The Relationship between Anxiety, Subjective and Objective Sleep, Chronotype and Circadian Rhythms with Depressive Symptoms in Insomnia Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Assessments
2.3.1. Polysomnographic Sleep Study
2.3.2. Dim Light Melatonin Onset
2.3.3. Questionnaires
2.4. Data Analysis
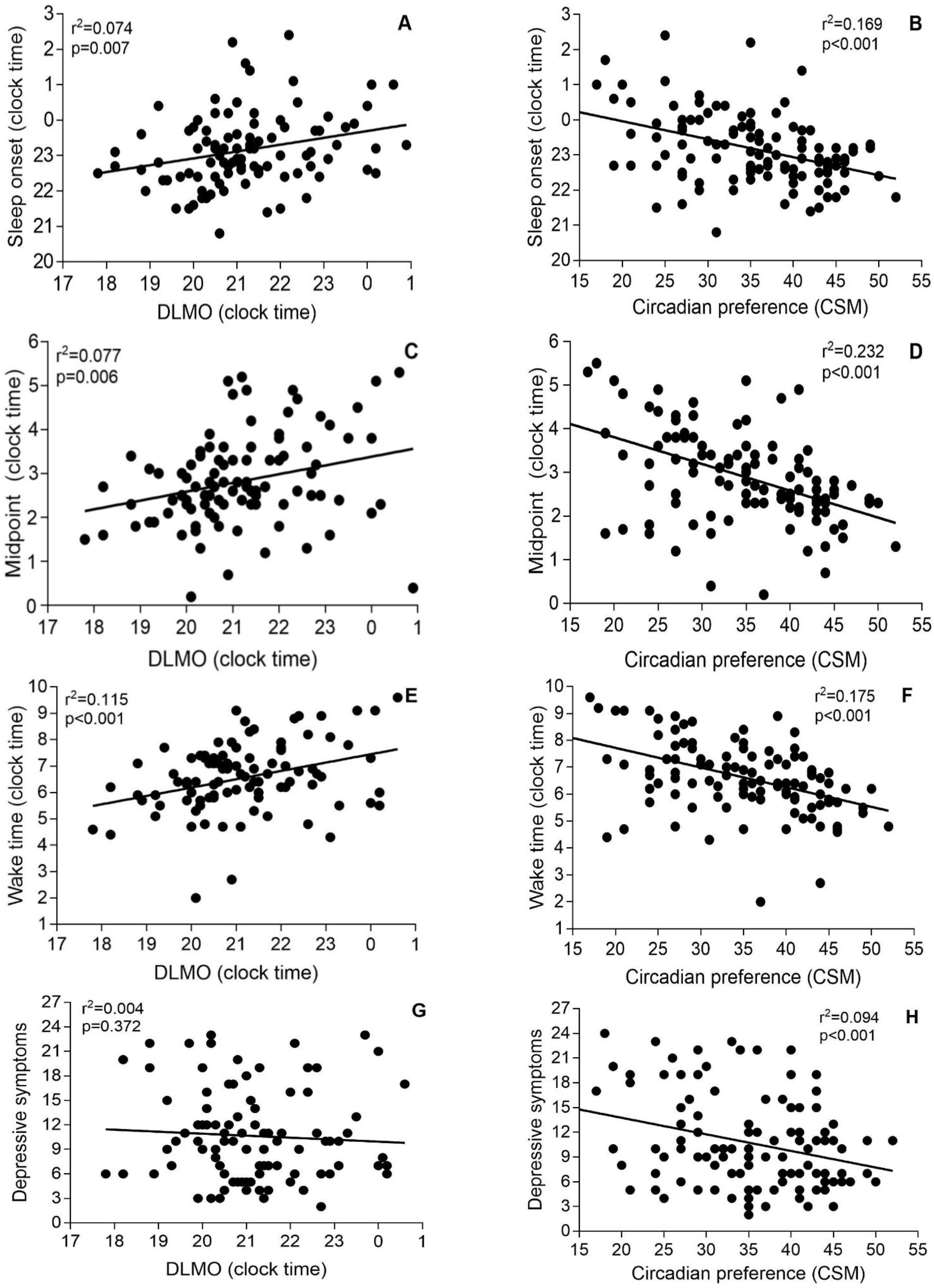
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morin, C.M.; Benca, R. Chronic insomnia. Lancet 2012, 379, 1129–1141. [Google Scholar] [CrossRef]
- Léger, D.; Guilleminault, C.; Bader, G.; Lévy, E.; Paillard, M. Medical and Socio-Professional Impact of Insomnia. Sleep 2002, 25, 621–625. [Google Scholar] [CrossRef]
- Miller, C.B.; Bartlett, D.J.; Mullins, A.; Dodds, K.; Gordon, C.J.; Kyle, S.; Kim, J.W.; D’Rozario, A.; Lee, R.S.; Comas, M.; et al. Clusters of Insomnia Disorder: An Exploratory Cluster Analysis of Objective Sleep Parameters Reveals Differences in Neurocognitive Functioning, Quantitative EEG, and Heart Rate Variability. Sleep 2016, 39, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Jansson-Fröjmark, M.; Lindblom, K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J. Psychosom. Res. 2008, 64, 443–449. [Google Scholar] [CrossRef]
- Palagini, L.; Hertenstein, E.; Riemann, D.; Nissen, C. Sleep, insomnia and mental health. J. Sleep Res. 2022, 31, e13628. [Google Scholar] [CrossRef]
- Robillard, R.; Carpenter, J.S.; Rogers, N.L.; Fares, S.; Grierson, A.B.; Hermens, D.F.; Naismith, S.L.; Mullin, S.J.; Feilds, K.-L.; Glozier, N.; et al. Circadian rhythms and psychiatric profiles in young adults with unipolar depressive disorders. Transl. Psychiatry 2018, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.A.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- Smith, C.S.; Reilly, C.; Midkiff, K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J. Appl. Psychol. 1989, 74, 728–738. [Google Scholar] [CrossRef]
- Jankowski, K. Composite Scale of Morningness: Psychometric properties, validity with Munich ChronoType Questionnaire and age/sex differences in Poland. Eur. Psychiatry 2015, 30, 166–171. [Google Scholar] [CrossRef]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between Clocks: Daily Temporal Patterns of Human Chronotypes. J. Biol. Rhythm. 2003, 18, 80–90. [Google Scholar] [CrossRef]
- De Souza, C.M.; Hidalgo, M.P.L. The midpoint of sleep on working days: A measure for chronodisruption and its association to individuals’ well-being. Chronobiol. Int. 2015, 32, 341–348. [Google Scholar] [CrossRef]
- Antypa, N.; Vogelzangs, N.; Meesters, Y.; Schoevers, R.; Penninx, B.W.J.H. Chronotype associations with depression and anxiety disorders in a large cohort study. Depress. Anxiety 2016, 33, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Au, J.; Reece, J. The relationship between chronotype and depressive symptoms: A meta-analysis. J. Affect. Disord. 2017, 218, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Merikanto, I.; Kronholm, E.; Peltonen, M.; Laatikainen, T.; Lahti, T.; Partonen, T. Relation of Chronotype to Sleep Complaints in the GeneralFinnish Population. Chronobiol. Int. 2012, 29, 311–317. [Google Scholar] [CrossRef]
- Lewy, A.J. Melatonin and Human Chronobiology. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2007; Volume 72, pp. 623–636. [Google Scholar]
- Kantermann, T.; Burgess, H.J. Average mid-sleep time as a proxy for circadian phase. PsyCh J. 2017, 6, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Kantermann, T.; Sung, H.; Burgess, H.J. Comparing the Morningness-Eveningness Questionnaire and Munich ChronoType Questionnaire to the Dim Light Melatonin Onset. J. Biol. Rhythm. 2015, 30, 449–453. [Google Scholar] [CrossRef]
- Roenneberg, T.; Pilz, L.K.; Zerbini, G.; Winnebeck, E.C. Chronotype and Social Jetlag: A (Self-) Critical Review. Biology 2019, 8, 54. [Google Scholar] [CrossRef]
- E Flynn-Evans, E.; A Shekleton, J.; Miller, B.; Epstein, L.J.; Kirsch, D.; A Brogna, L.; Burke, L.M.; Bremer, E.; Murray, J.M.; Gehrman, P.; et al. Circadian Phase and Phase Angle Disorders in Primary Insomnia. Sleep 2017, 40, 1–11. [Google Scholar] [CrossRef]
- Chan, J.W.Y.; Lam, S.P.; Li, S.X.; Yu, M.W.M.; Chan, N.Y.; Zhang, J.; Wing, Y.-K. Eveningness and Insomnia: Independent Risk Factors of Nonremission in Major Depressive Disorder. Sleep 2014, 37, 911–917. [Google Scholar] [CrossRef]
- Sheaves, B.; Porcheret, K.; Tsanas, A.; Espie, C.A.; Foster, R.G.; Freeman, D.; Harrison, P.J.; Wulff, K.; Goodwin, G.M. Insomnia, Nightmares, and Chronotype as Markers of Risk for Severe Mental Illness: Results from a Student Population. Sleep 2016, 39, 173–181. [Google Scholar] [CrossRef]
- Müller, M.J.; Kundermann, B.; Cabanel, N. Eveningness and poor sleep quality independently contribute to self-reported depression severity in psychiatric inpatients with affective disorder. Nord. J. Psychiatry 2015, 70, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The Consensus Sleep Diary: Standardizing Prospective Sleep Self-Monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef]
- Iber, C.; Ancoli-Israel, S.; Chesson, A.; Quan, S.F. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification, 1st ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Manconi, M.; Ferri, R.; Sagrada, C.; Punjabi, N.M.; Tettamanzi, E.; Zucconi, M.; Oldani, A.; Castronovo, V.; Ferini-Strambi, L. Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J. Sleep Res. 2010, 19, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Molina, T.A.; Burgess, H.J. Calculating the Dim Light Melatonin Onset: The Impact of Threshold and Sampling Rate. Chronobiol. Int. 2011, 28, 714–718. [Google Scholar] [CrossRef]
- Morin, C. Insomnia: Psychological Assessment and Management; Guilford Press: New York, NY, USA, 1993. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Morin, C.M.; Vallières, A.; Ivers, H. Dysfunctional Beliefs and Attitudes about Sleep (DBAS): Validation of a Brief Version (DBAS-16). Sleep 2007, 30, 1547–1554. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The gad-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Wright, H.R.; Lack, L.C.; Bootzin, R.R. Relationship between dim light melatonin onset and the timing of sleep in sleep onset insomniacs. Sleep Biol. Rhythm. 2006, 4, 78–80. [Google Scholar] [CrossRef]
- Jacobson, N.C.; Newman, M.G. Anxiety and depression as bidirectional risk factors for one another: A meta-analysis of longitudinal studies. Psychol. Bull. 2017, 143, 1155–1200. [Google Scholar] [CrossRef] [PubMed]
- Mason, E.C.; Grierson, A.B.; Sie, A.; Sharrock, M.J.; Li, I.; Chen, A.Z.; Newby, J.M. Co-occurring insomnia and anxiety: A randomized controlled trial of internet cognitive behavioral therapy for insomnia versus internet cognitive behavioral therapy for anxiety. Sleep 2022, 46, zsac205. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Lichstein, K.L.; Durrence, H.H.; Reidel, B.W.; Bush, A.J. Epidemiology of Insomnia, Depression, and Anxiety. Sleep 2005, 28, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.K.; Eastman, C.I. Sleep Logs of Young Adults with Self-Selected Sleep Times Predict the Dim Light Melatonin Onset. Chronobiol. Int. 2002, 19, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Manni, R.; Cremascoli, R.; De Icco, R.; Terzaghi, M. Chronotype in patients with epilepsy: A controlled study in 60 subjects with late-onset focal epilepsy. Epilepsy Behav. 2015, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Meliska, C.J.; Martínez, L.F.; López, A.M.; Sorenson, D.L.; Nowakowski, S.; Parry, B.L. Relationship of morningness-eveningness questionnaire score to melatonin and sleep timing, body mass index and atypical depressive symptoms in peri- and post-menopausal women. Psychiatry Res. 2011, 188, 88–95. [Google Scholar] [CrossRef]
- Steele, D.L.; Rajaratnam, S.M.W.; Redman, J.R.; Ponsford, J.L. The Effect of Traumatic Brain Injury on the Timing of Sleep. Chronobiol. Int. 2005, 22, 89–105. [Google Scholar] [CrossRef]
- Kitamura, S.; Hida, A.; Aritake, S.; Higuchi, S.; Enomoto, M.; Kato, M.; Vetter, C.; Roenneberg, T.; Mishima, K. Validity of the Japanese version of the Munich ChronoType Questionnaire. Chronobiol. Int. 2014, 31, 845–850. [Google Scholar] [CrossRef]
- Burgess, H.J.; Savic, N.; Sletten, T.; Roach, G.; Gilbert, S.S.; Dawson, D. The Relationship Between the Dim Light Melatonin Onset and Sleep on a Regular Schedule in Young Healthy Adults. Behav. Sleep Med. 2003, 1, 102–114. [Google Scholar] [CrossRef]
- Burgess, H.J.; Eastman, C.I. A late wake time phase delays the human dim light melatonin rhythm. Neurosci. Lett. 2006, 395, 191–195. [Google Scholar] [CrossRef]
- Reiter, A.M.; Sargent, C.; Roach, G.D. Finding DLMO: Estimating dim light melatonin onset from sleep markers derived from questionnaires, diaries and actigraphy. Chronobiol. Int. 2020, 37, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Bjorvatn, B.; Jernelöv, S.; Pallesen, S. Insomnia—A Heterogenic Disorder Often Comorbid With Psychological and Somatic Disorders and Diseases: A Narrative Review With Focus on Diagnostic and Treatment Challenges. Front. Psychol. 2021, 12, 639198. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.M.; Reid, K.J.; Zee, P.C. Chapter 40—Circadian Disorders of the Sleep-Wake Cycle. In Principles and Practice of Sleep Medicine, 6th ed.; Kryger, M., Roth, T., Dement, W.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 414–423.e5. [Google Scholar]
- Alvaro, P.K.; Roberts, R.M.; Harris, J.K. The independent relationships between insomnia, depression, subtypes of anxiety, and chronotype during adolescence. Sleep Med. 2014, 15, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Lack, L.C.; Mercer, J.D.; Wright, H. Circadian rhythms of early morning awakening insomniacs. J. Sleep Res. 1996, 5, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Di Milia, L.; Adan, A.; Natale, V.; Randler, C. Reviewing the Psychometric Properties of Contemporary Circadian Typology Measures. Chronobiol. Int. 2013, 30, 1261–1271. [Google Scholar] [CrossRef]
- DiNapoli, E.A.; Gebara, M.A.; Kho, T.; Butters, M.A.; Gildengers, A.G.; Albert, S.M.; Dew, M.A.; Erickson, K.I.; Reynolds, C.F., 3rd; Karp, J.F. Subjective-Objective Sleep Discrepancy in Older Adults with MCI and Subsyndromal Depression. J. Geriatr. Psychiatry Neurol. 2017, 30, 316–323. [Google Scholar] [CrossRef]
- Gould, C.E.; Karna, R.; Jordan, J.; Kawai, M.; Hirst, R.; Hantke, N.; Pirog, S.; Cotto, I.; Rose, S.M.S.-F.; Beaudreau, S.A.; et al. Subjective but Not Objective Sleep is Associated with Subsyndromal Anxiety and Depression in Community-Dwelling Older Adults. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2018, 26, 806–811. [Google Scholar] [CrossRef]
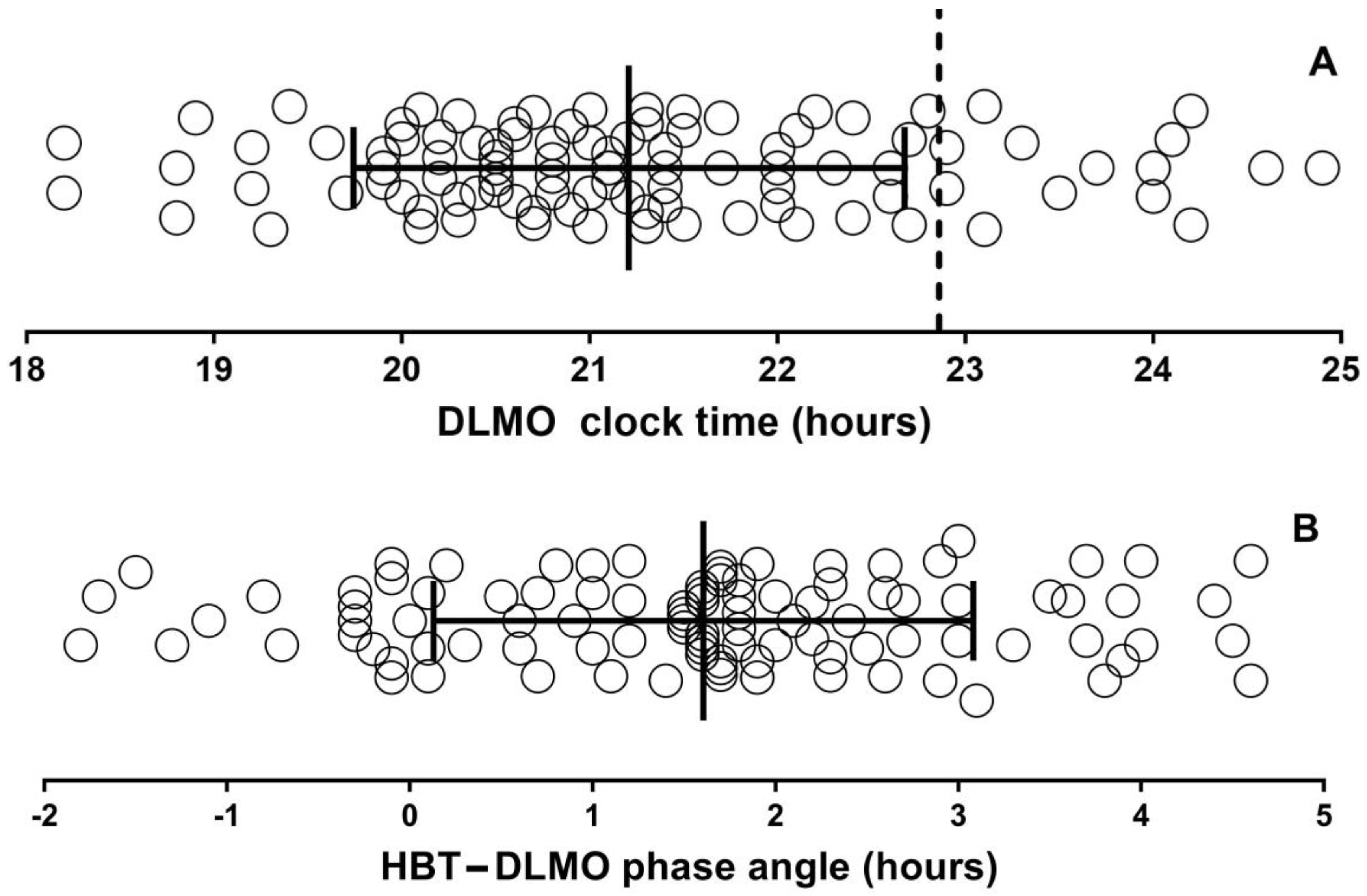
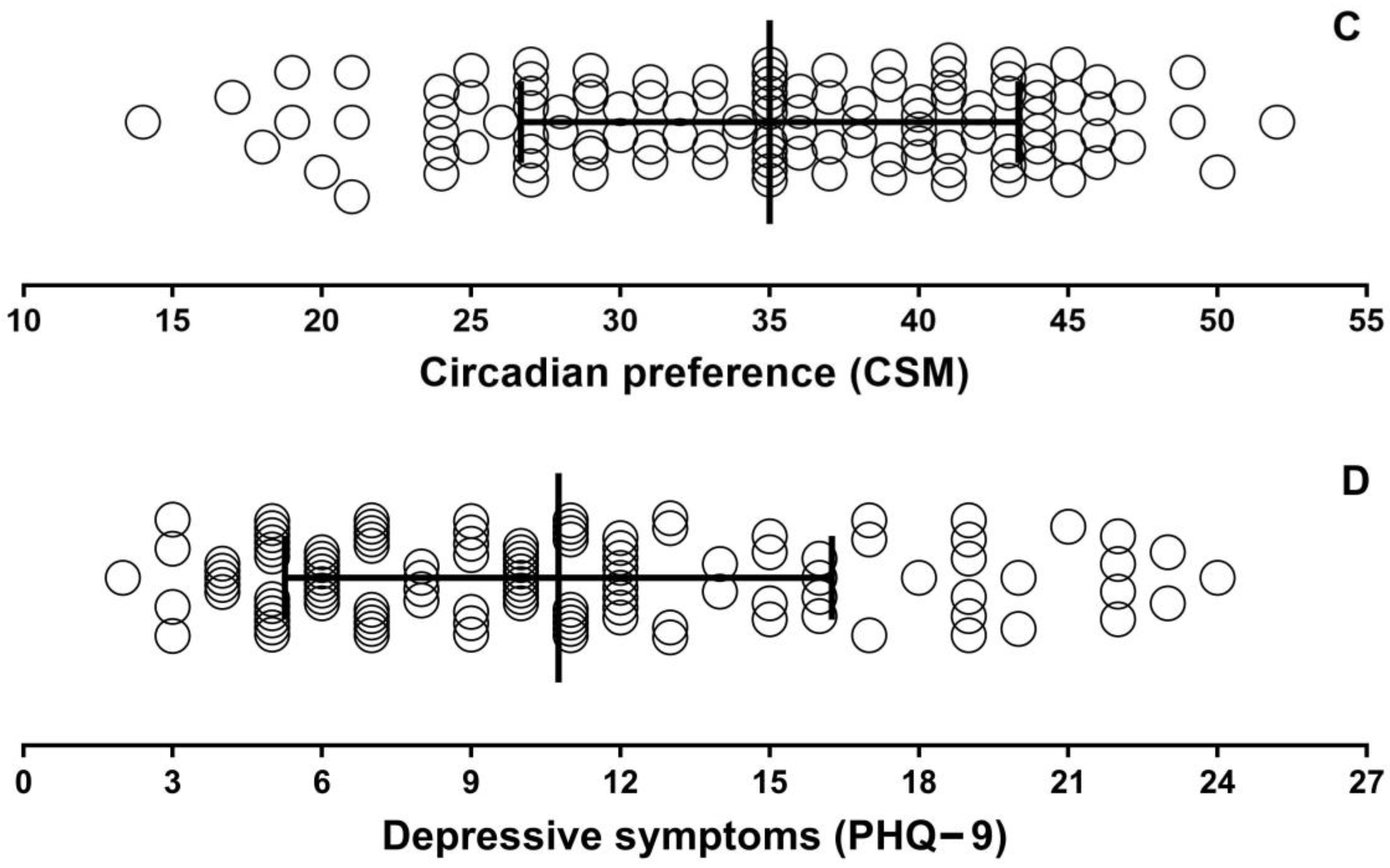

| Variable | Mean ± SD (Range) n = 115 |
|---|---|
| Age (years): SD | 47.1 ± 14.8 (18–80) |
| BMI (kg/m2) | 25.6 ± 5.3 (17.1–43.8) |
| Female: n (%) | 80 (70%) |
| ISI | 19.9 ± 4.2 (10–28) |
| PSQI | 13.1 ± 3.3 (5–20) |
| CSM | 35.0 ± 8.3 (14–52) |
| DBAS | 5.7 ± 1.6 (1–9) |
| GAD-7 | 7.2 ± 4.9 (0–21) |
| PHQ-9 | 10.8 ± 5.5 (2–24) |
| DLMO (hh:mm) (n = 98) | 21:12 ± 1:28 (17:28–0:32) |
| PSG: TST (min) | 344.2 ± 77.1 (156.0–518.5) |
| PSG: SOL (min) | 22.0 ± 26.4 (1.0–159.0) |
| PSG: WASO (min) | 86.0 ± 54.4 (7.0–249.5) |
| PSG: sleep efficiency (%) | 74.1 ± 14.4 (43.5–94.4) |
| Diary: TST (min) | 346.6 ± 87.2 (135.0–557.1) |
| Diary: SOL (min) | 43.0 ± 39.0 (5.6–180.0) |
| Diary: WASO (min) | 57.6 ± 50.0 (2–252.0) |
| Diary: sleep efficiency (%) | 75.7 ± 15.2 (39.1–98.2) |
| Phase angle HBTd—DLMO (min) | 96.3 ± 88.6 (−108–276) |
| Insomnia Subgroups | DLMO | Sleep Onset Time | r p Values | Midsleep | r p Values | Wake-Up Time | r p Values | Depressive Symptoms | r p Values |
|---|---|---|---|---|---|---|---|---|---|
| DLMO <22:00 (n = 69) | 20:30 (0:54) (17:48–21:48) | 23:00 (1:00) (20:48–2:12) | 0.196 p = 0.107 | 2:42 (0:54) (0:12–5:12) | 0.276 p = 0.022 * | 6:18 (1:12) (2:00–9:06) | 0.256 p = 0.034 * | 10.6 (5.4) (3–23) | −0.225 p = 0.063 |
| DLMO ≥22:00 (n = 29) | 23:00 (0:54) (22:00–0:54) | 1:30 (1:06) (21:30–2:24) | 0.097 p = 0.616 | 3:12 (1:12) (0:24–5:18) | −0.097 p = 0.943 | 6:54 (1:42) (1:36–9:36) | −0.205 p = 0.286 | 10.7 (5.9) (2–23) | 0.089 p = 0.645 |
| Predictors | Model 1 | Model 2 | Model 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | p | B | SE | β | p | B | SE | β | p | |
| Anxiety | 0.565 | 0.085 | 0.495 | <0.001 | 0.508 | 0.091 | 0.461 | <0.001 | 0.491 | 0.092 | 0.445 | <0.001 |
| Circadian preference | −0.072 | 0.056 | −0.110 | 0.202 | −0.075 | 0.058 | −0.124 | 0.196 | −0.066 | 0.059 | −0.109 | 0.265 |
| Dysfunctional beliefs | 0.425 | 0.264 | 0.120 | 0.111 | 0.334 | 0.272 | 0.102 | 0.222 | 0.368 | 0.276 | 0.112 | 0.185 |
| Previous depression | 2.073 | 0.935 | 0.174 | 0.029 | 1.804 | 0.96 | 0.161 | 0.63 | 1.865 | 0.951 | 0.166 | 0.053 |
| TSTdiary | −0.43 | 0.299 | −0.012 | 0.887 | 0.047 | 0.305 | 0.013 | 0.879 | ||||
| TSTPSG | −0.011 | 0.005 | −0.165 | 0.049 | ||||||||
| DLMO | 0.001 | 0.001 | 0.058 | 0.457 | ||||||||
| R2 | 0.529 | Adjusted 0.496 | 0.475 | Adjusted 0.430 | 0.508 | Adjusted 0.453 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comas, M.; Solis Flores, A.; Lovato, N.; Miller, C.B.; Bartlett, D.J.; Grunstein, R.R.; Chapman, J.; Gordon, C.J. The Relationship between Anxiety, Subjective and Objective Sleep, Chronotype and Circadian Rhythms with Depressive Symptoms in Insomnia Disorder. Brain Sci. 2023, 13, 613. https://doi.org/10.3390/brainsci13040613
Comas M, Solis Flores A, Lovato N, Miller CB, Bartlett DJ, Grunstein RR, Chapman J, Gordon CJ. The Relationship between Anxiety, Subjective and Objective Sleep, Chronotype and Circadian Rhythms with Depressive Symptoms in Insomnia Disorder. Brain Sciences. 2023; 13(4):613. https://doi.org/10.3390/brainsci13040613
Chicago/Turabian StyleComas, Maria, Alejandra Solis Flores, Nicole Lovato, Christopher B. Miller, Delwyn J. Bartlett, Ronald R. Grunstein, Julia Chapman, and Christopher J. Gordon. 2023. "The Relationship between Anxiety, Subjective and Objective Sleep, Chronotype and Circadian Rhythms with Depressive Symptoms in Insomnia Disorder" Brain Sciences 13, no. 4: 613. https://doi.org/10.3390/brainsci13040613
APA StyleComas, M., Solis Flores, A., Lovato, N., Miller, C. B., Bartlett, D. J., Grunstein, R. R., Chapman, J., & Gordon, C. J. (2023). The Relationship between Anxiety, Subjective and Objective Sleep, Chronotype and Circadian Rhythms with Depressive Symptoms in Insomnia Disorder. Brain Sciences, 13(4), 613. https://doi.org/10.3390/brainsci13040613





