Effectiveness of SGA-LAIs on Clinical, Cognitive, and Social Domains in Schizophrenia: Results from a Prospective Naturalistic Study
Abstract
1. Introduction
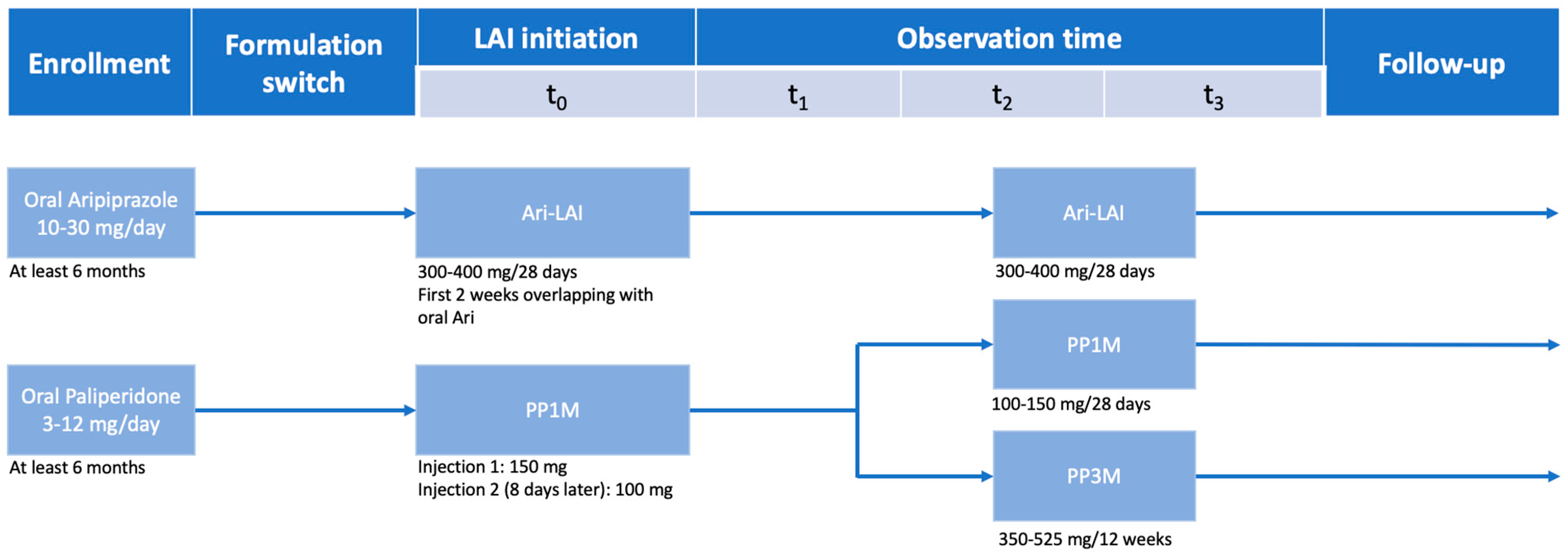
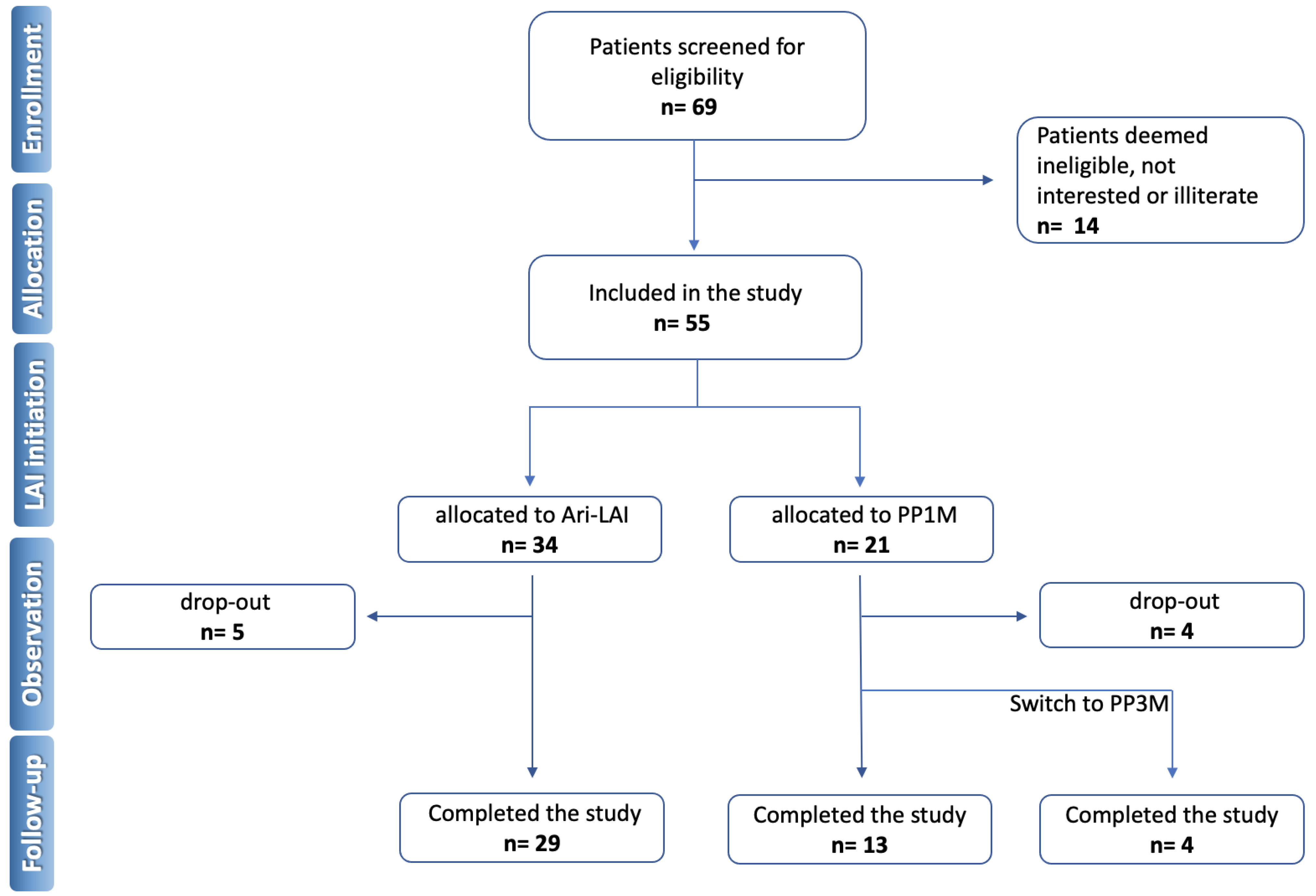
2. Materials and Methods
2.1. Assessment
2.1.1. Psychopathological Assessment
- Positive and Negative Syndrome Scale (PANSS) [19]: It is a rater-administrated scale that aims to evaluate the occurrence and severity of positive and negative general psychotic symptoms, and disorganization of thought and behavior. The tool counts 30 items, assessable with a value from 1 to 7, theoretically divided into three sub-scales: seven-item scales for positive and negative symptoms, and a 16 item scale covering general psychopathology.
2.1.2. Functional Assessment
- Quality of life scale (QoLS) [20]: It is a 16 item self-administered tool that allows the assessment of the self-perceived quality of life according to five conceptual domains: psychic and physical well-being, interpersonal relationships, civic and community activities, personal development, and recreational activities. Finally, the sum of individual items scores leads to a total score.
- Personal and Social Performance Scale (PSP) [21]: It is a rater-administrated tool scale that evaluates several dimensions of the patient’s overall personal functioning, assigning scores ranging between 1 and 6 to four domains: study and work activities, interpersonal relationships, self-care, aggression, and behavioral disorders. While the global functioning level is defined by a score ranging from a minimum of 1 to a maximum of 100.
2.1.3. Neuropsychological Assessment
- Stroop Color and Word Test (SCWT) [22]: It is a cognitive test that evaluates the subject’s ability to inhibit cognitive interference, interference control, verbal speed, flexibility, and attention. The cognitive interference is measured through the increase response latencies and/or errors (Stroop effect) in colors recognition even with confounding factors, and these changes provide an index of cognitive flexibility.
- Rey-Osterrieth Complex Figure Test (RCFT) [23]: It is a rater-administrated tool that assesses the individual’s short-term visual memory, visual organization, and visuospatial abilities. Additionally, the RCFT can be used to examine organizational strategies used during the copy task. This test also allows to calculate the Central Coherence Index (CCI), ranging from 0 (detailed) to 2 (global), which comes out from the order of the construction index (drawing of global or local elements in the first stage of the copy task) and the style index (the degree of continuity in the drawing process).
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Millan, M.J.; Goodwin, G.M.; Meyer-Lindenberg, A.; Ögren, S.O. Learning from the past and looking to the future: Emerging perspectives for improving the treatment of psychiatric disorders. Eur. Neuropsychopharmacol. 2015, 25, 599–656. [Google Scholar] [CrossRef] [PubMed]
- de Filippis, R.; De Fazio, P.; Gaetano, R.; Steardo, L.; Cedro, C.; Bruno, A.; Zoccali, R.A.; Muscatello, M.R.A. Current and emerging long-acting antipsychotics for the treatment of schizophrenia. Expert Opin. Drug Saf. 2021, 20, 771–790. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.W. Historical perspective on antipsychotic long-acting injections. Br. J. Psychiatry Suppl. 2009, 52, s7–s12. [Google Scholar] [CrossRef]
- Higashi, K.; Medic, G.; Littlewood, K.J.; Diez, T.; Granström, O.; De Hert, M. Medication adherence in schizophrenia: Factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther. Adv. Psychopharmacol. 2013, 3, 200–218. [Google Scholar] [CrossRef] [PubMed]
- de Filippis, R.; Aloi, M.; Pilieci, A.M.; Boniello, F.; Quirino, D.; Steardo, L., Jr.; Segura-Garcia, C.; De Fazio, P. Psychometric properties of the 9-item Shared Decision-Making Questionnaire (SDM-Q-9): Validation of the Italian version in a large psychiatric clinical sample. Clin. Neuropsychiatry J. Treat. Eval. 2022, 19, 264–271. [Google Scholar] [CrossRef]
- Aguglia, A.; Fusar-Poli, L.; Natale, A.; Amerio, A.; Espa, I.; Villa, V.; Martinotti, G.; Carrà, G.; Bartoli, F.; D’Agostino, A.; et al. Factors Associated with Medication Adherence to Long-Acting Injectable Antipsychotics: Results from the STAR Network Depot Study. Pharmacopsychiatry 2022, 55, 281–289. [Google Scholar] [CrossRef]
- Abdel-Baki, A.; Medrano, S.; Maranda, C.; Ladouceur, M.; Tahir, R.; Stip, E.; Potvin, S. Impact of early use of long-acting injectable antipsychotics on psychotic relapses and hospitalizations in first-episode psychosis. Int. Clin. Psychopharmacol. 2020, 35, 221–228. [Google Scholar] [CrossRef]
- Masand, P.S.; Gupta, S. Long-Acting Injectable Antipsychotics in the Elderly. Drugs Aging 2003, 20, 1099–1110. [Google Scholar] [CrossRef]
- Coles, A.S.; Knezevic, D.; George, T.P.; Correll, C.U.; Kane, J.M.; Castle, D. Long-Acting Injectable Antipsychotic Treatment in Schizophrenia and Co-occurring Substance Use Disorders: A Systematic Review. Front. Psychiatry 2021, 12, 808002. [Google Scholar] [CrossRef]
- Rubio, J.M.; Taipale, H.; Tanskanen, A.; Correll, C.U.; Kane, J.M.; Tiihonen, J. Long-term Continuity of Antipsychotic Treatment for Schizophrenia: A Nationwide Study. Schizophr. Bull. 2021, 47, 1611–1620. [Google Scholar] [CrossRef]
- Kishimoto, T.; Hagi, K.; Kurokawa, S.; Kane, J.M.; Correll, C.U. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: A systematic review and comparative meta-analysis of randomised, cohort, and pre–post studies. Lancet Psychiatry 2021, 8, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, I.; Tiihonen, J.; Kotzalidis, G.D.; Verdolini, N.; Murru, A.; Goikolea, J.M.; Valentí, M.; Aedo, A.; Vieta, E. Long-acting injectable antipsychotics (LAIs) for maintenance treatment of bipolar and schizoaffective disorders: A systematic review. Eur. Neuropsychopharmacol. 2019, 29, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Cicala, G.; de Filippis, R.; Barbieri, M.A.; Cutroneo, P.M.; De Fazio, P.; Schoretsanitis, G.; Spina, E. Tolerability Profile of Paliperidone Palmitate Formulations: A Pharmacovigilance Analysis of The EUDRAVigilance Database. Front. Psychiatry 2023, 14, 542. [Google Scholar] [CrossRef]
- Agid, O.; Remington, G.; Fung, C.; Nightingale, N.M.; Duclos, M.; Anger, G.J. Real-World Utilization Patterns of Long-Acting Injectable Antipsychotics in Canada: A Retrospective Study. Can. J. Psychiatry 2022, 67, 226–234. [Google Scholar] [CrossRef]
- Guinart, D.; Misawa, F.; Rubio, J.M.; Pereira, J.; de Filippis, R.; Gastaldon, C.; Kane, J.M.; Correll, C.U. A systematic review and pooled, patient-level analysis of predictors of mortality in neuroleptic malignant syndrome. Acta Psychiatr. Scand. 2021, 144, 329–341. [Google Scholar] [CrossRef]
- Magliocco, F.; de Filippis, R.; Aloi, M.; Staltari, F.A.; Gaetano, R.; Segura-Garcia, C.; De Fazio, P. Second-generation long-acting injections anti-psychotics improve executive functions in patients with schizophrenia: A 12-month real-world study. Int. J. Psychiatry Clin. Pract. 2020, 24, 201–207. [Google Scholar] [CrossRef]
- Russu, A.; Sliwa, J.K.; Ravenstijn, P.; Singh, A.; Mathews, M.; Kim, E.; Gopal, S. Maintenance dose conversion between oral risperidone and paliperidone palmitate 1 month: Practical guidance based on pharmacokinetic simulations. Int. J. Clin. Pract. 2018, 72, e13089. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Berzon, R.A.; Donnelly, M.A.; Simpson, R.L.; Simeon, G.P.; Tilson, H.H. Quality of life bibliography and indexes: 1994 update. Qual. Life Res. 1995, 4, 547–569. [Google Scholar] [CrossRef]
- Morosini, P.L.; Magliano, L.; Brambilla, L.; Ugolini, S.; Pioli, R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr. Scand. 2000, 101, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Golden, C. Stroop Color and Word Test: A Manual for Clinical and Experimental USes; Skoelting: Chiacago, IL, USA, 1978. [Google Scholar]
- Rey, A. L’examen psychologique dans les cas d’encéphalopathie traumatique. Arch. Psychol. 1941, 28, 215–285. [Google Scholar]
- Chrzanowski, W.K.; Marcus, R.N.; Torbeyns, A.; Nyilas, M.; McQuade, R.D. Effectiveness of long-term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: A 52-week, open-label comparison with olanzapine. Psychopharmacology 2006, 189, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-H.; Lin, W.-W.; Chen, S.-T.; Chen, K.-C.; Chen, K.-P.; Chiu, N.-Y.; Huang, C.; Chang, C.-J.; Lin, C.-H.; Lai, T.-J. A 64-week, multicenter, open-label study of aripiprazole effectiveness in the management of patients with schizophrenia or schizoaffective disorder in a general psychiatric outpatient setting. Ann. Gen. Psychiatry 2010, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Ceraso, A.; Lin, J.J.; Schneider-Thoma, J.; Siafis, S.; Tardy, M.; Komossa, K.; Heres, S.; Kissling, W.; Davis, J.M.; Leucht, S. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst. Rev. 2020, 2020, CD008016. [Google Scholar] [CrossRef]
- Morrissette, D.A.; Stahl, S.M. Optimizing Outcomes in Schizophrenia: Long-acting Depots and Long-term Treatment. CNS Spectr. 2012, 17, 10–21. [Google Scholar] [CrossRef]
- Karson, C.; Duffy, R.A.; Eramo, A.; Nylander, A.-G.; Offord, S.J. Long-term outcomes of antipsychotic treatment in patients with first-episode schizophrenia: A systematic review. Neuropsychiatr. Dis. Treat. 2016, 12, 57. [Google Scholar] [CrossRef]
- Kane, J.M.; Schooler, N.R.; Marcy, P.; Correll, C.U.; Achtyes, E.D.; Gibbons, R.D.; Robinson, D.G. Effect of Long-Acting Injectable Antipsychotics vs Usual Care on Time to First Hospitalization in Early-Phase Schizophrenia. JAMA Psychiatry 2020, 77, 1217–1224. [Google Scholar] [CrossRef]
- Aloi, M.; de Filippis, R.; Lavalle, F.G.; Chiappetta, E.; Viganò, C.; Segura-Garcia, C.; De Fazio, P. Effectiveness of integrated psychological therapy on clinical, neuropsychological, emotional and functional outcome in schizophrenia: A RCT study. J. Ment. Health 2018, 29, 524–531. [Google Scholar] [CrossRef]
- Ostuzzi, G.; Bertolini, F.; Tedeschi, F.; Vita, G.; Brambilla, P.; Fabro, L.; Gastaldon, C.; Papola, D.; Purgato, M.; Nosari, G.; et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: A network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry 2022, 21, 295–307. [Google Scholar] [CrossRef]
- Ostuzzi, G.; Bertolini, F.; Del Giovane, C.; Tedeschi, F.; Bovo, C.; Gastaldon, C.; Nosé, M.; Ogheri, F.; Papola, D.; Purgato, M.; et al. Maintenance Treatment with Long-Acting Injectable Antipsychotics for People with Nonaffective Psychoses: A Network Meta-Analysis. Am. J. Psychiatry 2021, 178, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Ostuzzi, G.; Vita, G.; Bertolini, F.; Tedeschi, F.; De Luca, B.; Gastaldon, C.; Nosé, M.; Papola, D.; Purgato, M.; Del Giovane, C.; et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: A systematic review and network meta-analysis. Lancet Psychiatry 2022, 9, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.; Zummo, J.; Desai, D.N.; Drake, C.; Hutton, B.; Kotb, A.; Weiden, P.J. Aripiprazole Lauroxil Compared with Paliperidone Palmitate in Patients with Schizophrenia: An Indirect Treatment Comparison. Value Health 2017, 20, 876–885. [Google Scholar] [CrossRef]
- Mason, K.; Barnett, J.; Pappa, S. Effectiveness of 2-year treatment with aripiprazole long-acting injectable and comparison with paliperidone palmitate. Ther. Adv. Psychopharmacol. 2021, 11, 204512532110294. [Google Scholar] [CrossRef]
- Di Lorenzo, R.; Ferri, P.; Cameli, M.; Rovesti, S.; Piemonte, C. Effectiveness of 1-year treatment with long-acting formulation of aripiprazole, haloperidol, or paliperidone in patients with schizophrenia: Retrospective study in a real-world clinical setting. Neuropsychiatr. Dis. Treat. 2019, 15, 183–198. [Google Scholar] [CrossRef]
- Correll, C.U.; Kim, E.; Sliwa, J.K.; Hamm, W.; Gopal, S.; Mathews, M.; Venkatasubramanian, R.; Saklad, S.R. Pharmacokinetic Characteristics of Long-Acting Injectable Antipsychotics for Schizophrenia: An Overview. CNS Drugs 2021, 35, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Pae, C.-U.; Wang, S.-M.; Han, C.; Bahk, W.-M.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Serretti, A.; Emsley, R. Comparison between long-acting injectable aripiprazole versus paliperidone palmitate in the treatment of schizophrenia. Int. Clin. Psychopharmacol. 2017, 32, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Shymko, G.; Grace, T.; Jolly, N.; Dobson, L.; Hacking, D.; Parmar, A.; Kapi, P.; Waters, F. Weight gain and metabolic screening in young people with early psychosis on long acting injectable antipsychotic medication (aripiprazole vs paliperidone). Early Interv. Psychiatry 2021, 15, 787–793. [Google Scholar] [CrossRef]
- Nasrallah, H.; Aquila, R.; Stanford, A.D.; Jamal, H.H.; Weiden, P.J.; Risinger, R. Metabolic and Endocrine Profiles during 1-Year Treatment of Outpatients with Schizophrenia with Aripiprazole Lauroxil. Psychopharmacol. Bull. 2017, 47, 35–43. [Google Scholar]
- Rocca, P.; Montemagni, C.; Frieri, T. Second-generation long-acting injectable antipsychotics in schizophrenia: Patient functioning and quality of life. Neuropsychiatr. Dis. Treat. 2016, 12, 917. [Google Scholar] [CrossRef]
- Pietrini, F.; Albert, U.; Ballerini, A.; Calò, P.; Maina, G.; Pinna, F.; Vaggi, M.; Boggian, I.; Fontana, M.; Moro, C.G.; et al. The modern perspective for long-acting injectables antipsychotics in the patient-centered care of schizophrenia. Neuropsychiatr. Dis. Treat. 2019, 15, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- de Filippis, R.; Gaetano, R.; Schoretsanitis, G.; Verde, G.; Oliveti, C.A.; Kane, J.M.; Segura-Garcia, C.; De Fazio, P. Clozapine Management in Schizophrenia Inpatients: A 5-Year Prospective Observational Study of Its Safety and Tolerability Profile. Neuropsychiatr. Dis. Treat. 2021, 17, 2141–2150. [Google Scholar] [CrossRef]
- Nasrallah, H.A. Triple advantages of injectable long acting second generation antipsychotics: Relapse prevention, neuroprotection, and lower mortality. Schizophr. Res. 2018, 197, 69–70. [Google Scholar] [CrossRef]
- Taipale, H.; Mittendorfer-Rutz, E.; Alexanderson, K.; Majak, M.; Mehtälä, J.; Hoti, F.; Jedenius, E.; Enkusson, D.; Leval, A.; Sermon, J.; et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr. Res. 2018, 197, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tao, S.; Coid, J.; Wei, W.; Wang, Q.; Yue, W.; Yan, H.; Tan, L.; Chen, Q.; Yang, G.; et al. The Role of Total White Blood Cell Count in Antipsychotic Treatment for Patients with Schizophrenia. Curr. Neuropharmacol. 2023, 21. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, O.; Essiambre, A.-M.; Béchard, L.; Roy, A.-A.; Huot-Lavoie, M.; Brodeur, S.; Chandrasena, R.; Thériault, C.; Crocker, C.; Melun, J.-P.; et al. Real-life effectiveness of transitioning from paliperidone palmitate 1-monthly to paliperidone palmitate 3-monthly long-acting injectable formulation. Ther. Adv. Psychopharmacol. 2022, 12, 204512532211360. [Google Scholar] [CrossRef]
- Karslioğlu, E.H.; Kolcu, Z.; Karslioğlu, N.I.; Çayköylü, A. Prospective analysis of serum prolactin levels, clinical symptomatology and sexual functions in patients with schizophrenia switched to paliperidone palmitate 3-monthly from paliperidone palmitate 1-monthly: Preliminary findings of the first 3 months. Hum. Psychopharmacol. Clin. Exp. 2022, 37, e2827. [Google Scholar] [CrossRef]
- Mauri, M.C.; Franco, G.; Minutillo, A.; Paletta, S.; Di Pace, C.; Reggiori, A.; Baldelli, S.; Cattaneo, D. The Switch from Paliperidone Long-Acting Injectable 1- to 3-Monthly. J. Clin. Psychopharmacol. 2022, 42, 23–30. [Google Scholar] [CrossRef]
- Savitz, A.J.; Xu, H.; Gopal, S.; Nuamah, I.; Ravenstijn, P.; Janik, A.; Schotte, A.; Hough, D.; Fleischhacker, W.W. Efficacy and Safety of Paliperidone Palmitate 3-Month Formulation for Patients with Schizophrenia: A Randomized, Multicenter, Double-Blind, Noninferiority Study. Int. J. Neuropsychopharmacol. 2016, 19, pyw018. [Google Scholar] [CrossRef]
- Ehlis, A.-C.; Herrmann, M.J.; Pauli, P.; Stoeber, G.; Pfuhlmann, B.; Fallgatter, A.J. Improvement of Prefrontal Brain Function in Endogenous Psychoses under Atypical Antipsychotic Treatment. Neuropsychopharmacology 2007, 32, 1669–1677. [Google Scholar] [CrossRef]
- de Filippis, R.; Aloi, M.; Bruni, A.; Gaetano, R.; Segura-Garcia, C.; De Fazio, P. Bipolar disorder and obsessive compulsive disorder: The comorbidity does not further impair the neurocognitive profile. J. Affect. Disord. 2018, 235, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Naber, D.; Hansen, K.; Forray, C.; Baker, R.A.; Sapin, C.; Beillat, M.; Peters-Strickland, T.; Nylander, A.-G.; Hertel, P.; Andersen, H.S.; et al. Qualify: A randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr. Res. 2015, 168, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Fagiolini, A.; Rocca, P.; De Giorgi, S.; Spina, E.; Amodeo, G.; Amore, M. Clinical trial methodology to assess the efficacy/effectiveness of long-acting antipsychotics: Randomized controlled trials vs naturalistic studies. Psychiatry Res. 2017, 247, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Potkin, S.G.; Loze, J.-Y.; Forray, C.; Baker, R.A.; Sapin, C.; Peters-Strickland, T.; Beillat, M.; Nylander, A.-G.; Hertel, P.; Schmidt, S.N.; et al. Multidimensional Assessment of Functional Outcomes in Schizophrenia: Results from QUALIFY, a Head-to-Head Trial of Aripiprazole Once-Monthly and Paliperidone Palmitate. Int. J. Neuropsychopharmacol. 2017, 20, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Nuechterlein, K.H.; Green, M.F.; Kern, R.S.; Baade, L.E.; Barch, D.M.; Cohen, J.D.; Essock, S.; Fenton, W.S.; Frese, F.J.; Gold, J.M.; et al. The MATRICS Consensus Cognitive Battery, Part 1: Test Selection, Reliability, and Validity. Am. J. Psychiatry 2008, 165, 203–213. [Google Scholar] [CrossRef] [PubMed]
| Ari-LAI (n = 29) | PP1M (n = 17) | Statistics | p | |||
|---|---|---|---|---|---|---|
| Age a | 42.5 | 15.3 | 43.1 | 13.7 | t = 0.141 | 0.888 |
| Sex b | χ2 = 3.490 | 0.062 | ||||
| Male | 16 | 55.2 | 14 | 82.4 | ||
| Female | 13 | 44.8 | 3 | 17.6 | ||
| Smoker (Yes) b | 13 | 44.8 | 12 | 70.6 | χ2 = 2.867 | 0.090 |
| Years of Education a | 11.1 | 3.2 | 12.2 | 3.5 | t = 1.153 | 0.255 |
| Marital Status b | χ2 = 2.646 | 0.450 | ||||
| Single | 20 | 69 | 13 | 76.4 | ||
| Married | 7 | 24.1 | 2 | 11.8 | ||
| Divorced | 2 | 6.9 | 2 | 11.8 | ||
| Employment b | χ2 = 2.714 | 0.744 | ||||
| Self-employment | 4 | 13.8 | 2 | 11.8 | ||
| Employed | 6 | 20.7 | 3 | 17.6 | ||
| Unemployed | 12 | 41.4 | 4 | 23.5 | ||
| Disabled | 5 | 17.2 | 6 | 35.3 | ||
| Student | 2 | 6.9 | 2 | 11.8 | ||
| Oral Treatment b | ||||||
| Antidepressants | 13 | 44.8 | 4 | 23.5 | χ2 = 2.087 | 0.149 |
| Antipsychotics | 5 | 17.2 | 2 | 11.7 | χ2 = 0.249 | 0.618 |
| Benzodiazepines | 9 | 31.0 | 8 | 47.1 | χ2 = 0.712 | 0.399 |
| Mood Stabilizers | 9 | 31.0 | 8 | 47.1 | χ2 = 1.181 | 0.277 |
| Patients with previous hospitalizations b | 16 | 55.2 | 9 | 52.9 | χ2 = 3.641 | 0.725 |
| Duration of illness (years) a | 15.3 | 8.1 | 12.5 | 6.5 | t = 1.343 | 0.242 |
| PANSS a | ||||||
| Total | 84.5 | 16.9 | 96.6 | 22.5 | t = 0.558 | 0.580 |
| Positive | 18.1 | 7.6 | 19.0 | 5.2 | t = −0.221 | 0.826 |
| Negative | 21.6 | 6.9 | 26.0 | 9.8 | t = 1.140 | 0.260 |
| General | 44.7 | 7.6 | 51.2 | 11.6 | t = 0.399 | 0.692 |
| QoLS a | 67.5 | 15.5 | 61.3 | 18.7 | t = −0.145 | 0.886 |
| PSP a | 53.2 | 11.7 | 48.4 | 16.5 | t = −0.435 | 0.665 |
| Stroop a | −2.1 | 6.9 | −3.2 | 8.4 | t = 1.268 | 0.212 |
| RCFT a | ||||||
| Accuracy | 32.1 | 6.0 | 30.9 | 3.8 | t = 2.136 | 0.083 |
| Percentage of Recall | 51.8 | 15.5 | 56.3 | 17.2 | t = −1.173 | 0.247 |
| Order | 1.8 | 0.9 | 1.5 | 0.9 | t = 0.317 | 0.753 |
| Style | 1.5 | 0.5 | 1.5 | .5 | t = 0.464 | 0.645 |
| CCI | 1.3 | 0.5 | 1.3 | 0.4 | t = 0.913 | 0.366 |
| OS | 4.7 | 2.0 | 3.8 | 1.5 | t = 1.509 | 0.139 |
| Scale | Subscale | Groups | t0 | t1 | t2 | t3 | F | p | ƞ2 a |
|---|---|---|---|---|---|---|---|---|---|
| PANSS Total | Total | Ari-LAI | 84.5 | 72.1 | 65.2 | 67.1 | Time: F = 10.990 | <0.001 | 0.622 |
| PP1M | 96.6 | 74.1 | 71.3 | 70.1 | Group: F = 0.821 | 0.498 | |||
| Positive | Ari-LAI | 18.1 | 14.9 | 13.2 | 14.2 | Time: F = 3.712 | 0.028 | 0.358 | |
| PP1M | 19 | 14.1 | 12.6 | 13.7 | Group: F = 0.159 | 0.923 | |||
| Negative | Ari-LAI | 21.6 | 19.4 | 17.7 | 18.2 | Time: F = 5.928 | 0.005 | 0.471 | |
| PP1M | 26 | 20.1 | 20.2 | 21 | Group: F = 0.768 | 0.525 | |||
| General | Ari-LAI | 44.7 | 38.3 | 34.5 | 34.7 | Time: F = 13.280 | <0.001 | 0.667 777 | |
| PP1M | 51.2 | 39.4 | 38.6 | 35.7 | Group: F = 1.303 | 0.301 | |||
| QoLS | Ari-LAI | 67.5 | 79.12 | 80.0 | 81.37 | Time: F = 5.856 | 0.016 | 0.422 | |
| PP1M | 61.3 | 70.45 | 76.2 | 77.40 | Group: F = 1.19 | 0.307 | |||
| PSP | Ari-LAI | 53.2 | 62.1 | 63.4 | 65.9 | Time: F = 9.530 | <0.001 | 0.588 | |
| PP1M | 48.4 | 58.4 | 61.4 | 60.6 | Group: F = 0.359 | 0.783 | |||
| Stroop | Ari-LAI | −2.1 | −1.5 | 2.2 | 2.6 | Time: F = 4.120 | 0.002 | 0.382 | |
| PP1M | −3.2 | −0.6 | 4.8 | −1 | Group: F = 0.572 | 0.064 | |||
| RCFT Accuracy | Accuracy | Ari-LAI | 32.1 | 34.4 | 34.1 | 33.9 | Time: F = 1.271 | 0.311 | |
| PP1M | 30.9 | 31.8 | 33.8 | 32.9 | Group: F = 0.599 | 0.145 | |||
| Percentage of Recall | Ari-LAI | 51.8 | 65.3 | 72.4 | 68.8 | Time: F = 4.968 | 0.001 | 0.427 | |
| PP1M | 56.3 | 53.8 | 61.4 | 56.2xf | Group: F = 0.599 | 0.623 | |||
| Order | Ari-LAI | 1.8 | 2.4 | 2.2 | 2 | Time: F = 2.225 | 0.117 | ||
| PP1M | 1.5 | 1.6 | 1.4 | 1.8 | Group: F = 1.212 | 0.331 | |||
| Style | Ari-LAI | 1.5 | 1.7 | 1.8 | 1.7 | Time: F = 1.190 | 0.339 | ||
| PP1M | 1.5 | 1.4 | 1.4 | 1.6 | Group: F = 1.842 | 0.172 | |||
| CCI | Ari-LAI | 1.3 | 1.6 | 1.6 | 1.5 | Time: F = 1.046 | 0.394 | ||
| PP1M | 1.3 | 1.2 | 1.2 | 1.3 | Group: F = 1.556 | 0.231 | |||
| OS | Ari-LAI | 4.7 | 5 | 4.9 | 5.5 | Time: F = 6.367 | 0.003 | 0.489 | |
| PP1M | 3.8 | 4.4 | 4.4 | 5.7 | Group: F = 0.918 | 0.450 |
| Variable | Groups | t0 | t3 | F | p | ƞ2 a |
|---|---|---|---|---|---|---|
| Triglycerides (mg/dL) | Ari-LAI | 160 | 146 | Time: F = 3.394 | 0.079 | |
| PP1M | 147 | 130 | Group: F = 0.062 | 0.806 | ||
| Total cholesterol (mg/dL) | Ari-LAI | 191 | 174 | Time: F = 11.375 | 0.003 | 0.331 |
| PP1M | 183 | 163 | Group: F = 0.112 | 0.74 | ||
| Prolactin (ng/mL) | Ari-LAI | 17.6 | 10.4 | Time: F = 3.827 | 0.067 | |
| PP1M | 41.1 | 34.2 | Group: F = 0.03 | 0.959 | ||
| Neutrophils (number 103/mm3) | Ari-LAI | 5.8 | 5.9 | Time: F = 0.02 | 0.964 | |
| PP1M | 4.9 | 4.8 | Group: F = 0.252 | 0.62 | ||
| Platelets (number 103/mm3) | Ari-LAI | 272 | 270 | Time: F = 0.36 | 0.852 | |
| PP1M | 240 | 245 | Group: F = 0.220 | 0.643 | ||
| TSH (µU/mL) | Ari-LAI | 2.3 | 2.1 | Time: F = 1.844 | 0.189 | |
| PP1M | 2.2 | 2.1 | Group: F = 0.380 | 0.544 |
| Scale | Subscale | Groups | t2 | t3 | F | p | ƞ2 |
|---|---|---|---|---|---|---|---|
| PANSS | Total | PP1M > PP3M | 65 | 51 | Time: F = 26.133 | 0.01 | 0.897 |
| Positive | PP1M > PP3M | 13 | 10 | Time: F = 8.593 | 0.061 | 0.741 | |
| Negative | PP1M > PP3M | 21 | 15 | Time: F = 10.475 | 0.05 | 0.777 | |
| General | PP1M > PP3M | 32 | 27 | Time: F = 13.636 | 0.03 | 0.82 | |
| PSP | PP1M > PP3M | 59 | 73 | Time: F = 30.351 | 0.01 | 0.91 | |
| Triglycerides (mg/dL) | PP1M > PP3M | 155 | 135 | Time: F = 19.469 | 0.022 | 0.866 | |
| Total cholesterol (mg/dL) | PP1M > PP3M | 190 | 176 | Time: F = 3.319 | 0.166 | 0.525 | |
| Prolactin (ng/mL) | PP1M > PP3M | 34.3 | 27.2 | Time: F = 8.610 | 0.06 | 0.742 | |
| Neutrophils (number 103/mm3) | PP1M > PP3M | 5.2 | 5.2 | Time: F = 0.009 | 0.932 | 0.003 | |
| Platelets (number 103/mm3) | PP1M > PP3M | 274 | 280 | Time: F = 0.549 | 0.513 | 0.155 | |
| TSH (µU/mL) | PP1M > PP3M | 2.22 | 2.07 | Time: F = 14.575 | 0.032 | 0.829 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Filippis, R.; Staltari, F.A.; Aloi, M.; Carbone, E.A.; Rania, M.; Destefano, L.; Steardo Jr., L.; Segura-Garcia, C.; De Fazio, P. Effectiveness of SGA-LAIs on Clinical, Cognitive, and Social Domains in Schizophrenia: Results from a Prospective Naturalistic Study. Brain Sci. 2023, 13, 577. https://doi.org/10.3390/brainsci13040577
de Filippis R, Staltari FA, Aloi M, Carbone EA, Rania M, Destefano L, Steardo Jr. L, Segura-Garcia C, De Fazio P. Effectiveness of SGA-LAIs on Clinical, Cognitive, and Social Domains in Schizophrenia: Results from a Prospective Naturalistic Study. Brain Sciences. 2023; 13(4):577. https://doi.org/10.3390/brainsci13040577
Chicago/Turabian Stylede Filippis, Renato, Filippo Antonio Staltari, Matteo Aloi, Elvira Anna Carbone, Marianna Rania, Laura Destefano, Luca Steardo Jr., Cristina Segura-Garcia, and Pasquale De Fazio. 2023. "Effectiveness of SGA-LAIs on Clinical, Cognitive, and Social Domains in Schizophrenia: Results from a Prospective Naturalistic Study" Brain Sciences 13, no. 4: 577. https://doi.org/10.3390/brainsci13040577
APA Stylede Filippis, R., Staltari, F. A., Aloi, M., Carbone, E. A., Rania, M., Destefano, L., Steardo Jr., L., Segura-Garcia, C., & De Fazio, P. (2023). Effectiveness of SGA-LAIs on Clinical, Cognitive, and Social Domains in Schizophrenia: Results from a Prospective Naturalistic Study. Brain Sciences, 13(4), 577. https://doi.org/10.3390/brainsci13040577











