriaxone relieves trigeminal neuropathic pain through suppression of spatiotemporal synaptic plasticity via restoration of glutamate transporter 1 in the medullary dorsal horn. Front. Cell. Neurosci. 2020, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain Off. J. Am. Pain Soc. 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.G.; Ko, S.W.; Wu, L.J.; Toyoda, H.; Xu, H.; Quan, J.; Li, J.; Jia, Y.; Ren, M.; Xu, Z.C.; et al. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 8923–8930. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Y.; Lao, L.; Saito, R.; Li, A.; Backman, C.M.; Berman, B.M.; Ren, K.; Wei, P.K.; Zhang, R.X. Spinal interleukin-17 promotes thermal hyperalgesia and NMDA NR1 phosphorylation in an inflammatory pain rat model. Pain 2013, 154, 294–305. [Google Scholar] [CrossRef]
- Alotaibi, G.; Rahman, S. Effects of glial glutamate transporter activator in formalin-induced pain behaviour in mice. Eur. J. Pain 2019, 23, 765–783. [Google Scholar] [CrossRef]
- Daulhac, L.; Mallet, C.; Courteix, C.; Etienne, M.; Duroux, E.; Privat, A.M.; Eschalier, A.; Fialip, J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol. Pharmacol. 2006, 70, 1246–1254. [Google Scholar] [CrossRef]
- Yashpal, K.; Fisher, K.; Chabot, J.G.; Coderre, T.J. Differential effects of NMDA and group I mGluR antagonists on both nociception and spinal cord protein kinase C translocation in the formalin test and a model of neuropathic pain in rats. Pain 2001, 94, 17–29. [Google Scholar] [CrossRef]
- Jensen, A.A.; Fahlke, C.; Bjørn-Yoshimoto, W.E.; Bunch, L. Excitatory amino acid transporters: Recent insights into molecular mechanisms, novel modes of modulation and new therapeutic possibilities. Curr. Opin. Pharmacol. 2015, 20, 116–123. [Google Scholar] [CrossRef]
- Wilkie, C.M.; Barron, J.C.; Brymer, K.J.; Barnes, J.R.; Nafar, F.; Parsons, M.P. The effect of GLT-1 upregulation on extracellular glutamate dynamics. Front. Cell. Neurosci. 2021, 15, 661412. [Google Scholar] [CrossRef]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 2019, 161, 107559. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C. Glutamate uptake. Prog. Neurobiol. 2001, 65, 1–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, R.; Xu, M.J.; Sha, J.; Xu, G.Y.; Wu, J.; Zhang, P.A. MiRNA-107 contributes to inflammatory pain by down-regulating GLT-1 expression in rat spinal dorsal horn. Eur. J. Pain 2021, 25, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Furness, D.; Dehnes, Y.; Akhtar, A.; Rossi, D.; Hamann, M.; Grutle, N.; Gundersen, V.; Holmseth, S.; Lehre, K.; Ullensvang, K. A quantitative assessment of glutamate uptake into hippocampal synaptic terminals and astrocytes: New insights into a neuronal role for excitatory amino acid transporter 2 (EAAT2). Neuroscience 2008, 157, 80–94. [Google Scholar] [CrossRef]
- Rose, C.R.; Felix, L.; Zeug, A.; Dietrich, D.; Reiner, A.; Henneberger, C. Astroglial glutamate signaling and uptake in the hippocampus. Front. Mol. Neurosci. 2018, 10, 451. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Dykes-Hoberg, M.; Pardo, C.A.; Bristol, L.A.; Jin, L.; Kuncl, R.W.; Kanai, Y.; Hediger, M.A.; Wang, Y.; Schielke, J.P. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 1996, 16, 675–686. [Google Scholar] [CrossRef]
- Ramos, K.M.; Lewis, M.T.; Morgan, K.N.; Crysdale, N.Y.; Kroll, J.L.; Taylor, F.R.; Harrison, J.A.; Sloane, E.M.; Maier, S.F.; Watkins, L.R. Spinal upregulation of glutamate transporter GLT-1 by ceftriaxone: Therapeutic efficacy in a range of experimental nervous system disorders. Neuroscience 2010, 169, 1888–1900. [Google Scholar] [CrossRef]
- Suto, T.; Severino, A.L.; Eisenach, J.C.; Hayashida, K. Gabapentin increases extracellular glutamatergic level in the locus coeruleus via astroglial glutamate transporter-dependent mechanisms. Neuropharmacology 2014, 81, 95–100. [Google Scholar] [CrossRef]
- Morioka, N.; Zhang, F.F.; Nakamura, Y.; Kitamura, T.; Hisaoka-Nakashima, K.; Nakata, Y. Tumor necrosis factor-mediated downregulation of spinal astrocytic connexin43 leads to increased glutamatergic neurotransmission and neuropathic pain in mice. Brain Behav. Immun. 2015, 49, 293–310. [Google Scholar] [CrossRef]
- Guo, W.; Imai, S.; Zou, S.; Yang, J.; Watanabe, M.; Wang, J.; Dubner, R.; Wei, F.; Ren, K. Altered glial glutamate transporter expression in descending circuitry and the emergence of pain chronicity. Mol. Pain 2019, 15, 1744806918825044. [Google Scholar] [CrossRef]
- Kimura, M.; Eisenach, J.C.; Hayashida, K. Gabapentin loses efficacy over time after nerve injury in rats: Role of glutamate transporter-1 in the locus coeruleus. Pain 2016, 157, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.A.; Baron, D.A.; Kim, J.K.; Unterwald, E.M.; Rawls, S.M. β-Lactam antibiotic produces a sustained reduction in extracellular glutamate in the nucleus accumbens of rats. Amino Acids 2011, 40, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, C.S.; Corley, G.; Kovalevich, J.; Yen, W.; Langford, D.; Rawls, S.M. Ceftriaxone attenuates locomotor activity induced by acute and repeated cocaine exposure in mice. Neurosci. Lett. 2013, 556, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Maciel, I.S.; Silva, R.B.; Morrone, F.B.; Calixto, J.B.; Campos, M.M. Synergistic effects of celecoxib and bupropion in a model of chronic inflammation-related depression in mice. PLoS ONE 2013, 8, e77227. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-J.; Toyoda, H.; Zhao, M.-G.; Lee, Y.-S.; Tang, J.; Ko, S.W.; Jia, Y.H.; Shum, F.W.; Zerbinatti, C.V.; Bu, G. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J. Neurosci. 2005, 25, 11107–11116. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Rahman, S. Effects of alpha-7 nicotinic acetylcholine receptor positive allosteric modulator on lipopolysaccharide-induced neuroinflammatory pain in mice. Eur. J. Pharmacol. 2016, 783, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.; Pogrel, J.; Chung, J.; Yaksh, T. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-Y.; Hsieh, C.-L.; Huang, C.-P.; Lin, Y.-W. Electroacupuncture attenuates CFA-induced inflammatory pain by suppressing Nav1. 8 through S100B, TRPV1, opioid, and adenosine pathways in mice. Sci. Rep. 2017, 7, 42531. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.; Tie, L.; Yao, H.; Jiang, W.; Ma, X.; Li, X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006, 1122, 56–64. [Google Scholar] [CrossRef]
- Franklin, K.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, Compact: The Coronal Plates and Diagrams; Elsevier Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004, 20, 467–473. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, Y.; Wang, L.; Huang, J.; Wen, J.; Lv, H.; Wu, X.; Wan, C.; Yu, C.; Zhang, W.; et al. Thymosin Alpha-1 Inhibits Complete Freund’s Adjuvant-Induced Pain and Production of Microglia-Mediated Pro-inflammatory Cytokines in Spinal Cord. Neurosci. Bull. 2019, 35, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, P.; Gegelashvili, G.; Munro, G.; Heegaard, A.; Bjerrum, O. The β-lactam clavulanic acid mediates glutamate transport-sensitive pain relief in a rat model of neuropathic pain. Eur. J. Pain 2018, 22, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Sweitzer, S.M.; Colburn, R.W.; Rutkowski, M.; DeLeo, J.A. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999, 829, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001, 24, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Padi, S.S.; Kulkarni, S.K. Minocycline prevents the development of neuropathic pain, but not acute pain: Possible anti-inflammatory and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 601, 79–87. [Google Scholar] [CrossRef]
- Gu, N.; Peng, J.; Murugan, M.; Wang, X.; Eyo, U.B.; Sun, D.; Ren, Y.; DiCicco-Bloom, E.; Young, W.; Dong, H.; et al. Spinal Microgliosis Due to Resident Microglial Proliferation Is Required for Pain Hypersensitivity after Peripheral Nerve Injury. Cell Rep. 2016, 16, 605–614. [Google Scholar] [CrossRef]
- Raghavendra, V.; Tanga, F.; Rutkowski, M.D.; DeLeo, J.A. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: Mechanistic implications of spinal glia and proinflammatory cytokines. Pain 2003, 104, 655–664. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Glia: A novel drug discovery target for clinical pain. Nat. Rev. Drug Discov. 2003, 2, 973–985. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Bae, J.-C.; Kim, J.-Y.; Lee, H.-L.; Lee, K.-M.; Kim, D.-S.; Cho, H.-J. Activation of p38 MAP kinase in the rat dorsal root ganglia and spinal cord following peripheral inflammation and nerve injury. Neuroreport 2002, 13, 2483–2486. [Google Scholar] [CrossRef]
- Tsuda, M.; Mizokoshi, A.; Shigemoto-Mogami, Y.; Koizumi, S.; Inoue, K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia 2004, 45, 89–95. [Google Scholar] [CrossRef]
- Willemen, H.L.; Eijkelkamp, N.; Wang, H.; Dantzer, R.; Dorn, G.W., II; Kelley, K.W.; Heijnen, C.J.; Kavelaars, A. Microglial/macrophage GRK2 determines duration of peripheral IL-1beta-induced hyperalgesia: Contribution of spinal cord CX3CR1, p38 and IL-1 signaling. Pain 2010, 150, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in pain: Detrimental and protective roles in pathogenesis and resolution of pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Irving, E.A.; Barone, F.C.; Reith, A.D.; Hadingham, S.J.; Parsons, A.A. Differential activation of MAPK/ERK and p38/SAPK in neurones and glia following focal cerebral ischaemia in the rat. Brain Res. Mol. Brain Res. 2000, 77, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Waxman, E.A.; Lynch, D.R. N-methyl-D-aspartate receptor subtype mediated bidirectional control of p38 mitogen-activated protein kinase. J. Biol. Chem. 2005, 280, 29322–29333. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Cafferty, W.B.; Marchand, F. Immune and glial cell factors as pain mediators and modulators. Exp. Neurol. 2005, 192, 444–462. [Google Scholar] [CrossRef] [PubMed]
- Lujia, Y.; Xin, L.; Shiquan, W.; Yu, C.; Shuzhuo, Z.; Hong, Z. Ceftriaxone pretreatment protects rats against cerebral ischemic injury by attenuating microglial activation-induced IL-1beta expression. Int. J. Neurosci. 2014, 124, 657–665. [Google Scholar] [CrossRef]
- Amin, B.; Hajhashemi, V.; Hosseinzadeh, H.; Abnous, K. Antinociceptive evaluation of ceftriaxone and minocycline alone and in combination in a neuropathic pain model in rat. Neuroscience 2012, 224, 15–25. [Google Scholar] [CrossRef]
- Hu, Y.; Li, W.; Lu, L.; Cai, J.; Xian, X.; Zhang, M.; Li, Q.; Li, L. An anti-nociceptive role for ceftriaxone in chronic neuropathic pain in rats. Pain 2010, 148, 284–301. [Google Scholar] [CrossRef]
- Nicholson, K.J.; Gilliland, T.M.; Winkelstein, B.A. Upregulation of GLT-1 by treatment with ceftriaxone alleviates radicular pain by reducing spinal astrocyte activation and neuronal hyperexcitability. J. Neurosci. Res. 2014, 92, 116–129. [Google Scholar] [CrossRef]
- Yang, M.; Roman, K.; Chen, D.-F.; Wang, Z.-G.; Lin, Y.; Stephens, R.L. GLT-1 overexpression attenuates bladder nociception and local/cross-organ sensitization of bladder nociception. Am. J. Physiol. Ren. Physiol. 2011, 300, F1353–F1359. [Google Scholar] [CrossRef]
- Morioka, N.; Fujii, S.; Kondo, S.; Zhang, F.F.; Miyauchi, K.; Nakamura, Y.; Hisaoka-Nakashima, K.; Nakata, Y. Downregulation of spinal astrocytic connexin43 leads to upregulation of interleukin-6 and cyclooxygenase-2 and mechanical hypersensitivity in mice. Glia 2018, 66, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gao, J.; Zhao, M.; Cui, J.; Li, Y.; Yang, X.; Jing, X.; Wu, Z. A novel cognitive impairment mechanism that astrocytic p-connexin 43 promotes neuronic autophagy via activation of P2X7R and down-regulation of GLT-1 expression in the hippocampus following traumatic brain injury in rats. Behav. Brain Res. 2015, 291, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Chever, O.; Pannasch, U.; Ezan, P.; Rouach, N. Astroglial connexin 43 sustains glutamatergic synaptic efficacy. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130596. [Google Scholar] [CrossRef] [PubMed]
- Pearson, V.L.; Rothwell, N.J.; Toulmond, S. Excitotoxic brain damage in the rat induces interleukin-1β protein in microglia and astrocytes: Correlation with the progression of cell death. Glia 1999, 25, 311–323. [Google Scholar] [CrossRef]
- Noda, M.; Nakanishi, H.; Nabekura, J.; Akaike, N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Li, A.; Liu, B.; Wang, L.; Ren, K.; Zhang, H.; Berman, B.M.; Lao, L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain 2008, 135, 232–239. [Google Scholar] [CrossRef]
- Zhu, G.; Okada, M.; Yoshida, S.; Mori, F.; Ueno, S.; Wakabayashi, K.; Kaneko, S. Effects of interleukin-1beta on hippocampal glutamate and GABA releases associated with Ca2+-induced Ca2+ releasing systems. Epilepsy Res. 2006, 71, 107–116. [Google Scholar] [CrossRef]
- Yan, X.; Yadav, R.; Gao, M.; Weng, H.R. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia 2014, 62, 1093–1109. [Google Scholar] [CrossRef]
- Miller, B.; Sarantis, M.; Traynelis, S.F.; Attwell, D. Potentiation of NMDA receptor currents by arachidonic acid. Nature 1992, 355, 722–725. [Google Scholar] [CrossRef]
- Viviani, B.; Bartesaghi, S.; Gardoni, F.; Vezzani, A.; Behrens, M.M.; Bartfai, T.; Binaglia, M.; Corsini, E.; Di Luca, M.; Galli, C.L.; et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 8692–8700. [Google Scholar] [CrossRef]
- Lynch, M.A.; Voss, K.L. Arachidonic acid increases inositol phospholipid metabolism and glutamate release in synaptosomes prepared from hippocampal tissue. J. Neurochem. 1990, 55, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, E.; Herrero, I.; Miras-Portugal, M.T.; Sanchez-Prieto, J. Role of arachidonic acid in the facilitation of glutamate release from rat cerebrocortical synaptosomes independent of metabotropic glutamate receptor responses. Neurosci. Lett. 1994, 174, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Weng, H.R. Endogenous interleukin-1beta in neuropathic rats enhances glutamate release from the primary afferents in the spinal dorsal horn through coupling with presynaptic N-methyl-D-aspartic acid receptors. J. Biol. Chem. 2013, 288, 30544–30557. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Sheng, W.S.; Ehrlich, L.C.; Peterson, P.K.; Chao, C.C. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation 2000, 7, 153–159. [Google Scholar] [CrossRef]
- Berger, U.V.; Hediger, M.A. Distribution of the glutamate transporters GLT-1 (SLC1A2) and GLAST (SLC1A3) in peripheral organs. Anat. Embryol. 2006, 211, 595–606. [Google Scholar] [CrossRef]
- Delgado, J.M. Cerebral structures involved in transmission and elaboration of noxious stimulation. J. Neurophysiol. 1955, 18, 261–275. [Google Scholar] [CrossRef]
- Dutar, P.; Lamour, Y.; Jobert, A. Activation of identified septo-hippocampal neurons by noxious peripheral stimulation. Brain Res. 1985, 328, 15–21. [Google Scholar] [CrossRef]
- Lozovaya, N.; Kopanitsa, M.; Boychuk, Y.; Krishtal, O. Enhancement of glutamate release uncovers spillover-mediated transmission by N-methyl-D-aspartate receptors in the rat hippocampus. Neuroscience 1999, 91, 1321–1330. [Google Scholar] [CrossRef]
- Cao, H.; Gao, Y.-J.; Ren, W.-H.; Li, T.-T.; Duan, K.-Z.; Cui, Y.-H.; Cao, X.-H.; Zhao, Z.-Q.; Ji, R.-R.; Zhang, Y.-Q. Activation of extracellular signal-regulated kinase in the anterior cingulate cortex contributes to the induction and expression of affective pain. J. Neurosci. 2009, 29, 3307–3321. [Google Scholar] [CrossRef]
- Fontainhas, A.M.; Wang, M.; Liang, K.J.; Chen, S.; Mettu, P.; Damani, M.; Fariss, R.N.; Li, W.; Wong, W.T. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS ONE 2011, 6, e15973. [Google Scholar] [CrossRef]
- Nair, A.; Bonneau, R.H. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J. Neuroimmunol. 2006, 171, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zheng, H.; Shao, X.; Wang, W.; Yao, Q.; Li, Z. Excitotoxicity of TNFalpha derived from KA activated microglia on hippocampal neurons in vitro and in vivo. J. Neurochem. 2010, 114, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Limatola, C.; Ransohoff, R.M. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front. Cell. Neurosci. 2014, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; Lauro, C.; Cipriani, R.; Chece, G.; Ponzetta, A.; Di Angelantonio, S.; Ragozzino, D.; Limatola, C. CX3CL1 protects neurons against excitotoxicity enhancing GLT-1 activity on astrocytes. J. Neuroimmunol. 2013, 263, 75–82. [Google Scholar] [CrossRef]
- Yang, J.-L.; Xu, B.; Li, S.-S.; Zhang, W.-S.; Xu, H.; Deng, X.-M.; Zhang, Y.-Q. Gabapentin reduces CX3CL1 signaling and blocks spinal microglial activation in monoarthritic rats. Mol. Brain 2012, 5, 18. [Google Scholar] [CrossRef]
- Gonçalves-Ribeiro, J.; Pina, C.C.; Sebastião, A.M.; Vaz, S.H. Glutamate Transporters in Hippocampal LTD/LTP: Not Just Prevention of Excitotoxicity. Front. Cell. Neurosci. 2019, 13, 357. [Google Scholar] [CrossRef]
- Kobayashi, K.; Manabe, T.; Takahashi, T. Presynaptic Long-Term Depression at the Hippocampal Mossy Fiber-CA3 Synapse. Science 1996, 273, 648–650. [Google Scholar] [CrossRef]
- Rosa, A.S.; Freitas, M.F.; Rocha, I.R.; Chacur, M. Gabapentin decreases microglial cells and reverses bilateral hyperalgesia and allodynia in rats with chronic myositis. Eur. J. Pharmacol. 2017, 799, 111–117. [Google Scholar] [CrossRef]
- Tawfik, V.L.; Regan, M.R.; Haenggeli, C.; Lacroix-Fralish, M.L.; Nutile-McMenemy, N.; Perez, N.; Rothstein, J.D.; DeLeo, J.A. Propentofylline-induced astrocyte modulation leads to alterations in glial glutamate promoter activation following spinal nerve transection. Neuroscience 2008, 152, 1086–1092. [Google Scholar] [CrossRef]
- Mao, J.; Price, D.D.; Hayes, R.L.; Lu, J.; Mayer, D.J. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hyperalgesia in rats with painful peripheral mononeuropathy. Brain Res. 1992, 598, 271–278. [Google Scholar] [CrossRef]
- Joels, M.; Fernhout, B. Decreased population spike in CA1 hippocampal area of adrenalectomized rats after repeated synaptic stimulation. J. Neuroendocrinol. 1993, 5, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Abraham, I.; Juhasz, G.; Kekesi, K.A.; Kovacs, K.J. Effect of intrahippocampal dexamethasone on the levels of amino acid transmitters and neuronal excitability. Brain Res. 1996, 733, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Virgin, C.E., Jr.; Ha, T.P.; Packan, D.R.; Tombaugh, G.C.; Yang, S.H.; Horner, H.C.; Sapolsky, R.M. Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: Implications for glucocorticoid neurotoxicity. J. Neurochem. 1991, 57, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Morioka, T.; Kalehua, A.N. MK-801 prevents microglial reaction in rat hippocampus after forebrain ischemia. Neuroreport 1992, 3, 146–148. [Google Scholar] [CrossRef]
- Thomas, D.M.; Kuhn, D.M. MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res. 2005, 1050, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.-X.; Zhou, L.-Y.; Wang, X.-Y.; Song, Y.-J.; Han, H.-H.; Zhang, T.-S.; Wang, Y.-J.; Shi, Q.; Xu, H.; Liang, Q.-Q. MK-801 attenuates lesion expansion following acute brain injury in rats: A meta-analysis. Neural Regen. Res. 2019, 14, 1919. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, G.; Khan, A.; Ronan, P.J.; Lutfy, K.; Rahman, S. Glial Glutamate Transporter Modulation Prevents Development of Complete Freund’s Adjuvant-Induced Hyperalgesia and Allodynia in Mice. Brain Sci. 2023, 13, 807. https://doi.org/10.3390/brainsci13050807
Alotaibi G, Khan A, Ronan PJ, Lutfy K, Rahman S. Glial Glutamate Transporter Modulation Prevents Development of Complete Freund’s Adjuvant-Induced Hyperalgesia and Allodynia in Mice. Brain Sciences. 2023; 13(5):807. https://doi.org/10.3390/brainsci13050807
Chicago/Turabian StyleAlotaibi, Ghallab, Amna Khan, Patrick J. Ronan, Kabirullah Lutfy, and Shafiqur Rahman. 2023. "Glial Glutamate Transporter Modulation Prevents Development of Complete Freund’s Adjuvant-Induced Hyperalgesia and Allodynia in Mice" Brain Sciences 13, no. 5: 807. https://doi.org/10.3390/brainsci13050807
APA StyleAlotaibi, G., Khan, A., Ronan, P. J., Lutfy, K., & Rahman, S. (2023). Glial Glutamate Transporter Modulation Prevents Development of Complete Freund’s Adjuvant-Induced Hyperalgesia and Allodynia in Mice. Brain Sciences, 13(5), 807. https://doi.org/10.3390/brainsci13050807






