Hemispheric Differences of 1 Hz rTMS over Motor and Premotor Cortex in Modulation of Neural Processing and Hand Function
Abstract
1. Introduction
1.1. Modulation of Neural Networks and of Hand Motor Function by rTMS
1.2. Modulation of Hand Function by rTMS
1.3. Stimulation Location Dependent Effects of rTMS
2. Methods
2.1. Participants
2.2. Study Design
2.3. Evaluations
2.3.1. Neurophysiological Evaluations
MEP Amplitude
CSP Duration
ISP Duration
2.3.2. Hand Motor Function Evaluation
2.4. Interventions
2.5. Analysis
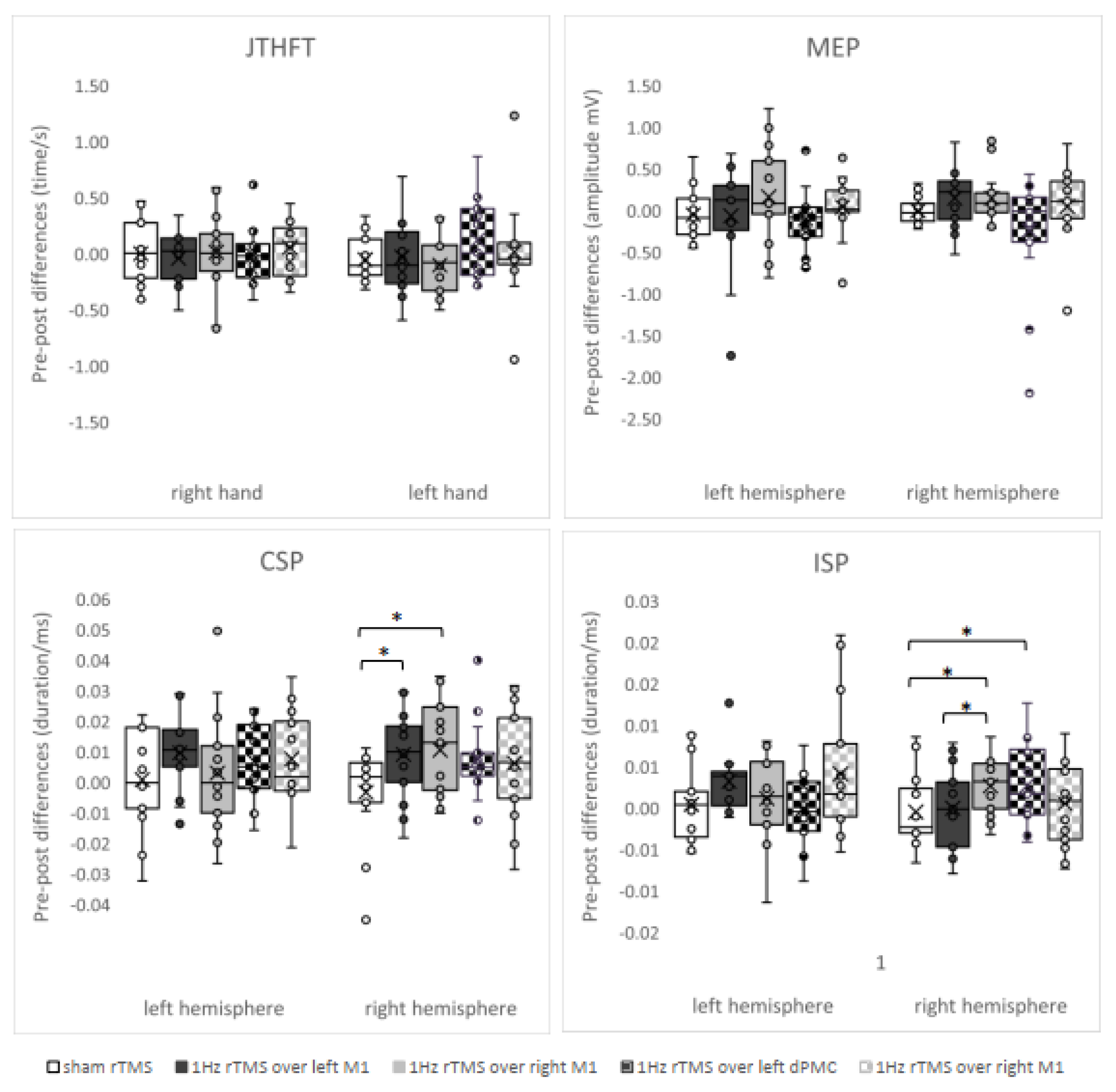
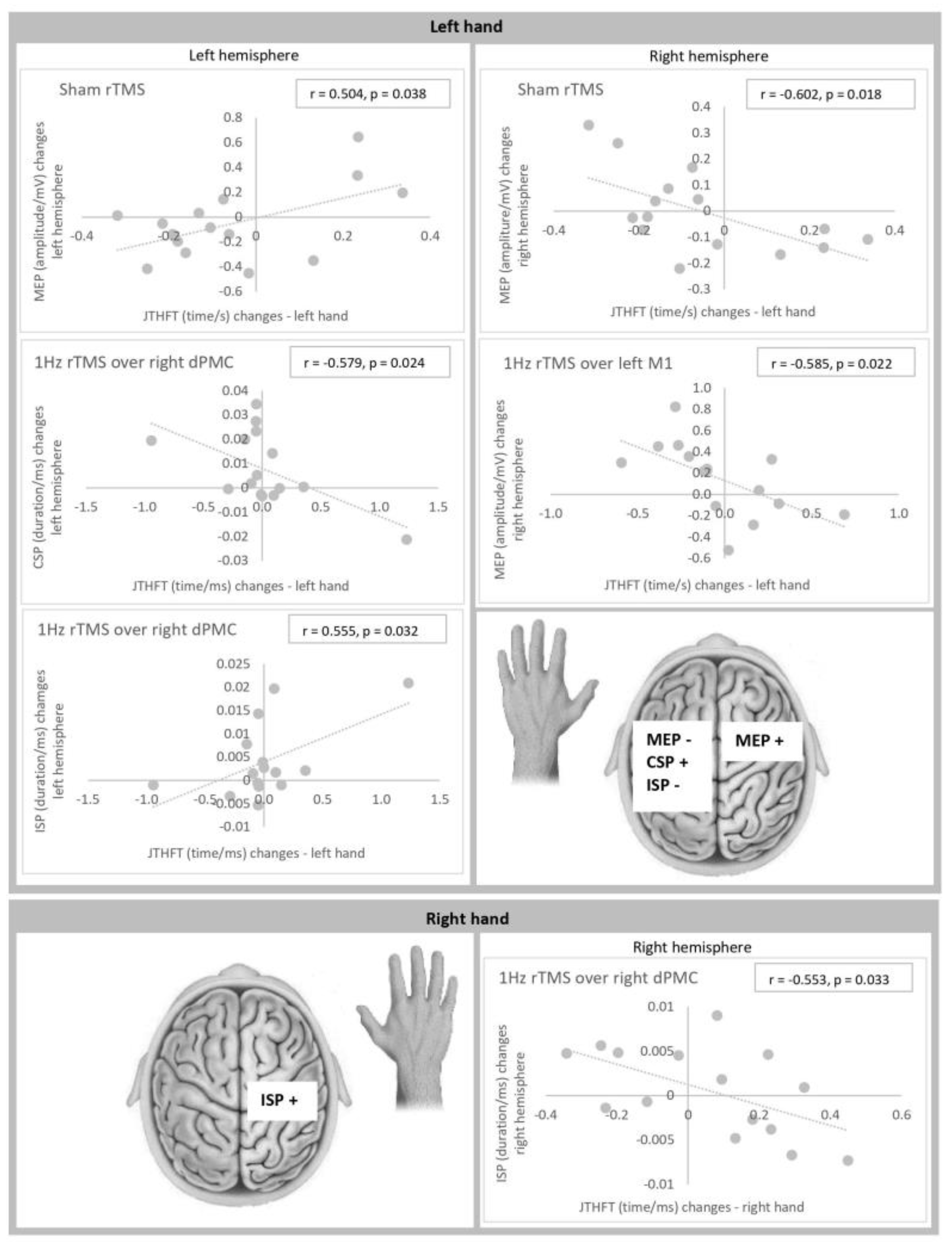
3. Results
3.1. ANOVAs
3.2. Correlations
4. Discussion
4.1. rTMS-Induced Motor Effects
4.2. rTMS-Induced Changes of Corticospinal Excitability and Relationships to Motor Performance
4.3. rTMS-Induced Changes of CSP Duration and Relationships to Motor Performance
4.4. rTMS-Induced Changes of ISP and Relationships to Motor Performance
4.5. Stimulation Location Dependent Effects
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoogendam, J.M.; Ramakers, G.M.; Di Lazzaro, V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010, 3, 95–118. [Google Scholar] [CrossRef]
- Chung, S.W.; Hill, A.T.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. Use of theta-burst stimulation in changing excitability of motor cortex: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 63, 43–64. [Google Scholar] [CrossRef]
- Peinemann, A.; Reimer, B.; Löer, C.; Quartarone, A.; Münchau, A.; Conrad, B.; Siebner, H.R. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin. Neurophysiol. 2004, 115, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Fierro, B.; Piazza, A.; Brighina, F.; La Bua, V.; Buffa, D.; Oliveri, M. Modulation of intracortical inhibition induced by low- and high-frequency repetitive transcranial magnetic stimulation. Exp. Brain Res. 2001, 138, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.; Siebner, H.R. Repetitive transkranielle Magnetstimulation. In Das rTMS Buch; Siebner, H.R., Ziemann, U., Eds.; Springer: Heidelberg, Germany, 2007; pp. 499–509. [Google Scholar]
- Maeda, F.; Keenan, J.P.; Tormos, J.M.; Topka, H.; Pascual-Leone, A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp. Brain Res. 2000, 133, 425–430. [Google Scholar] [CrossRef]
- Hamada, M.; Murase, N.; Hasan, A.; Balaratnam, M.; Rothwell, J.C. The role of interneuron networks in driving human motor cortical plasticity. Cereb. Cortex 2013, 23, 1593–1605. [Google Scholar] [CrossRef]
- Hordacre, B.; Goldsworthy, M.R.; Vallence, A.-M.; Darvishi, S.; Moezzi, B.; Hamada, M.; Rothwell, J.C.; Ridding, M.C. Variability in neural excitability and plasticity induction in the human cortex: A brain stimulation study. Brain Stimul. 2017, 10, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Ridding, M.C.; Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010, 588, 2291–2304. [Google Scholar] [CrossRef]
- Corp, D.T.; Bereznicki, H.G.; Clark, G.M.; Youssef, G.J.; Fried, P.J.; Jannati, A.; Davies, C.B.; Gomes-Osman, J.; Stamm, J.; Chung, S.W.; et al. Large-scale analysis of interindividual variability in theta-burst stimulation data: Results from the ‘Big TMS Data Collaboration’. Brain Stimul. 2020, 13, 1476–1488. [Google Scholar] [CrossRef]
- Corp, D.T.; Bereznicki, H.G.; Clark, G.M.; Youssef, G.J.; Fried, P.J.; Jannati, A.; Davies, C.B.; Gomes-Osman, J.; Kirkovski, M.; Albein-Urios, N.; et al. Large-scale analysis of interindividual variability in single and paired-pulse TMS data. Clin. Neurophysiol. 2021, 132, 2639–2653. [Google Scholar] [CrossRef]
- Watanabe, M.; Matsunaga, T.; Okudera, Y.; Sato, M.; Hatakeyama, K.; Chida, S.; Takahashi, Y.; Shimada, Y. Optimum Stimulation Frequency of High-Frequency Repetitive Transcranial Magnetic Stimulation for Upper-Limb Function in Healthy Subjects. Int. J. Phys. Med. Rehabil. 2015, 3, 6. [Google Scholar] [CrossRef]
- Koch, G.; Esposito, R.; Motta, C.; Casula, E.P.; Di Lorenzo, F.; Bonnì, S.; Cinnera, A.M.; Ponzo, V.; Maiella, M.; Picazio, S.; et al. Improving visuo-motor learning with cerebellar theta burst stimulation: Behavioral and neurophysiological evidence. Neuroimage 2020, 208, 116424. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Lee, H.J.; Lee, J.; Na, Y.; Chang, W.H.; Kim, Y.H. Optimal stimulation site for rTMS to improve motor function: Anatomical hand knob vs. hand motor hotspot. Neurosci. Lett. 2021, 740, 135424. [Google Scholar] [CrossRef] [PubMed]
- Schramm, S.; Albers, L.; Ille, S.; Schröder, A.; Meyer, B.; Sollmann, N.; Krieg, S.M. Navigated transcranial magnetic stimulation of the supplementary motor cortex disrupts fine motor skills in healthy adults. Sci. Rep. 2019, 9, 17744. [Google Scholar] [CrossRef]
- Mirdamadi, J.L.; Block, H.J. Somatosensory versus cerebellar contributions to proprioceptive changes associated with motor skill learning: A theta burst stimulation study. Cortex 2021, 140, 98–109. [Google Scholar] [CrossRef]
- Jäncke, L.; Steinmetz, H.; Benilow, S.; Ziemann, U. Slowing fastest finger movements of the dominant hand with low-frequency rTMS of the hand area of the primary motor cortex. Exp. Brain Res. 2004, 155, 196–203. [Google Scholar]
- Ishibashi, K.; Ishii, D.; Yamamoto, S.; Okamoto, Y.; Wakatabi, M.; Kohno, Y. Asymmetry of Interhemispheric Connectivity during Rapid Movements of Right and Left Hands: A TMS-EEG Study. J. Mot. Behav. 2021, 54, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kimura, D. Acquisition of a motor skill after left-hemisphere damage. Brain 1977, 100, 527–542. [Google Scholar] [CrossRef]
- Lüdemann-Podubecká, J.; Bösl, K.; Theilig, S.; Wiederer, R.; Nowak, D.A. The Effectiveness of 1 Hz rTMS Over the Primary Motor Area of the Unaffected Hemisphere to Improve Hand Function After Stroke Depends on Hemispheric Dominance. Brain Stimul. 2015, 8, 823–830. [Google Scholar] [CrossRef]
- Lüdemann-Podubecká, J.; Bösl, K.; Nowak, D.A. Inhibition of the contralesional dorsal premotor cortex improves motor function of the affected hand following stroke. Eur. J. Neurol. 2016, 23, 823–830. [Google Scholar] [CrossRef]
- Keel, J.C.; Smith, M.J.; Wassermann, E.M. A safety screening questionnaire for transcranial magnetic stimulation. Clin. Neurophysiol. 2001, 112, 720. [Google Scholar] [CrossRef]
- Kojima, S.; Onishi, H.; Sugawara, K.; Kirimoto, H.; Suzuki, M.; Tamaki, H. Modulation of the cortical silent period elicited by single- and paired-pulse transcranial magnetic stimulation. BMC Neurosci. 2013, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, K.E.; Swanson, C.W.; Fling, B.W.; Seidler, R.D. TMS-induced silent periods: A review of methods and call for consistency. J. Neurosci. Methods 2020, 346, 108950. [Google Scholar] [CrossRef]
- Garvey, M.A.; Ziemann, U.; Becker, D.A.; Barker, C.A.; Bartko, J.J. New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clin. Neurophysiol. 2001, 112, 1451–1460. [Google Scholar] [CrossRef]
- Jebsen, R.H.; Taylor, N.; Trieschmann, R.B.; Trotter, M.J.; Howard, L.A. An objective and standardized test of hand function. Arch. Phys. Med. Rehabil. 1969, 50, 311–319. [Google Scholar]
- Bestmann, S.; Baudewig, J.; Siebner, H.R.; Rothwell, J.C.; Frahm, J. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage 2005, 28, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Johansen-Berg, H.; Rushworth, M.F.; Bogdanovic, M.D.; Kischka, U.; Wimalaratna, S.; Matthews, P.M. The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl. Acad. Sci. USA 2002, 99, 14518–14523. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Conchou, F.; Loubinoux, I.; Castel-Lacanal, E.; Le Tinnier, A.; Gerdelat-Mas, A.; Faure-Marie, N.; Gros, H.; Thalamas, C.; Calvas, F.; Berry, I.; et al. Neural substrates of low-frequency repetitive transcranial magnetic stimulation during movement in healthy subjects and acute stroke patients. A PET study. Hum. Brain Mapp. 2009, 30, 2542–2557. [Google Scholar] [CrossRef]
- Schambra, H.M.; Sawaki, L.; Cohen, L.G. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin. Neurophysiol. 2003, 114, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.; Kamm, T.; Tergau, F.; Ulm, G.; Paulus, W. Repetitive paired-pulse transcranial magnetic stimulation affects corticospinal excitability and finger tapping in Parkinson’s disease. Clin. Neurophysiol. 2002, 113, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Avanzino, L.; Bove, M.; Trompetto, C.; Tacchino, A.; Ogliastro, C.; Abbruzzese, G. 1-Hz repetitive TMS over ipsilateral motor cortex influences the performance of sequential finger movements of different complexity. Eur. J. Neurosci. 2008, 27, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M. Effect of slow repetitive TMS of the motor cortex on ipsilateral sequential simple finger movements and motor skill learning. Restor. Neurol. Neurosci. 2010, 28, 437–448. [Google Scholar] [CrossRef]
- Boyd, L.A.; Linsdell, M.A. Excitatory repetitive transcranial magnetic stimulation to left dorsal premotor cortex enhances motor consolidation of new skills. BMC Neurosci. 2009, 10, 72. [Google Scholar] [CrossRef]
- Lega, C.; Stephan, M.A.; Zatorre, R.J.; Penhune, V. Testing the Role of Dorsal Premotor Cortex in Auditory-Motor Association Learning Using Transcranical Magnetic Stimulation (TMS). PLoS ONE 2016, 11, e0163380. [Google Scholar] [CrossRef]
- Davare, M.; Andres, M.; Cosnard, G.; Thonnard, J.L.; Olivier, E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J. Neurosci. 2006, 26, 2260–2268. [Google Scholar] [CrossRef]
- Lang, N.; Harms, J.; Weyh, T.; Lemon, R.N.; Paulus, W.; Rothwell, J.C.; Siebner, H.R. Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clin. Neurophysiol. 2006, 117, 2292–2301. [Google Scholar] [CrossRef]
- Oxley, T.; Fitzgerald, P.B.; Brown, T.L.; de Castella, A.; Daskalakis, Z.J.; Kulkarni, J. Repetitive transcranial magnetic stimulation reveals abnormal plastic response to premotor cortex stimulation in schizophrenia. Biol. Psychiatry 2004, 56, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Gerschlager, W.; Siebner, H.R.; Rothwell, J.C. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology 2001, 57, 449–455. [Google Scholar] [CrossRef]
- Rizzo, V.; Siebner, H.R.; Modugno, N.; Pesenti, A.; Münchau, A.; Gerschlager, W.; Webb, R.M.; Rothwell, J.C. Shaping the excitability of human motor cortex with premotor rTMS. J. Physiol. 2004, 554, 483–495. [Google Scholar] [CrossRef]
- Bäumer, T.; Lange, R.; Liepert, J.; Weiller, C.; Siebner, H.R.; Rothwell, J.C.; Münchau, A. Repeated premotor rTMS leads to cumulative plastic changes of motor cortex excitability in humans. Neuroimage 2003, 20, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Schlaghecken, F.; Münchau, A.; Bloem, B.R.; Rothwell, J.; Eimer, M. Slow frequency repetitive transcranial magnetic stimulation affects reaction times, but not priming effects, in a masked prime task. Clin. Neurophysiol. 2003, 114, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.K.; Kim, B.O.; Song, H.A.T. Effect of Stimulation Polarity of Transcranial Direct Current Stimulation on Non-dominant Hand Function. Ann. Rehabil. Med. 2012, 36, 1–7. [Google Scholar] [CrossRef]
- Williams, J.A.; Pascual-Leone, A.; Fregni, F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys. Ther. 2010, 90, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, Y.H.; Chang, W.H.; Kwon, T.G.; Shin, Y.I. Interhemispheric modulation of dual-mode, noninvasive brain stimulation on motor function. Ann. Rehabil. Med. 2014, 38, 297–303. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Dileone, M.; Pilato, F.; Capone, F.; Musumeci, G.; Ranieri, F.; Ricci, V.; Bria, P.; Di Iorio, R.; de Waure, C.; et al. Modulation of motor cortex neuronal networks by rTMS: Comparison of local and remote effects of six different protocols of stimulation. J. Neurophysiol. 2011, 105, 2150–2156. [Google Scholar] [CrossRef]
- Gilio, F.; Rizzo, V.; Siebner, H.R.; Rothwell, J.C. Effects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J. Physiol. 2003, 551, 563–573. [Google Scholar] [CrossRef]
- Avanzino, L.; Teo, J.T.; Rothwell, J.C. Intracortical circuits modulate transcallosal inhibition in humans. J. Physiol. 2007, 583, 99–114. [Google Scholar] [CrossRef]
- Cincotta, M.; Giovannelli, F.; Borgheresi, A.; Balestrieri, F.; Zaccara, G.; Inghilleri, M.; Berardelli, A. Modulatory effects of high-frequency repetitive transcranial magnetic stimulation on the ipsilateral silent period. Exp. Brain Res. 2006, 171, 490–496. [Google Scholar] [CrossRef]
- Chen, M.; Deng, H.; Schmidt, R.L.; Kimberley, T.J. Low-Frequency Repetitive Transcranial Magnetic Stimulation Targeted to Premotor Cortex Followed by Primary Motor Cortex Modulates Excitability Differently Than Premotor Cortex or Primary Motor Cortex Stimulation Alone. Neuromodulation 2015, 18, 678–685. [Google Scholar] [CrossRef]
- Garvey, M.A.; Ziemann, U.; Bartko, J.J.; Denckla, M.B.; Barker, C.A.; Wassermann, E.M. Cortical correlates of neuromotor development in healthy children. Clin. Neurophysiol. 2003, 114, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Ciechanski, P.; Zewdie, E.; Kirton, A. Developmental profile of motor cortex transcallosal inhibition in children and adolescents. J. Neurophysiol. 2017, 118, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, R.; Yamada, K.; Kinomura, S.; Yamaguchi, T.; Matsui, H.; Yoshioka, S.; Fukuda, H. Regional cerebral blood flow changes of cortical motor areas and prefrontal areas in humans related to ipsilateral and contralateral hand movement. Brain Res. 1993, 623, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Ashe, J.; Hendrich, K.; Ellermann, J.M.; Merkle, H.; Uğurbil, K.; Georgopoulos, A.P. Functional magnetic resonance imaging of motor cortex: Hemispheric asymmetry and handedness. Science 1993, 261, 615–617. [Google Scholar] [CrossRef]
- Duque, J.; Murase, N.; Celnik, P.; Hummel, F.; Harris-Love, M.; Mazzocchio, R.; Olivier, E.; Cohen, L.G. Intermanual Differences in movement-related interhemispheric inhibition. J. Cogn. Neurosci. 2007, 19, 204–213. [Google Scholar] [CrossRef]
| Placebo rTMS | 1 Hz rTMS over Left M1 | 1 Hz rTMS over Right M1 | 1 Hz rTMS over Left dPMC | 1 Hz rTMS over Right dPMC | |||
|---|---|---|---|---|---|---|---|
| JTHFT (time, s) | right hand | pre | 4.31 ± 0.46 | 4.38 ± 0.49 | 4.33 ± 0.50 | 4.40 ± 0.54 | 4.38 ± 0.38 |
| post | 4.31 ± 0.57 | 4.34 ± 0.48 | 4.34 ± 0.45 | 4.39 ± 0.63 | 4.43 ± 0.05 | ||
| left hand | pre | 4.42 ± 0.53 | 4.54 ± 0.64 | 4.57 ± 0.48 | 4.53 ± 0.65 | 4.50 ± 0.39 | |
| post | 4.37 ± 0.51 | 4.51 ± 0.62 | 4.47 ± 0.43 | 4.65 ± 0.70 | 4.52 ± 0.49 | ||
| MEP (size, mV) | left hemisphere | pre | 0.62 ± 0.66 | 0.69 ± 0.85 | 0.69 ± 0.62 | 0.85 ± 0.75 | 0.56 ± 0.55 |
| post | 0.57 ± 0.62 | 0.63 ± 0.52 | 0.85 ± 0.64 | 0.75 ± 0.60 | 0.60 ± 0.39 | ||
| right hemisphere | pre | 0.42 ± 0.34 | 0.48 ± 0.34 | 0.29 ± 0.22 b | 0.64 ± 0.65 | 0.46 ± 0.37 b | |
| post | 0.42 ± 0.40 | 0.62 ± 0.37 | 0.43 ± 0.27 | 0.40 ± 0.29 | 0.51 ± 0.43 | ||
| CSP (duration, ms) | left hemisphere | pre | 0.147 ± 0.036 b | 0.150 ± 0.033 | 0.156 ± 0.038 | 0.156 ± 0.040 | 0.156 ± 0.035 b |
| post | 0.148 ± 0.039 | 0.169 ± 0.039 | 0.159 ± 0.033 | 0.163 ± 0.038 | 0.164 ± 0.029 | ||
| right hemisphere | pre | 0.160 ± 0.038 | 0.162 ± 0.033 | 0.158 ± 0.036 | 0.157 ± 0.043 | 0.162 ± 0.031 | |
| post | 0.157 ± 0.035 | 0.171 ± 0.030 * | 0.169 ± 0.030 * | 0.164 ± 0.043 | 0.169 ± 0.036 | ||
| ISP (duration, ms) | left hemisphere | pre | 0.028 ± 0.004 | 0.030 ± 0.003 | 0.030 ± 0.004 | 0.030 ± 0.006 | 0.029 ± 0.006 |
| post | 0.028 ± 0.004 | 0.033 ± 0.004 | 0.032 ± 0.004 | 0.030 ± 0.005 | 0.033 ± 0.007 | ||
| right hemisphere | pre | 0.031 ± 0.003 | 0.032 ± 0.004 | 0.030 ± 0.004 | 0.030 ± 0.005 | 0.032 ± 0.005 | |
| post | 0.030 ± 0.004 | 0.032 ± 0.005 a | 0.033 ± 0.005 *a | 0.032 ± 0.005 * | 0.032 ± 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veldema, J.; Nowak, D.A.; Bösl, K.; Gharabaghi, A. Hemispheric Differences of 1 Hz rTMS over Motor and Premotor Cortex in Modulation of Neural Processing and Hand Function. Brain Sci. 2023, 13, 752. https://doi.org/10.3390/brainsci13050752
Veldema J, Nowak DA, Bösl K, Gharabaghi A. Hemispheric Differences of 1 Hz rTMS over Motor and Premotor Cortex in Modulation of Neural Processing and Hand Function. Brain Sciences. 2023; 13(5):752. https://doi.org/10.3390/brainsci13050752
Chicago/Turabian StyleVeldema, Jitka, Dennis Alexander Nowak, Kathrin Bösl, and Alireza Gharabaghi. 2023. "Hemispheric Differences of 1 Hz rTMS over Motor and Premotor Cortex in Modulation of Neural Processing and Hand Function" Brain Sciences 13, no. 5: 752. https://doi.org/10.3390/brainsci13050752
APA StyleVeldema, J., Nowak, D. A., Bösl, K., & Gharabaghi, A. (2023). Hemispheric Differences of 1 Hz rTMS over Motor and Premotor Cortex in Modulation of Neural Processing and Hand Function. Brain Sciences, 13(5), 752. https://doi.org/10.3390/brainsci13050752





