IL-10/β-Endorphin-Mediated Neuroimmune Modulation on Microglia during Antinociception
Abstract
1. Introduction
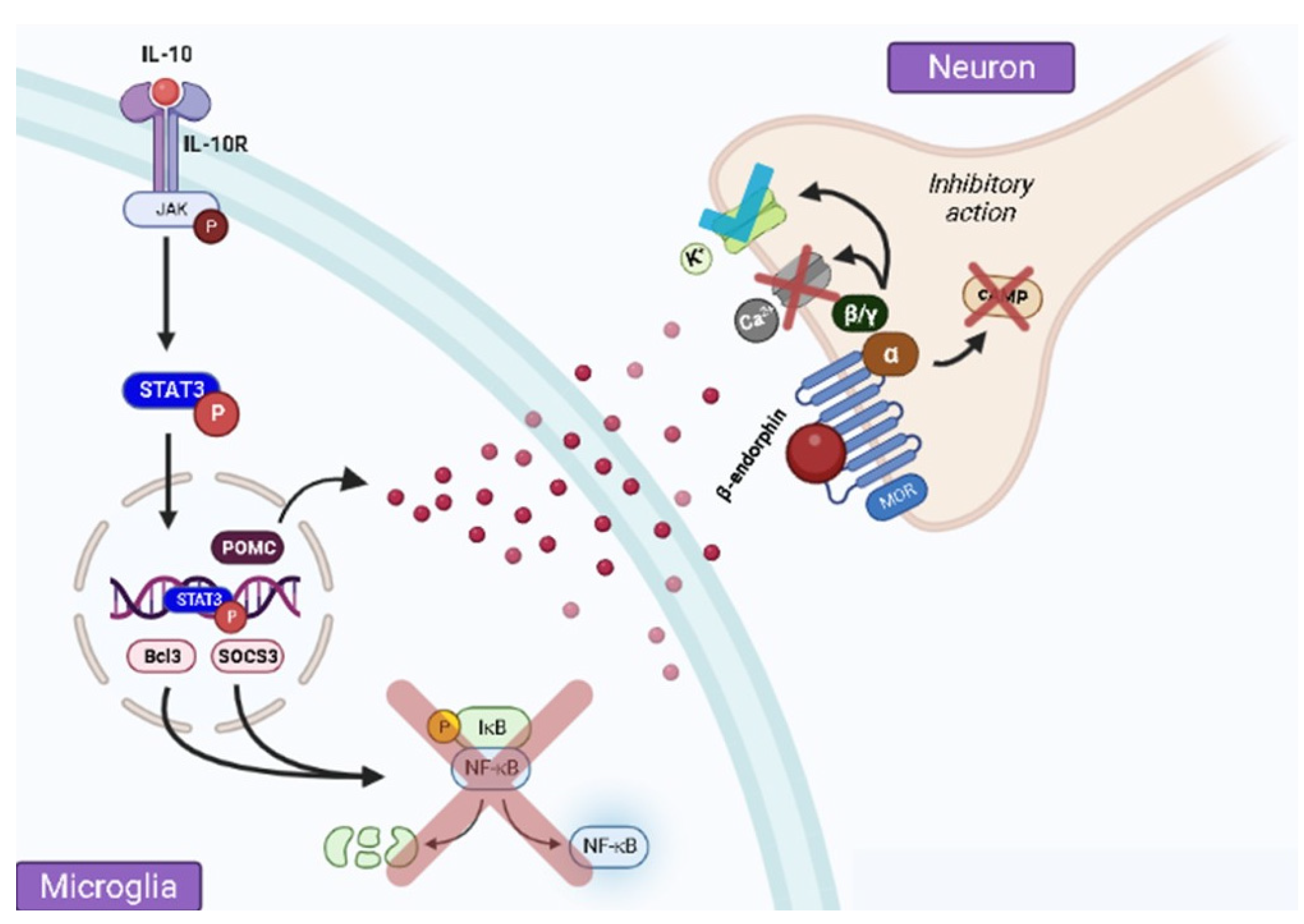
2. Pain Control by IL-10 via β-Endorphin Release at the Spinal Level
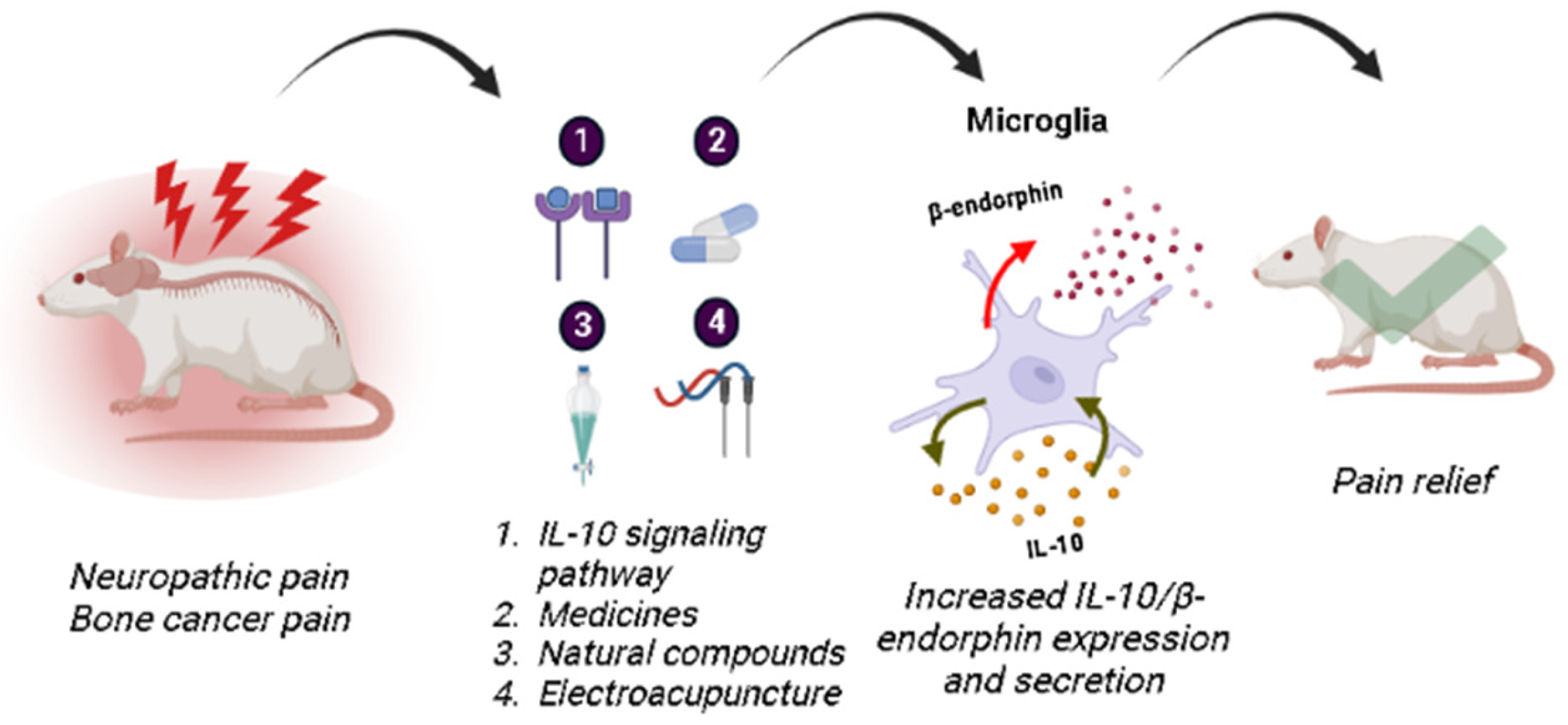
3. Spinal Microglial Activation Increases IL-10/β-Endorphin Levels
4. β-Endorphin Does Not Participate in IL-10-Induced Antinociception at the Peripheral Level
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain 2022, 163, 1740–1750. [Google Scholar] [CrossRef]
- Lee, G.I.; Neumeister, M.W. Pain: Pathways and Physiology. Clin. Plast. Surg. 2020, 47, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Treede, R.D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, N.M.; Chen, C.; Gilam, G.; Mackey, S.; Scherrer, G. Brain Circuits for Pain and Its Treatment. Sci. Transl. Med. 2021, 13, eabj7360. [Google Scholar] [CrossRef]
- Dubin, A.E. Patapoutian. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2012, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed]
- Haberberger, R.V.; Barry, C.; Dominguez, N.; Matusica, D. Human Dorsal Root Ganglia. Front. Cell. Neurosci. 2019, 13, 271. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Pace, M.C.; Passavanti, M.B.; De Nardis, L.; Bosco, F.; Sansone, P.; Pota, V.; Barbarisi, M.; Palagiano, A.; Iannoti, F.A.; Panza, E.; et al. Nociceptor plasticity: A closer look. J. Cell. Physiol. 2017, 233, 2824–2838. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Nwonu, C.N.S. Neuronal Cell Mechanisms of Pain. West Afr. J. Med. 2022, 39, 1075–1983. [Google Scholar] [PubMed]
- Araque, A.; Navarrete, M. Glial cells in neuronal network function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2375–2381. [Google Scholar] [CrossRef] [PubMed]
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and pathology. Acta Neuropathol. 2012, 119, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Weber, B.; Barros, L.F. The Astrocyte: Powerhouse and Recycling Center. Cold Spring Harb. Perspect. Biol. 2015, 7, a020396. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Chen, Q.; Eusman, M.A.; Pal, A.; Eguchi, S.; Wu, L.; Ma, D. The Role of Microglia in the Pathobiology of Neuropathic Pain Development: What Do We Know? Br. J. Anaesth. 2017, 118, 504–516. [Google Scholar] [CrossRef]
- Akhmetzyanova, E.; Kletenkov, K.; Mukhamedshina, Y.; Rizvanov, A. Different Approaches to Modulation of Microglia Phenotypes After Spinal Cord Injury. Front. Syst. Neurosci. 2019, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Chen, Y.; Fu, B.; Jiang, J.; Zhang, M. Infant Nerve Injury Induces Delayed Microglial Polarization to the M1 Phenotype, and Exercise Reduces Delayed Neuropathic Pain by Modulating Microglial Activity. Neuroscience 2017, 349, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Moore, K.W.; Malefyt, R.W.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, X.; Insolera, R.; Fink, D.J.; Mata, M. Interleukin-10 provides direct trophic support to neurons. J. Neurochem. 2009, 110, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.-F.; Zhu, H.-Q.; Wei, X.-H.; Wang, J.; Li, Y.-Y.; Pang, R.-P.; Liu, X.-G. Interleukin-10 down-regulates voltage gated sodium channels in rat dorsal root ganglion neurons. Exp. Neurol. 2013, 247, 466–475. [Google Scholar] [CrossRef]
- Pilozzi, A.; Carro, C.; Huang, X. Roles of β-Endorphin in Stress, Behavior, Neuroinflammation, and Brain Energy Metabolism. Int. J. Mol. Sci. 2021, 22, 338. [Google Scholar] [CrossRef]
- Hartwig, A.C. Peripheral beta-endorphin and pain modulation. Anesth. Prog. 1991, 38, 75–78. [Google Scholar]
- Sabat, R.; Grütz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of Interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Wu, H.Y.; Mao, X.F.; Tang, X.Q.; Ali, U.; Apryani, E.; Liu, H.; Li, X.Y.; Wang, Y.X. Spinal Interleukin-10 Produces Antinociception in Neuropathy through Microglial β-Endorphin Expression, Separated from Antineuroinflammation. Brain Behav. Immun. 2018, 73, 504–519. [Google Scholar] [CrossRef]
- Wu, H.Y.; Tang, X.Q.; Mao, X.F.; Wang, Y.X. Autocrine Interleukin-10 Mediates Glucagon-Like Peptide-1 Receptor-Induced Spinal Microglial β-Endorphin Expression. J. Neurosci. 2017, 37, 11701–11714. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, J.T.; Traynor, J.R. Opioid Receptor Interacting Proteins and the Control of Opioid Signaling. Curr. Pharm. Des. 2013, 19, 7333–7347. [Google Scholar] [CrossRef] [PubMed]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ju, P.; Wang, W.; Wei, J.; Wang, W.; Zhao, M.; Ahmad, K.A.; Wang, Y.; Chen, J. Microglial Activation of GLP-1R Signaling in Neuropathic Pain Promotes Gene Expression Adaption Involved in Inflammatory Responses. Neural. Plast. 2021, 2021, 9923537. [Google Scholar] [CrossRef]
- Ma, L.; Peng, S.; Wei, J.; Zhao, M.; Ahmad, K.A.; Chen, J.; Wang, Y.X. Spinal Microglial β- Endorphin Signaling Mediates IL-10 and Exenatide-Induced Inhibition of Synaptic Plasticity in Neuropathic Pain. CNS Neurosci. Ther. 2021, 27, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, H.; Mao, X.; Li, X.; Wang, Y. The GLP-1 Receptor Herbal Agonist Morroniside Attenuates Neuropathic Pain via Spinal Microglial Expression of IL-10 and β-Endorphin. Biochem. Biophys. Res. Commun. 2020, 530, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Q.; Yin, M.; Wang, Z.Y.; Liu, H.; Ao, J.P.; Wang, Y.X. Cynandione A Alleviates Neuropathic Pain Through α7-NAChR-Dependent IL-10/β-Endorphin Signaling Complexes. Front. Pharmacol. 2021, 11, 614450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Han, Q.Q.; Deng, M.Y.; Zhao, M.J.; Apryani, E.; Shoaib, R.M.; Wei, D.Q.; Wang, Y.X. Lemairamin, Isolated from the Zanthoxylum Plants, Alleviates Pain Hypersensitivity via Spinal α7 Nicotinic Acetylcholine Receptors. Biochem. Biophys. Res. Commun. 2020, 525, 1087–1094. [Google Scholar] [CrossRef]
- Apryani, E.; Ali, U.; Wang, Z.Y.; Wu, H.Y.; Mao, X.F.; Ahmad, K.A.; Li, X.Y.; Wang, Y.X. The Spinal Microglial IL-10/β-Endorphin Pathway Accounts for Cinobufagin-Induced Mechanical Antiallodynia in Bone Cancer Pain Following Activation of A7-Nicotinic Acetylcholine Receptors. J. Neuroinflammation 2020, 17, 75. [Google Scholar] [CrossRef]
- Mao, X.F.; Wu, H.Y.; Tang, X.-Q.; Ali, U.; Liu, H.; Wang, Y.-X. Activation of GPR40 Produces Mechanical Antiallodynia via the Spinal Glial Interleukin-10/β-Endorphin Pathway. J. Neuroinflam. 2019, 16, 84. [Google Scholar] [CrossRef]
- Deng, M.Y.; Ahmad, K.A.; Han, Q.Q.; Wang, Z.Y.; Shoaib, R.M.; Li, X.Y.; Wang, Y.X. Thalidomide Alleviates Neuropathic Pain through Microglial IL-10/β-Endorphin Signaling Pathway. Biochem. Pharmacol. 2021, 192, 114727. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.A.; Shoaib, R.M.; Ahsan, M.Z.; Deng, M.Y.; Ma, L.; Apryani, E.; Li, X.Y.; Wang, Y.X. Microglial IL-10 and β-Endorphin Expression Mediates Gabapentinoids Antineuropathic Pain. Brain Behav. Immun. 2021, 95, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Apryani, E.; Wu, H.Y.; Mao, X.F.; Liu, H.; Wang, Y.X. Low Frequency Electroacupuncture Alleviates Neuropathic Pain by Activation of Spinal Microglial IL-10/β-Endorphin Pathway. Biomed. Pharmacother. 2020, 125, 109898. [Google Scholar] [CrossRef] [PubMed]
- Asatsuma-Okumura, T.; Ito, T.; Handa, H. Molecular Mechanisms of the Teratogenic Effects of Thalidomide. Pharmaceuticals 2020, 13, 95. [Google Scholar] [CrossRef]
- Cheng, J.K.; Chiou, L.C. Mechanisms of the Antinociceptive Action of Gabapentin. J. Pharmacol. Sci. 2006, 100, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M.; Porreca, F.; Taylor, C.P.; Cheung, R.; Thorpe, A.J.; Clair, A. The Diverse Therapeutic Actions of Pregabalin: Is a Single Mechanism Responsible for Several Pharmacological Activities? Trends Pharmacol. Sci. 2013, 34, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhao, H.; Ma, C.; Bao, F.; Zhang, J.; Wang, D.H.; Zhang, Y.X.; He, W. Effects of Electroacupuncture on Depression and the Production of Glial Cell Line-Derived Neurotrophic Factor Compared with Fluoxetine: A Randomized Controlled Pilot Study. J. Altern. Complement. Med. 2013, 19, 733–739. [Google Scholar] [CrossRef]
- Saadé, N.E.; Jabbur, S.J. Nociceptive behavior in animal models for peripheral neuropathy: Spinal and supraspinal mechanisms. Prog. Neurobiol. 2008, 86, 22–47. [Google Scholar] [CrossRef]
- D-Souza, R.S.; Barman, R.; Joseph, A.; Abd-Elsayed, A. Evidence-Based Treatment of Painful Diabetic Neuropathy: A Systematic Review. Curr. Pain Headache Rep. 2022, 26, 583–593. [Google Scholar] [CrossRef]
- Abdel-Wahhab, K.G.; Daoud, E.M.; El Gendy, A.; Mourad, H.H.; Mannaa, F.A.; Saber, M.M. Efficiencies of Low-Level Laser Therapy (LLLT) and Gabapentin in the Management of Peripheral Neuropathy: Diabetic Neuropathy. Appl. Biochem. Biotechnol. 2018, 186, 161–173. [Google Scholar] [CrossRef]
- Liou, J.T.; Mao, C.C.; Ching-Wah Sum, D.; Liu, F.C.; Lai, Y.S.; Li, J.C.; Day, Y.J. Peritoneal Administration of Met-RANTES Attenuates Inflammatory and Nociceptive Responses in a Murine Neuropathic Pain Model. J. Pain 2013, 14, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Leiguarda, C.; Potilinski, C.; Rubione, J.; Tate, P.; Villar, M.J.; Montaner, A.; Bisagno, V.; Constandil, L.; Brumovsky, P.R. IMT504 Provides Analgesia by Modulating Cell Infiltrate and Inflammatory Milieu in a Chronic Pain Model. J. Neuroimmune Pharmacol. 2021, 16, 651–666. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.R.; Kitching, A.R.; Tipping, P.G.; Holdsworth, S.R. Interleukin-10 Inhibits Macrophage-Induced Glomerular Injury. J. Am. Soc. Nephrol. 2000, 11, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, C.; Seybold, K.; Petzi, S.; Wasmeier, G.; Raaz, D.; Yilmaz, A.; Anger, T.; Daniel, W.G.; Garlichs, C.D. Interleukin-10 Improves Left Ventricular Function in Rats with Heart Failure Subsequent to Myocardial Infarction. Eur. J. Heart Fail. 2008, 10, 733–739. [Google Scholar] [CrossRef]
- Mannaa, F.A.E.; Abdel-Wahhab, K.G.E.D.; Daoud, E.M.; El Gendy, A.A.R.; Saber, M.M.; Fadl, N.N. Effectiveness of Low-Power Laser Therapy in Improvement of the Peripheral Neuropathy Induced by Xenobiotics in Rats. Biochem. Biophys. Rep. 2021, 27, 101085. [Google Scholar] [CrossRef]
- Suchting, R.; Colpo, G.D.; Rocha, N.P.; Ahn, H. The Effect of Transcranial Direct Current Stimulation on Inflammation in Older Adults With Knee Osteoarthritis: A Bayesian Residual Change Analysis. Biol. Res. Nurs. 2020, 22, 57–63. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, T.; Zhang, Y.; Wang, X.; Liu, J.; Chen, W.; Wei, C.; Liu, D.; Wu, W.; Lan, T.; et al. IRG1/itaconate increases IL-10 release to alleviate mechanical and thermal hypersensitivity in mice after nerve injury. Front. Immunol. 2022, 13, 6189. [Google Scholar] [CrossRef]
- Strelko, C.L.; Lu, W.; Dufort, F.J.; Seyfried, T.N.; Chiles, T.C.; Rabinowitz, J.D.; Roberts, M.F. Itaconic acid is a mammalian metabolite induced during macrophage activation. J. Am. Chem. Soc. 2011, 133, 16383–16389. [Google Scholar] [CrossRef]
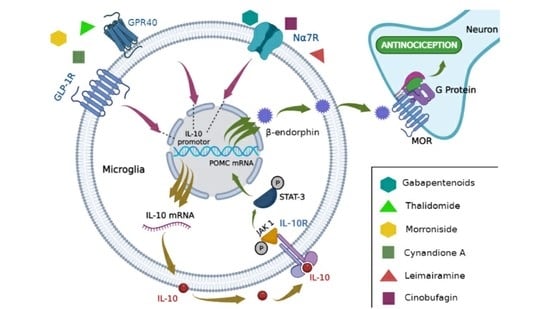
| Increased IL-10/β-Endorphin Signaling in Microglia | IL-10/β-Endorphin Impact on Pain Decrease | Refs. | |
|---|---|---|---|
| IL-10 signaling pathway | GLP-1 R activation by exenatin/IL-10: GLP-1 activation inhibits glutamatergic transmission and pain hypersensitivity via β-endorphin MOR for IL-10 signaling | ↓ mechanical allodynia and thermal hyperalgesia in neuropathic rats ↓ excitatory postsynaptic currents dorsal horn neurons of lamina II | [34,35] |
| GPR40 receptor activation by the GW9508 agonist: ↑ expression of IL-10/β-endorphin genes and proteins | ↓ mechanical allodynia and hyperalgesia in rats with neuropathic pain | [40] | |
| Administration of intrathecal IL-10: ↑ β-endorphin expression ↑ POMC mRNA expression ↑ pSTAT3 | ↓ mechanical allodynia and thermal hyperalgesia in male and female neuropathic rats | [31] | |
| Medicines | Gabapentinoids: ↑IL-10/β-endorphin mRNA expression | ↓ mechanical allodynia of rats with neuropathic pain | [42] |
| Thalidomide: ↑ IL-10/β-endorphin expression | ↓ mechanical allodynia and thermal hyperalgesia in rats with neuropathic pain | [41] | |
| Natural compounds | α7-nAChR receptor activation by Cynandione A: ↑ IL-10/POMC mRNA expression ↑ IL-10/β-endorphin protein expression ↑ pSTAT3 ↑ pPKA, p38, and CREB | ↓ mechanical allodynia in neuropathic pain rats | [37] |
| GLP-1R Receptor Activation by Morroniside: ↑ IL-10/β-endorphin/POMC gene expression | ↓ mechanical allodynia in neuropathic pain rats | [36] | |
| α7-nAChRs receptor activation by Lemairamin: ↑ IL-10/β-endorphin protein expression ↑ IL-10 mRNA expression | ↓ mechanical allodynia of rats with neuropathic pain and bone cancer pain | [38] | |
| α7-nAChRs receptor activation by Cinobufagin: ↑ IL-10/POMC mRNA expression ↑ IL-10/β-endorphin protein expression | ↓ mechanical allodynia of rats with bone cancer pain | [39] | |
| Non-pharmacological treatment | Electroacupuncture: ↑ IL-10/β-endorphin gene and protein expression ↑ POMC gene expression | ↓ mechanical allodynia of rats with neuropathic pain | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belo, T.C.A.; Santos, G.X.; da Silva, B.E.G.; Rocha, B.L.G.; Abdala, D.W.; Freire, L.A.M.; Rocha, F.S.; Galdino, G. IL-10/β-Endorphin-Mediated Neuroimmune Modulation on Microglia during Antinociception. Brain Sci. 2023, 13, 789. https://doi.org/10.3390/brainsci13050789
Belo TCA, Santos GX, da Silva BEG, Rocha BLG, Abdala DW, Freire LAM, Rocha FS, Galdino G. IL-10/β-Endorphin-Mediated Neuroimmune Modulation on Microglia during Antinociception. Brain Sciences. 2023; 13(5):789. https://doi.org/10.3390/brainsci13050789
Chicago/Turabian StyleBelo, Thiago Caetano Andrade, Gabriela Xavier Santos, Bruno Eduardo Gabriel da Silva, Bruno Lopes Gonçalves Rocha, Dennis William Abdala, Larissa Alves Moreira Freire, Fernanda Santos Rocha, and Giovane Galdino. 2023. "IL-10/β-Endorphin-Mediated Neuroimmune Modulation on Microglia during Antinociception" Brain Sciences 13, no. 5: 789. https://doi.org/10.3390/brainsci13050789
APA StyleBelo, T. C. A., Santos, G. X., da Silva, B. E. G., Rocha, B. L. G., Abdala, D. W., Freire, L. A. M., Rocha, F. S., & Galdino, G. (2023). IL-10/β-Endorphin-Mediated Neuroimmune Modulation on Microglia during Antinociception. Brain Sciences, 13(5), 789. https://doi.org/10.3390/brainsci13050789