Noncontrast Computed Tomography Markers Associated with Hematoma Expansion: Analysis of a Multicenter Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Treatment
2.3. Imaging Evaluation
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Study Patients
3.2. Association between NCCT Markers and Hematoma Expansion for Different Definitions
3.3. Independent Predictive Value of Significant Predictors for HE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordonnier, C.; Demchuk, A.; Ziai, W.; Anderson, C.S. Intracerebral haemorrhage: Current approaches to acute management. Lancet 2018, 392, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.M.; Broderick, J.; Hennerici, M.; Brun, N.C.; Diringer, M.N.; Mayer, S.A.; Begtrup, K.; Steiner, T. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006, 66, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Luna, D.; Rubiera, M.; Ribo, M.; Coscojuela, P.; Piñeiro, S.; Pagola, J.; Hernandez-Guillamon, M.; Ibarra, B.; Romero, F.; Alvarez-Sabin, J.; et al. Ultraearly hematoma growth predicts poor outcome after acute intracerebral hemorrhage. Neurology 2011, 77, 1599–1604. [Google Scholar] [CrossRef]
- Wang, W.J.; Lu, J.J.; Liu, L.P.; Jia, J.K.; Zhao, X.Q. Ultraearly Hematoma Growth in Acute Spontaneous Intracerebral Hemorrhage Predicts Early and Long-Term Poor Clinical Outcomes: A Prospective, Observational Cohort Study. Front. Neurol. 2021, 12, 747551. [Google Scholar] [CrossRef] [PubMed]
- Demchuk, A.M.; Dowlatshahi, D.; Rodriguez-Luna, D.; Molina, C.A.; Blas, Y.S.; Dzialowski, I.; Kobayashi, A.; Boulanger, J.M.; Lum, C.; Gubitz, G.; et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): A prospective observational study. Lancet Neurol. 2012, 11, 307–314. [Google Scholar] [CrossRef]
- Wada, R.; Aviv, R.I.; Fox, A.J.; Sahlas, D.J.; Gladstone, D.J.; Tomlinson, G.; Symons, S.P. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke 2007, 38, 1257–1262. [Google Scholar] [CrossRef]
- Delgado Almandoz, J.E.; Yoo, A.J.; Stone, M.J.; Schaefer, P.W.; Goldstein, J.N.; Rosand, J.; Oleinik, A.; Lev, M.H.; Gonzalez, R.G.; Romero, J.M. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: The spot sign score. Stroke 2009, 40, 2994–3000. [Google Scholar] [CrossRef]
- Dowlatshahi, D.; Brouwers, H.B.; Demchuk, A.M.; Hill, M.D.; Aviv, R.I.; Ufholz, L.A.; Reaume, M.; Wintermark, M.; Hemphill, J.C., 3rd; Murai, Y.; et al. Predicting Intracerebral Hemorrhage Growth with the Spot Sign: The Effect of Onset-to-Scan Time. Stroke 2016, 47, 695–700. [Google Scholar] [CrossRef]
- Phan, T.G.; Krishnadas, N.; Lai, V.W.Y.; Batt, M.; Slater, L.A.; Chandra, R.V.; Srikanth, V.; Ma, H. Meta-Analysis of Accuracy of the Spot Sign for Predicting Hematoma Growth and Clinical Outcomes. Stroke 2019, 50, 2030–2036. [Google Scholar] [CrossRef]
- Orito, K.; Hirohata, M.; Nakamura, Y.; Takeshige, N.; Aoki, T.; Hattori, G.; Sakata, K.; Abe, T.; Uchiyama, Y.; Sakamoto, T.; et al. Leakage Sign for Primary Intracerebral Hemorrhage: A Novel Predictor of Hematoma Growth. Stroke 2016, 47, 958–963. [Google Scholar] [CrossRef]
- Fu, F.; Sun, S.; Liu, L.; Gu, H.; Su, Y.; Li, Y. Iodine Sign as a Novel Predictor of Hematoma Expansion and Poor Outcomes in Primary Intracerebral Hemorrhage Patients. Stroke 2018, 49, 2074–2080. [Google Scholar] [CrossRef]
- Boulouis, G.; Morotti, A.; Charidimou, A.; Dowlatshahi, D.; Goldstein, J.N. Noncontrast Computed Tomography Markers of Intracerebral Hemorrhage Expansion. Stroke 2017, 48, 1120–1125. [Google Scholar] [CrossRef]
- Morotti, A.; Boulouis, G.; Dowlatshahi, D.; Li, Q.; Barras, C.D.; Delcourt, C.; Yu, Z.; Zheng, J.; Zhou, Z.; Aviv, R.I.; et al. Standards for Detecting, Interpreting, and Reporting Noncontrast Computed Tomographic Markers of Intracerebral Hemorrhage Expansion. Ann. Neurol. 2019, 86, 480–492. [Google Scholar] [CrossRef]
- Selariu, E.; Zia, E.; Brizzi, M.; Abul-Kasim, K. Swirl sign in intracerebral haemorrhage: Definition, prevalence, reliability and prognostic value. BMC Neurol. 2012, 12, 109. [Google Scholar] [CrossRef]
- Pfleger, M.J.; Hardee, E.P.; Contant, C.F., Jr.; Hayman, L.A. Sensitivity and specificity of fluid-blood levels for coagulopathy in acute intracerebral hematomas. AJNR Am. J. Neuroradiol. 1994, 15, 217–223. [Google Scholar]
- Barras, C.D.; Tress, B.M.; Christensen, S.; MacGregor, L.; Collins, M.; Desmond, P.M.; Skolnick, B.E.; Mayer, S.A.; Broderick, J.P.; Diringer, M.N.; et al. Density and shape as CT predictors of intracerebral hemorrhage growth. Stroke 2009, 40, 1325–1331. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, G.; Huang, Y.J.; Dong, M.X.; Lv, F.J.; Wei, X.; Chen, J.J.; Zhang, L.J.; Qin, X.Y.; Xie, P. Blend Sign on Computed Tomography: Novel and Reliable Predictor for Early Hematoma Growth in Patients with Intracerebral Hemorrhage. Stroke 2015, 46, 2119–2123. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, G.; Xiong, X.; Wang, X.C.; Yang, W.S.; Li, K.W.; Wei, X.; Xie, P. Black Hole Sign: Novel Imaging Marker That Predicts Hematoma Growth in Patients with Intracerebral Hemorrhage. Stroke 2016, 47, 1777–1781. [Google Scholar] [CrossRef]
- Dowlatshahi, D.; Demchuk, A.M.; Flaherty, M.L.; Ali, M.; Lyden, P.L.; Smith, E.E. Defining hematoma expansion in intracerebral hemorrhage: Relationship with patient outcomes. Neurology 2011, 76, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, J.; Xu, H.; He, W.; Li, Y.; Jiao, L.; Xiang, Y.; Zhan, C.; Chen, J.; Yang, X.; et al. Association Between Eosinophilic Leukocyte Count and Hematoma Expansion in Acute Spontaneous Intracerebral Hemorrhage. Front. Neurol. 2019, 10, 1164. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wei, H.; Liu, Y.; Du, L.; Xia, J. Association between leukocyte subpopulations and hematoma expansion after spontaneous intracerebral hemorrhage: A retrospective cohort study. Front. Neurol. 2022, 13, 992851. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef]
- Morotti, A.; Phuah, C.L.; Anderson, C.D.; Jessel, M.J.; Schwab, K.; Ayres, A.M.; Pezzini, A.; Padovani, A.; Gurol, M.E.; Viswanathan, A.; et al. Leukocyte Count and Intracerebral Hemorrhage Expansion. Stroke 2016, 47, 1473–1478. [Google Scholar] [CrossRef]
- Blacquiere, D.; Demchuk, A.M.; Al-Hazzaa, M.; Deshpande, A.; Petrcich, W.; Aviv, R.I.; Rodriguez-Luna, D.; Molina, C.A.; Silva Blas, Y.; Dzialowski, I.; et al. Intracerebral Hematoma Morphologic Appearance on Noncontrast Computed Tomography Predicts Significant Hematoma Expansion. Stroke 2015, 46, 3111–3116. [Google Scholar] [CrossRef]
- Boulouis, G.; Morotti, A.; Brouwers, H.B.; Charidimou, A.; Jessel, M.J.; Auriel, E.; Pontes-Neto, O.; Ayres, A.; Vashkevich, A.; Schwab, K.M.; et al. Association Between Hypodensities Detected by Computed Tomography and Hematoma Expansion in Patients with Intracerebral Hemorrhage. JAMA Neurol. 2016, 73, 961–968. [Google Scholar] [CrossRef]
- Fujii, Y.; Tanaka, R.; Takeuchi, S.; Koike, T.; Minakawa, T.; Sasaki, O. Hematoma enlargement in spontaneous intracerebral hemorrhage. J. Neurosurg. 1994, 80, 51–57. [Google Scholar] [CrossRef]
- Brott, T.; Broderick, J.; Kothari, R.; Barsan, W.; Tomsick, T.; Sauerbeck, L.; Spilker, J.; Duldner, J.; Khoury, J. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997, 28, 1–5. [Google Scholar] [CrossRef]
- Li, Z.; You, M.; Long, C.; Bi, R.; Xu, H.; He, Q.; Hu, B. Hematoma Expansion in Intracerebral Hemorrhage: An Update on Prediction and Treatment. Front. Neurol. 2020, 11, 702. [Google Scholar] [CrossRef]
- Roh, D.; Boehme, A.; Young, C.; Roth, W.; Gutierrez, J.; Flaherty, M.; Rosand, J.; Testai, F.; Woo, D.; Elkind, M.S.V. Hematoma expansion is more frequent in deep than lobar intracerebral hemorrhage. Neurology 2020, 95, e3386–e3393. [Google Scholar] [CrossRef]
- Wang, W.; Jin, W.; Feng, H.; Wu, G.; Wang, W.; Jia, J.; Ji, R.; Wang, A.; Zhao, X. Higher Cerebral Blood Flow Predicts Early Hematoma Expansion in Patients with Intracerebral Hemorrhage: A Clinical Study. Front. Neurol. 2021, 12, 735771. [Google Scholar] [CrossRef]
- Brouwers, H.B.; Chang, Y.; Falcone, G.J.; Cai, X.; Ayres, A.M.; Battey, T.W.; Vashkevich, A.; McNamara, K.A.; Valant, V.; Schwab, K.; et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014, 71, 158–164. [Google Scholar] [CrossRef]
- Roh, D.J.; Albers, D.J.; Magid-Bernstein, J.; Doyle, K.; Hod, E.; Eisenberger, A.; Murthy, S.; Witsch, J.; Park, S.; Agarwal, S.; et al. Low hemoglobin and hematoma expansion after intracerebral hemorrhage. Neurology 2019, 93, e372–e380. [Google Scholar] [CrossRef] [PubMed]
- Morotti, A.; Boulouis, G.; Charidimou, A.; Li, Q.; Poli, L.; Costa, P.; De Giuli, V.; Leuci, E.; Mazzacane, F.; Busto, G.; et al. Hematoma Expansion in Intracerebral Hemorrhage with Unclear Onset. Neurology 2021, 96, e2363–e2371. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Luna, D.; Coscojuela, P.; Rubiera, M.; Hill, M.D.; Dowlatshahi, D.; Aviv, R.I.; Silva, Y.; Dzialowski, I.; Lum, C.; Czlonkowska, A.; et al. Ultraearly hematoma growth in active intracerebral hemorrhage. Neurology 2016, 87, 357–364. [Google Scholar] [CrossRef]
- Liu, J.; Xu, H.; Chen, Q.; Zhang, T.; Sheng, W.; Huang, Q.; Song, J.; Huang, D.; Lan, L.; Li, Y.; et al. Prediction of hematoma expansion in spontaneous intracerebral hemorrhage using support vector machine. EBioMedicine 2019, 43, 454–459. [Google Scholar] [CrossRef]
- Di Napoli, M.; Parry-Jones, A.R.; Smith, C.J.; Hopkins, S.J.; Slevin, M.; Masotti, L.; Campi, V.; Singh, P.; Papa, F.; Popa-Wagner, A.; et al. C-reactive protein predicts hematoma growth in intracerebral hemorrhage. Stroke 2014, 45, 59–65. [Google Scholar] [CrossRef]
- Camps-Renom, P.; Alejaldre-Monforte, A.; Delgado-Mederos, R.; Martínez-Domeño, A.; Prats-Sánchez, L.; Pascual-Goñi, E.; Martí-Fàbregas, J. Does prior antiplatelet therapy influence hematoma volume and hematoma growth following intracerebral hemorrhage? Results from a prospective study and a meta-analysis. Eur. J. Neurol. 2017, 24, 302–308. [Google Scholar] [CrossRef]
- Kazui, S.; Naritomi, H.; Yamamoto, H.; Sawada, T.; Yamaguchi, T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke 1996, 27, 1783–1787. [Google Scholar] [CrossRef]
- Yao, X.; Wu, B.; Xu, Y.; Siwila-Sackman, E.; Selim, M. Day-night variability of hematoma expansion in patients with spontaneous intracerebral hemorrhage. J. Biol. Rhythm. 2015, 30, 242–250. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, D.; Liu, J.; Zhang, M.; Xu, H.; Xiang, Y.; Zhan, C.; Zhang, Y.; Huang, S.; Yang, Y. Clinical-radiomics Nomogram for Risk Estimation of Early Hematoma Expansion after Acute Intracerebral Hemorrhage. Acad. Radiol. 2021, 28, 307–317. [Google Scholar] [CrossRef]
- Nehme, A.; Ducroux, C.; Panzini, M.A.; Bard, C.; Bereznyakova, O.; Boisseau, W.; Deschaintre, Y.; Diestro, J.D.B.; Guilbert, F.; Jacquin, G.; et al. Non-contrast CT markers of intracerebral hematoma expansion: A reliability study. Eur. Radiol. 2022, 32, 6126–6135. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Huang, C.; Dong, J.; Yang, X.; Xiang, J.; Mao, Y.; Dong, Q.; Tang, Y. Minimal Computed Tomography Attenuation Value Within the Hematoma is Associated with Hematoma Expansion and Poor Outcome in Intracerebral Hemorrhage Patients. Neurocritical Care 2019, 31, 455–465. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, W.; Huang, S.; Lin, F.; He, Q.; Zheng, Y.; Gao, Z.; Cai, L.; Ye, G.; Chen, R.; et al. Quantitative hematoma heterogeneity associated with hematoma growth in patients with early intracerebral hemorrhage. Front. Neurol. 2022, 13, 999223. [Google Scholar] [CrossRef]
- Teng, L.; Ren, Q.; Zhang, P.; Wu, Z.; Guo, W.; Ren, T. Artificial Intelligence Can Effectively Predict Early Hematoma Expansion of Intracerebral Hemorrhage Analyzing Noncontrast Computed Tomography Image. Front. Aging Neurosci. 2021, 13, 632138. [Google Scholar] [CrossRef]
- Fisher, C.M. Pathological observations in hypertensive cerebral hemorrhage. J. Neuropathol. Exp. Neurol. 1971, 30, 536–550. [Google Scholar] [CrossRef]
- New, P.F.; Aronow, S. Attenuation measurements of whole blood and blood fractions in computed tomography. Radiology 1976, 121 Pt 1, 635–640. [Google Scholar] [CrossRef]
- Parizel, P.M.; Makkat, S.; Van Miert, E.; Van Goethem, J.W.; van den Hauwe, L.; De Schepper, A.M. Intracranial hemorrhage: Principles of CT and MRI interpretation. Eur. Radiol. 2001, 11, 1770–1783. [Google Scholar] [CrossRef]
| Variable | Total (n = 158) |
|---|---|
| Age, y, mean (SD) | 61.0 (12.5) |
| Male sex, n (%) | 128 (81.0) |
| Hypertension, n (%) | 110 (69.6) |
| Diabetes mellitus, n (%) | 29 (18.3) |
| Oral anticoagulants, n (%) | 3 (1.9%) |
| Oral antiplatelet drugs, n (%) | 4 (2.5%) |
| Admission SBP, mmHg, mean (SD) | 163.6 (27.7) |
| Admission DBP, mmHg, mean (SD) | 95.4 (16.4) |
| Baseline GCS, median (IQR) | 13 (10–15) |
| Deep ICH, n (%) | 130 (82.3) |
| IVH, n (%) | 47 (29.7) |
| Hydrocephalus, n (%) | 19 (12.0) |
| Density category | |
| Homogeneous, n (%) | 142 (89.9) |
| Heterogeneous, n (%) | 16 (10.1) |
| Swirl sign, n (%) | 38 (24.1) |
| Black hole sign, n (%) | 15 (9.5) |
| Blend sign, n (%) | 12 (7.6) |
| Blood-fluid level, n (%) | 4 (2.5) |
| Time to baseline CT, h, median (IQR) | 3.5 (2.0–6.3) |
| ICH volume, mL, median (IQR) | 24.3 (14.5–36.5) |
| UHG, mL/h, median (IQR) | 5.5 (3.3–13.6) |
| WBC, 109/L, mean (SD) | 9.5 (3.3) |
| HGB, g/L, mean (SD) | 142.3 (18.0) |
| PLT, 109/L, mean (SD) | 212.6 (63.7) |
| GLU, mmol/L, mean (SD) | 7.4 (3.4) |
| Variable | Hematoma Expansion for BCT within 6 h and FCT within 24 h (n = 92) | Hematoma Expansion for BCT within 6 h and FCT within 72 h (n = 118) | ||||
|---|---|---|---|---|---|---|
| Yes (n = 18) | No (n = 74) | p-Value | Yes (n = 25) | No (n = 93) | p-Value | |
| Age, y, mean (SD) | 59.8 (13.3) | 61.0 (12.3) | 0.733 | 57.4 (12.6) | 60.9 (12.0) | 0.206 |
| Male Sex, n (%) | 16 (88.9) | 57 (77.0) | 0.429 | 22 (88.0) | 74 (79.6) | 0.502 |
| Hypertension, n (%) | 12 (66.7) | 56 (75.7) | 0.630 | 15 (60.0) | 68 (73.1) | 0.202 |
| Diabetes mellitus, n (%) | 3 (16.7) | 6 (8.1) | 0.513 | 5 (20.0) | 7 (7.5) | 0.145 |
| Admission SBP, mmHg, mean (SD) | 168.9 (30.7) | 167.7 (26.2) | 0.856 | 163.8 (29.8) | 163.8 (26.2) | 0.998 |
| Admission DBP, mmHg, mean (SD) | 97.1 (17.8) | 98.6 (15.7) | 0.725 | 91.2 (16.7) | 98.1 (16.1) | 0.063 |
| Baseline GCS, median (IQR) | 13 (9–15) | 13 (10–15) | 0.960 | 14 (9.5–15) | 13 (10–15) | 0.699 |
| Deep ICH, n (%) | 16 (88.9) | 62 (83.8) | 0.861 | 23 (92.0) | 79 (84.9) | 0.558 |
| IVH, n (%) | 3 (16.7) | 23 (31.1) | 0.223 | 5 (20.0) | 24 (25.8) | 0.549 |
| Hydrocephalus, n (%) | 2 (11.1) | 9 (12.2) | 0.902 | 1 (4.0) | 12 (12.9) | 0.367 |
| Heterogeneity, n (%) | 7 (38.9) | 3 (4.1) | <0.001 | 8 (32.0) | 4 (4.3) | <0.001 |
| Swirl sign, n (%) | 6 (33.3) | 16 (21.6) | 0.461 | 7 (28.0) | 24 (25.8) | 0.825 |
| Black hole sign, n (%) | 3 (16.7) | 4 (5.4) | 0.262 | 3 (12.0) | 8 (8.6) | 0.896 |
| Blend sign, n (%) | 3 (16.7) | 4 (5.4) | 0.262 | 7 (28.0) | 4 (4.3) | 0.001 |
| Blood-fluid level, n (%) | 1 (5.6) | 2 (2.7) | 0.484 | 1 (4.0) | 3 (3.2) | 0.849 |
| Time to baseline CT, h, median (IQR) | 1.8 (1.3–2.9) | 2.8 (1.8–4.1) | 0.005 | 2.0 (1.6–3.4) | 2.8 (1.9–4.1) | 0.023 |
| ICH volume, mL, median (IQR) | 24.9 (16.9–36.0) | 21.0 (12.9–31.3) | 0.340 | 24.3 (15.9–33.6) | 21.6(13.5–31.5) | 0.453 |
| UHG, mL/h, median (IQR) | 14.2 (6.8–30.3) | 7.2 (3.8–14.9) | 0.022 | 10.8 (5.1–26.3) | 7.2 (4.0–15.5) | 0.047 |
| WBC, 109/L, mean (SD) | 9.6 (4.8) | 9.5 (2.9) | 0.869 | 8.8 (4.6) | 9.6 (3.0) | 0.320 |
| HGB, g/L, mean (SD) | 142.6 (18.8) | 140.4 (19.0) | 0.665 | 143.9 (17.4) | 141.2 (18.2) | 0.497 |
| PLT, 109/L, mean (SD) | 208.6 (77.3) | 223.4 (57.8) | 0.367 | 199.4 (74.3) | 216.5 (61.4) | 0.242 |
| GLU, mmol/L, mean (SD) | 8.5 (4.5) | 7.6 (3.4) | 0.394 | 8.3 (4.5) | 7.4 (3.2) | 0.244 |
| Variable | BCT within 6 h and FCT within 24 h (n = 92) | BCT within 6 h and FCT within 72 h (n = 118) | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 0.993 (0.952–1.035) | 0.730 | 0.975 (0.939–1.014) | 0.206 |
| Sex | 0.419 (0.087–2.008) | 0.277 | 0.531 (0.144–1.963) | 0.343 |
| Hypertension | 0.643 (0.211–1.960) | 0.437 | 0.551 (0.219–1.387) | 0.206 |
| Diabetes mellitus | 2.267 (0.509–10.102) | 0.283 | 3.071 (0.883–10.683) | 0.078 |
| Admission SBP | 1.002 (0.983–1.021) | 0.854 | 1.000 (0.984–1.017) | 0.998 |
| Admission DBP | 0.994 (0.962–1.027) | 0.722 | 0.973 (0.945–1.002) | 0.067 |
| Baseline GCS | 0.961 (0.812–1.136) | 0.641 | 0.997 (0.860–1.157) | 0.973 |
| Deep ICH | 1.548 (0.314–7.628) | 0.591 | 2.038 (0.431–9.627) | 0.369 |
| IVH | 0.443 (0.117–1.683) | 0.232 | 0.719 (0.243–2.126) | 0.551 |
| Hydrocephalus | 0.903 (0.177–4.593) | 0.902 | 0.281 (0.035–2.274) | 0.234 |
| Heterogeneity | 15.061 (3.380–67.106) | <0.001 | 10.471 (2.832–38.711) | <0.001 |
| Swirl sign | 1.812 (0.588–5.586) | 0.300 | 1.118 (0.416–3.006) | 0.825 |
| Black hole sign | 3.500 (0.708–17.291) | 0.124 | 1.449 (0.355–5.918) | 0.606 |
| Blend sign | 3.500 (0.708–17.291) | 0.124 | 8.653 (2.291–32.677) | 0.001 |
| Blood-fluid level | 2.118 (0.181–24.736) | 0.550 | 1.250 (0.124–12.562) | 0.850 |
| Time to baseline CT | 0.504 (0.309–0.821) | 0.006 | 0.665 (0.465–0.949) | 0.025 |
| ICH volume | 1.015 (0.985–1.045) | 0.337 | 1.011 (0.983–1.040) | 0.442 |
| UHG | 1.048 (1.008–1.090) | 0.017 | 1.044 (1.008–1.081) | 0.016 |
| WBC | 1.013 (0.870–1.180) | 0.867 | 0.930 (0.805–1.073) | 0.318 |
| HGB | 1.006 (0.978–1.035) | 0.661 | 1.009 (0.983–1.035) | 0.493 |
| PLT | 0.996 (0.987–1.005) | 0.363 | 0.996 (0.989–1.003) | 0.241 |
| GLU | 1.058 (0.930–1.203) | 0.394 | 1.069 (0.954–1.198) | 0.248 |
| Unadjusted | Adjusted * | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| BCT within 6 h and FCT within 24 h | ||||
| Heterogeneity | 40.536 (5.366–306.223) | <0.001 | 88.445(5.387–1452.191) | 0.002 |
| Time to baseline CT | 0.278 (0.106–0.729) | 0.009 | 0.234 (0.077–0.712) | 0.011 |
| UHG | 0.971 (0.910–1.036) | 0.368 | 0.973 (0.909–1.042) | 0.437 |
| BCT within 6 h and FCT within 72 h | ||||
| Heterogeneity | 8.833 (2.165–36.037) | 0.002 | 31.703 (3.036–331.026) | 0.004 |
| Blend sign | 7.121 (1.651–30.709) | 0.008 | 6.985 (1.364–35.776) | 0.020 |
| Time to baseline CT | 0.722 (0.414–1.259) | 0.251 | 0.660 (0.360–1.209) | 0.179 |
| UHG | 1.011 (0.956–1.071) | 0.695 | 1.024 (0.963–1.088) | 0.447 |
| BCT within 12 h and FCT within 24 h | ||||
| Heterogeneity | 11.259 (1.873–67.687) | 0.008 | 10.478 (1.657–66.252) | 0.013 |
| Black hole sign | 1.061 (0.136–8.293) | 0.955 | 1.214 (0.161–9.173) | 0.851 |
| UHG | 1.044 (1.003–1.087) | 0.036 | 1.044 (0.990–1.100) | 0.110 |
| BCT within 12 h and FCT within 72 h | ||||
| Diabetes mellitus | 3.255 (0.838–12.645) | 0.088 | 3.259 (0.836–12.702) | 0.089 |
| IVH | 0.368 (0.108–1.261) | 0.112 | 0.367 (0.105–1.281) | 0.116 |
| Heterogeneity | 6.473 (1.579–26.541) | 0.009 | 6.465 (1.569–26.637) | 0.010 |
| Black hole sign | 1.292 (0.296–5.634) | 0.733 | 1.292 (0.296–5.635) | 0.733 |
| Blend sign | 6.197 (1.428–26.886) | 0.015 | 6.203 (1.424–27.014) | 0.015 |
| UHG | 1.044 (1.004–1.086) | 0.029 | 1.045 (0.995–1.097) | 0.081 |
| Variable | Hematoma Expansion for BCT within 12 h and FCT within 24 h (n = 115) | Hematoma Expansion for BCT within 12 h and FCT within 72 h (n = 158) | ||||
|---|---|---|---|---|---|---|
| Yes (n = 22) | No (n = 93) | p-Value | Yes (n = 31) | No (n = 127) | p-Value | |
| Age, y, mean (SD) | 59.6 (12.6) | 60.8 (12.2) | 0.669 | 57.7 (12.5) | 61.8 (12.4) | 0.106 |
| Male Sex, n (%) | 20 (90.9) | 71 (76.3) | 0.222 | 28 (90.3) | 100 (78.7) | 0.140 |
| Hypertension, n (%) | 16 (72.7) | 66 (71.0) | 0.870 | 20 (64.5) | 90 (70.9) | 0.491 |
| Diabetes mellitus, n (%) | 3 (13.6) | 7 (7.5) | 0.621 | 20 (64.5) | 9 (7.1) | <0.001 |
| Admission SBP, mmHg, mean (SD) | 171.4 (31.8) | 165.2 (27.1) | 0.354 | 165.1 (30.5) | 163.3 (27.1) | 0.747 |
| Admission DBP, mmHg, mean (SD) | 97.2 (20.3) | 96.4 (15.6) | 0.849 | 97.0 (19.0) | 95.0 (15.7) | 0.552 |
| Baseline GCS, median (IQR) | 13 (9.75–15) | 13 (10–15) | 0.878 | 14 (15–10) | 13 (15–10) | 0.542 |
| Deep ICH, n (%) | 19 (86.4) | 75 (80.6) | 0.751 | 28 (90.3%) | 102 (80.3%) | 0.191 |
| IVH, n (%) | 4 (18.2) | 33 (35.5) | 0.118 | 4 (12.9) | 43 (33.9) | 0.022 |
| Hydrocephalus, n (%) | 3 (13.6) | 13 (14.0) | 0.967 | 3 (9.7) | 16 (12.6) | 0.888 |
| Heterogeneity, n (%) | 8 (36.4) | 4 (4.3) | <0.001 | 10 (32.3) | 6 (4.7) | <0.001 |
| Swirl sign, n (%) | 7 (31.8) | 20 (21.5) | 0.305 | 8 (25.8) | 30 (23.6) | 0.799 |
| Black hole sign, n (%) | 5 (22.7) | 5 (5.4) | 0.030 | 6 (19.3) | 9 (7.1) | 0.024 |
| Blend sign, n (%) | 3 (13.6) | 4 (4.3) | 0.250 | 8 (25.8) | 4 (3.1) | <0.001 |
| Blood–fluid level, n (%) | 1 (4.5) | 2 (2.2) | 0.474 | 2 (6.5) | 2 (1.6) | 0.173 |
| Time to baseline CT, h, median (IQR) | 2.1 (1.6–3.7) | 3.5 (2.2–5.5) | 0.030 | 2.1 (4.5–1.6) | 3.7 (6.5–2.3) | 0.018 |
| ICH volume, mL, median (IQR) | 27.4 (19.1–44.4) | 23.3 (13.7–36.8) | 0.146 | 26.2 (40.0–17.8) | 23.8 (36.4–14.1) | 0.278 |
| UHG, mL/h, median (IQR) | 11.2 (4.8–25.5) | 5.6 (3.3–12.8) | 0.010 | 8.8 (4.2–24.8) | 5.2 (3.3–11.7) | 0.022 |
| WBC, 109/L, mean (SD) | 9.1 (4.7) | 9.5 (2.9) | 0.619 | 8.7 (4.4) | 9.7 (3.0) | 0.142 |
| HGB, g/L, mean (SD) | 138.7 (21.3) | 141.6 (18.0) | 0.507 | 141.1 (19.4) | 142.6 (17.7) | 0.676 |
| PLT, 109/L, mean (SD) | 201.6 (80.4) | 219.7 (56.5) | 0.219 | 200.1 (78.4) | 215.6 (59.5) | 0.223 |
| GLU, mmol/L, mean (SD) | 8.6 (4.9) | 7.5 (3.2) | 0.166 | 8.3 (4.8) | 7.2 (2.9) | 0.248 |
| Variable | BCT within 12 h and FCT within 24 h (n = 115) | BCT within 12 h and FCT within 72 h (n = 158) | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 0.992 (0.954–1.031) | 0.666 | 0.973 (0.941–1.006) | 0.108 |
| Sex | 0.323 (0.070–1.491) | 0.147 | 0.397 (0.112–1.405) | 0.152 |
| Hypertension | 1.091 (0.386–3.085) | 0.870 | 0.747 (0.326–1.713) | 0.492 |
| Diabetes mellitus | 1.940 (0.459–8.195) | 0.367 | 3.147 (1.027–9.639) | 0.045 |
| Admission SBP | 1.008 (0.991–1.025) | 0.352 | 1.002 (0.988–1.017) | 0.745 |
| Admission DBP | 1.003 (0.975–1.031) | 0.847 | 1.007 (0.984–1.032) | 0.549 |
| Baseline GCS | 0.971 (0.827–1.141) | 0.721 | 1.011 (0.879–1.164) | 0.876 |
| Deep ICH | 1.520 (0.405–5.701) | 0.535 | 2.288 (0.643–8.133) | 0.201 |
| IVH | 0.404 (0.126–1.294) | 0.127 | 0.289 (0.095–0.880) | 0.029 |
| Hydrocephalus | 0.972 (0.252–3.753) | 0.967 | 0.743 (0.202–2.730) | 0.655 |
| Heterogeneity | 12.714 (3.376–47.878) | <0.001 | 9.603 (3.155–29.231) | <0.001 |
| Swirl sign | 1.703 (0.611–4.745) | 0.308 | 1.125 (0.456–2.774) | 0.799 |
| Black hole sign | 5.176 (1.350–19.847) | 0.016 | 3.147 (1.027–9.639) | 0.045 |
| Blend sign | 3.513 (0.726–17.001) | 0.118 | 10.696 (2.973–38.474) | <0.001 |
| Blood-fluid level | 2.167 (0.188–25.030) | 0.536 | 4.310 (0.583–31.886) | 0.152 |
| Time to baseline CT | 0.855 (0.692–1.056) | 0.146 | 0.878 (0.751–1.026) | 0.101 |
| ICH volume | 1.022 (0.996–1.049) | 0.102 | 1.016 (0.992–1.041) | 0.199 |
| UHG | 1.047 (1.010–1.086) | 0.012 | 1.045 (1.012–1.079) | 0.007 |
| WBC | 0.963 (0.829–1.117) | 0.616 | 0.906 (0.795–1.033) | 0.142 |
| HGB | 0.992 (0.968–1.016) | 0.504 | 0.995 (0.974–1.017) | 0.674 |
| PLT | 0.995 (0.987–1.003) | 0.218 | 0.996 (0.990–1.002) | 0.223 |
| GLU | 1.081 (0.966–1.210) | 0.174 | 1.082 (0.977–1.199) | 0.129 |
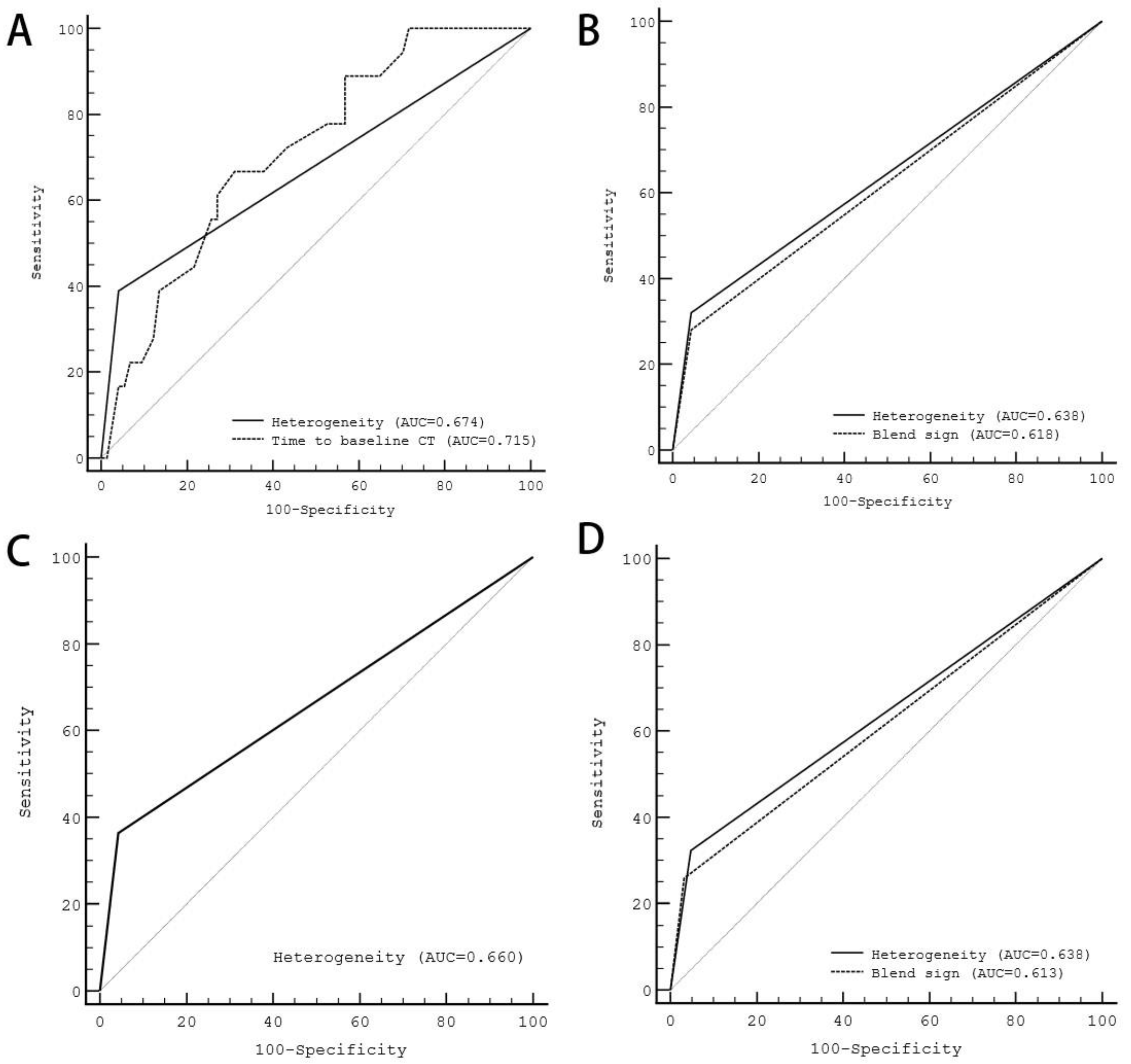
| Variable | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|
| BCT within 6 h and FCT within 24 h | 38.9 | 96.0 | 70.0 | 86.6 | 0.674 |
| BCT within 6 h and FCT within 72 h | 32.0 | 95.7 | 66.7 | 84.0 | 0.638 |
| BCT within 12 h and FCT within 24 h | 36.4 | 95.7 | 66.7 | 86.4 | 0.660 |
| BCT within 12 h and FCT within 72 h | 32.3 | 95.3 | 62.5 | 85.2 | 0.638 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Zhao, M.; Lin, Y.; Zeng, J.; He, Q.; Zheng, Y.; Ma, K.; Lin, F.; Kang, D. Noncontrast Computed Tomography Markers Associated with Hematoma Expansion: Analysis of a Multicenter Retrospective Study. Brain Sci. 2023, 13, 608. https://doi.org/10.3390/brainsci13040608
Yu L, Zhao M, Lin Y, Zeng J, He Q, Zheng Y, Ma K, Lin F, Kang D. Noncontrast Computed Tomography Markers Associated with Hematoma Expansion: Analysis of a Multicenter Retrospective Study. Brain Sciences. 2023; 13(4):608. https://doi.org/10.3390/brainsci13040608
Chicago/Turabian StyleYu, Lianghong, Mingpei Zhao, Yuanxiang Lin, Jiateng Zeng, Qiu He, Yan Zheng, Ke Ma, Fuxin Lin, and Dezhi Kang. 2023. "Noncontrast Computed Tomography Markers Associated with Hematoma Expansion: Analysis of a Multicenter Retrospective Study" Brain Sciences 13, no. 4: 608. https://doi.org/10.3390/brainsci13040608
APA StyleYu, L., Zhao, M., Lin, Y., Zeng, J., He, Q., Zheng, Y., Ma, K., Lin, F., & Kang, D. (2023). Noncontrast Computed Tomography Markers Associated with Hematoma Expansion: Analysis of a Multicenter Retrospective Study. Brain Sciences, 13(4), 608. https://doi.org/10.3390/brainsci13040608






