L-DOPA Improves Ventilation but Not the Ventilatory Response to Hypercapnia in a Reserpine Model of Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Model and Drugs
2.3. Ventilation Measurements
2.4. Open-Field Test
2.5. Protocol
2.6. Statistical Analysis
3. Results
3.1. Behavioral Analysis
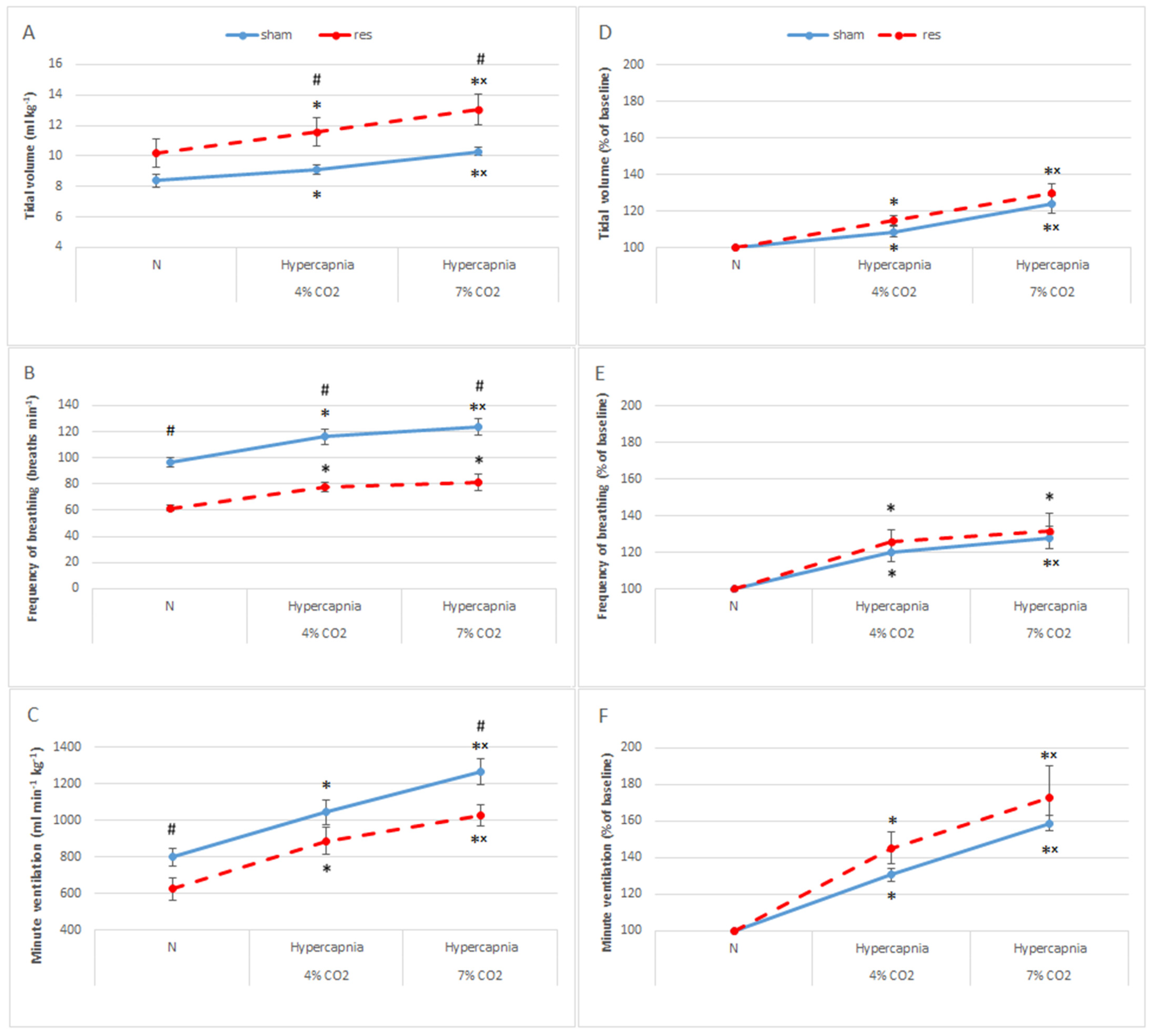
3.2. Normocapnic Breathing and Respiratory Response to Hypercapnia after Reserpine Treatment
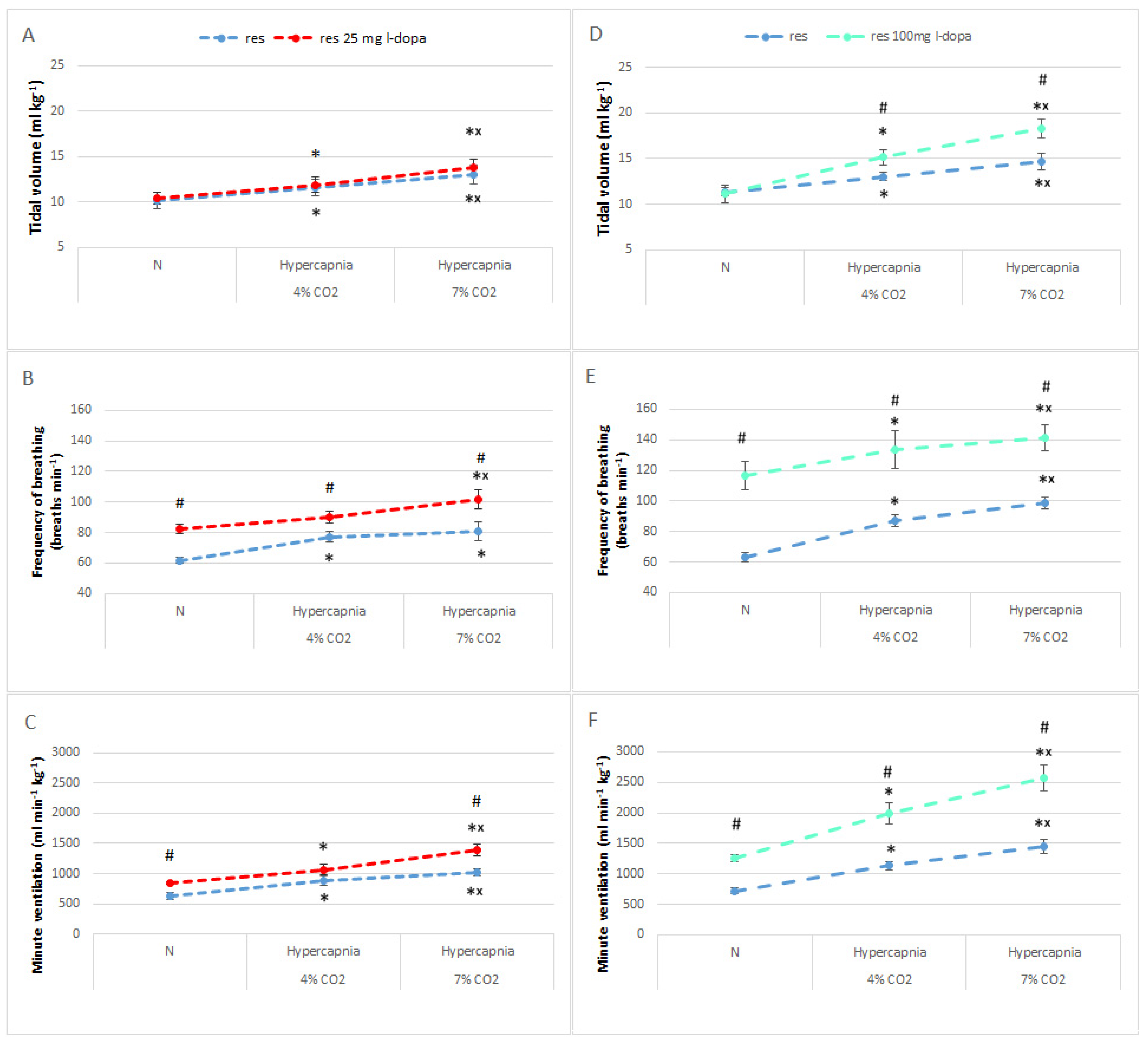
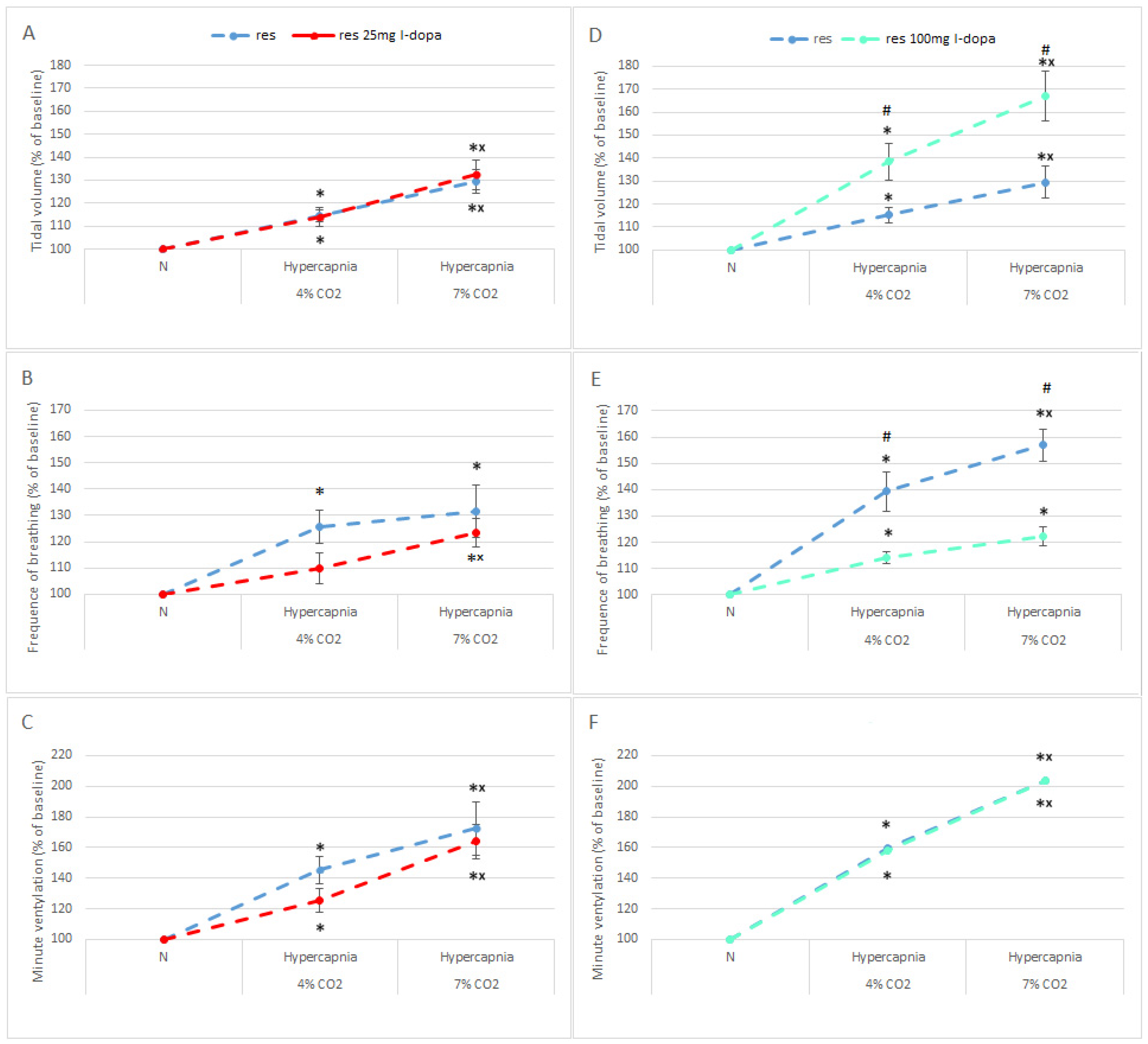
3.3. Normocapnic Breathing and Respiratory Response to Hypercapnia in RES Rats after L-DOPA Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Triarhou, L.C. Introduction. Dopamine and Parkinson’s disease. Adv. Exp. Med. Biol. 2002, 517, 1–14. [Google Scholar] [PubMed]
- Dragicevic, E.; Schiemann, J.; Liss, B. Dopamine midbrain neurons in health and Parkinson’s disease: Emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience 2015, 284, 798–814. [Google Scholar] [CrossRef] [PubMed]
- Aquino, Y.C.; Cabral, L.M.; Miranda, N.C.; Naccarato, M.C.; Falquetto, B.; Moreira, T.S.; Takakura, A.C. Respiratory disorders of Parkinson’s disease. J. Neurophysiol. 2022, 127, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kaczyńska, K.; Orłowska, M.E.; Andrzejewski, K. Respiratory Abnormalities in Parkinson’s Disease: What Do We Know from Studies in Humans and Animal Models? Int. J. Mol. Sci. 2022, 23, 3499. [Google Scholar] [CrossRef]
- Seccombe, L.M.; Giddings, H.L.; Rogers, P.G.; Corbett, A.J.; Hayes, M.W.; Peters, M.J.; Veitch, E.M. Abnormal ventilatory control in Parkinson’s disease—Further evidence for non-motor dysfunction. Respir. Physiol. Neurobiol. 2011, 179, 300–304. [Google Scholar] [CrossRef]
- Onodera, H.; Okabe, S.; Kikuchi, Y.; Tsuda, T.; Itoyama, Y. Impaired chemosensitivity and perception of dyspnoea in Parkinson’s disease. Lancet 2000, 356, 739–740. [Google Scholar] [CrossRef]
- Feinsilver, S.H.; Friedman, J.H.; Rosen, J.M. Respiration and sleep in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1986, 49, 964. [Google Scholar] [CrossRef]
- Andrzejewski, K.; Budzińska, K.; Kaczyńska, K. Effect of 6-OHDA on hypercapnic ventilatory response in the rat model of Parkinson’s disease. Physiol. Res. 2019, 68, 285–293. [Google Scholar] [CrossRef]
- Tuppy, M.; Barna, B.F.; Alves-Dos-Santos, L.; Britto, L.R.; Chiavegatto, S.; Moreira, T.S.; Takakura, A.C. Respiratory deficits in a rat model of Parkinson’s disease. Neuroscience 2015, 297, 194–204. [Google Scholar] [CrossRef]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef]
- Colpaert, F.C. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacology 1987, 26, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Skalisz, L.L.; Beijamini, V.; Joca, S.L.; Vital, M.A.B.F.; Da Cunha, C.; Andreatini, R. Evaluation of the face validity of reserpine administration as an animal model of depression—Parkinson’s disease association. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.R.; Cunha, J.A.; Dierschnabel, A.L.; Campêlo, C.L.; Leão, A.H.; Silva, A.F.; Engelberth, R.C.; Izídio, G.S.; Cavalcante, J.S.; Abílio, V.C.; et al. Cognitive, motor and tyrosine hydroxylase temporal impairment in a model of parkinsonism induced by reserpine. Behav. Brain Res. 2013, 253, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Leao, A.H.; Sarmento-Silva, A.J.; Santos, J.R.; Ribeiro, A.M.; Silva, R.H. Molecular, Neurochemical, and Behavioral Hallmarks of Reserpine as a Model for Parkinson’s Disease. New Perspect. Long Standing Model. Brain Pathol. 2015, 25, 377–390. [Google Scholar] [CrossRef]
- Bernstein, A.I.; Stout, K.A.; Miller, G.W. The vesicular monoamine transporter 2: An underexplored pharmacological target. Neurochem. Int. 2014, 73, 89–97. [Google Scholar] [CrossRef]
- Fernandes, V.S.; Santos, J.R.; Leão, A.H.; Medeiros, A.M.; Melo, T.G.; Izídio, G.S.; Cabral, A.; Ribeiro, R.A.; Abílio, V.C.; Ribeiro, A.M.; et al. Repeated treatment with a low dose of reserpine as a progressive model of Parkinson’s disease. Behav. Brain Res. 2012, 231, 154–163. [Google Scholar] [CrossRef]
- Jampolska, M.; Andrzejewski, K.; Zaremba, M.; Joniec-Maciejak, I.; Kaczyńska, K. Deficiency of Biogenic Amines Modulates the Activity of Hypoglossal Nerve in the Reserpine Model of Parkinson’s Disease. Cells 2021, 10, 531. [Google Scholar] [CrossRef]
- Widerlöv, E. Dose-dependent pharmacokinetics of alpha-methyl-p-tyrosine (alpha-MT) and comparison of catecholamine turnover rates after two doses of alpha-MT. J. Neural Transm. 1979, 44, 145–158. [Google Scholar] [CrossRef]
- Hsiao, C.; Lahiri, S.; Mokashi, A. Peripheral and central dopamine receptors in respiratory control. Respir. Physiol. 1989, 76, 327–336. [Google Scholar] [CrossRef]
- McNamara, M.C.; Lawson, E.E. Ontogeny of biogenic amines in respiratory nuclei of the rabbit brainstem. Brain Res. 1983, 283, 181–185. [Google Scholar] [CrossRef]
- Milner, T.A.; Joh, T.H.; Pickel, V.M. Tyrosine hydroxylase in the rat parabrachial region: Ultrastructural localization and extrinsic sources of immunoreactivity. J. Neurosci. 1986, 6, 2585–2603. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.J.; Pilowsky, P.; Minson, J.; Arnolda, L.; Chalmers, J.; Llewelyn-Smith, I.J. Close appositions between tyrosine hydroxylase immunoreactive boutons and respiratory neurons in the rat ventrolateral medulla. J. Comp. Neurol. 1994, 340, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nsegbe, E.; Wallen-Mackenzie, A.; Dauger, S.; Roux, J.C.; Shvarev, Y.; Lagerkranz, H.; Perlmann, T.; Herlenius, E. Congenital hypoventilation and impaired hypoxic response in Nurr1 mutant mice. J. Physiol. 2004, 556, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, K.; Jampolska, M.; Zaremba, M.; Joniec-Maciejak, I.; Boguszewski, P.M.; Kaczyńska, K. Respiratory pattern and phrenic and hypoglossal nerve activity during normoxia and hypoxia in 6-OHDA-induced bilateral model of Parkinson’s disease. J. Physiol. Sci. 2020, 70, 16. [Google Scholar] [CrossRef]
- Andrzejewski, K.; Budzińska, K.; Zaremba, M.; Kaczyńska, K. Hypoxic ventilatory response after dopamine D2 receptor blockade in unilateral rat model of Parkinson’s disease. Neuroscience 2016, 316, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Serebrovskaya, T.; Karaban, I.; Mankovskaya, I.; Bernardi, L.; Passino, C.; Appenzeller, O. Hypoxic ventilatory responses and gas exchange in patients with Parkinson’s disease. Respiration 1998, 65, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Lalley, P.M. Opioidergic and dopaminergic modulation of respiration. Respir. Physiol. Neurobiol. 2008, 164, 160–167. [Google Scholar] [CrossRef]
- Lundberg, D.; Breese, G.R.; Mueller, R.A. Dopaminergic interaction with the respiratory control system in the rat. Eur. J. Pharmacol. 1979, 54, 153–159. [Google Scholar] [CrossRef]
- Hedner, J.; Hedner, T.; Jonason, J.; Lundberg, D. Evidence for a dopamine interaction with the central respiratory control system in the rat. Eur. J. Pharmacol. 1982, 81, 603–615. [Google Scholar] [CrossRef]
- Vincent, S.G.; Waddell, A.E.; Caron, M.G.; Walker, J.K.; Fisher, J.T. A murine model of hyperdopaminergic state displays altered respiratory control. FASEB J. 2007, 21, 1463–1471. [Google Scholar] [CrossRef]
- Yaffe, D.; Forrest, L.R.; Schuldiner, S. The ins and outs of vesicular monoamine transporters. J. Gen. Physiol. 2018, 150, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, V.; Bícego, K.C.; Almeida, M.C.; Gargaglioni, L.H. Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflug. Arch. 2008, 455, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Malheiros-Lima, M.R.; Totola, L.T.; Takakura, A.C.; Moreira, T.S. Impaired chemosensory control of breathing after depletion of bulbospinal catecholaminergic neurons in rats. Pflug. Arch. 2018, 470, 277–293. [Google Scholar] [CrossRef]
- Li, A.; Emond, L.; Nattie, E. Brainstem catecholaminergic neurons modulate both respiratory and cardiovascular function. Adv. Exp. Med. Biol. 2008, 605, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Nattie, E.E.; Li, A.; Richerson, G.B.; Lappi, D.A. Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J. Physiol. 2004, 556, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewski, K.; Kaczyńska, K.; Zaremba, M. Serotonergic system in hypoxic ventilatory response in unilateral rat model of Parkinson’s disease. J. Biomed. Sci. 2017, 24, 24. [Google Scholar] [CrossRef]
- Pho, H.; Amorim, M.R.; Qiu, Q.; Shin, M.K.; Kim, L.J.; Anokye-Danso, F.; Jun, J.J.; Ahima, R.S.; Branco, L.G.S.; Kuhn, D.M.; et al. The effect of brain serotonin deficiency on breathing is magnified by age. Physiol. Rep. 2022, 10, e15245. [Google Scholar] [CrossRef]
- Olson, L.G.; Saunders, N.A. The effect of central and peripheral dopamine-agonists on ventilation in the mouse. Respir. Physiol. 1985, 61, 335–345. [Google Scholar] [CrossRef]
- Bialkowska, M.; Boguszewski, P.; Pokorski, M. Breathing in Parkinsonism in the Rat. Adv. Exp. Med. Biol. 2016, 884, 1–11. [Google Scholar] [CrossRef]
- Alachkar, A.; Brotchie, J.M.; Jones, O.T. Locomotor response to L-DOPA in reserpine-treated rats following central inhibition of aromatic L-amino acid decarboxylase: Further evidence for non-dopaminergic actions of L-DOPA and its metabolites. Neurosci. Res. 2010, 68, 44–50. [Google Scholar] [CrossRef]

| Distance (cm) | Moving (s) | Mobile (s) | |
|---|---|---|---|
| SHAM | 554 ± 73 | 62 ± 8.8 | 58 ± 6.8 |
| SHAM + L-DOPA | 420 ± 93 | 47 ± 9.2 | 48 ± 8.3 |
| RES | 1.62 ± 0.33 ## | 0.08 ± 0 # | 0.08 ± 0 # |
| RES + L-DOPA 25 mg | 1.58 ± 0.73 ## | 0.18 ± 0.06 * # | 0.25 ± 0.17 ## |
| RES + L-DOPA 100 mg | 32 ± 8.2 ** ## ++ | 2.28 ± 1.0 * # | 8.2 ± 3.25 ** ## + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jampolska, M.; Andrzejewski, K.; Boguszewski, P.M.; Kaczyńska, K. L-DOPA Improves Ventilation but Not the Ventilatory Response to Hypercapnia in a Reserpine Model of Parkinson’s Disease. Brain Sci. 2023, 13, 775. https://doi.org/10.3390/brainsci13050775
Jampolska M, Andrzejewski K, Boguszewski PM, Kaczyńska K. L-DOPA Improves Ventilation but Not the Ventilatory Response to Hypercapnia in a Reserpine Model of Parkinson’s Disease. Brain Sciences. 2023; 13(5):775. https://doi.org/10.3390/brainsci13050775
Chicago/Turabian StyleJampolska, Monika, Kryspin Andrzejewski, Paweł M. Boguszewski, and Katarzyna Kaczyńska. 2023. "L-DOPA Improves Ventilation but Not the Ventilatory Response to Hypercapnia in a Reserpine Model of Parkinson’s Disease" Brain Sciences 13, no. 5: 775. https://doi.org/10.3390/brainsci13050775
APA StyleJampolska, M., Andrzejewski, K., Boguszewski, P. M., & Kaczyńska, K. (2023). L-DOPA Improves Ventilation but Not the Ventilatory Response to Hypercapnia in a Reserpine Model of Parkinson’s Disease. Brain Sciences, 13(5), 775. https://doi.org/10.3390/brainsci13050775





