Interpretable Machine Learning Model Predicting Early Neurological Deterioration in Ischemic Stroke Patients Treated with Mechanical Thrombectomy: A Retrospective Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Patient Variables and Data Definitions
2.3. Statistical Analysis
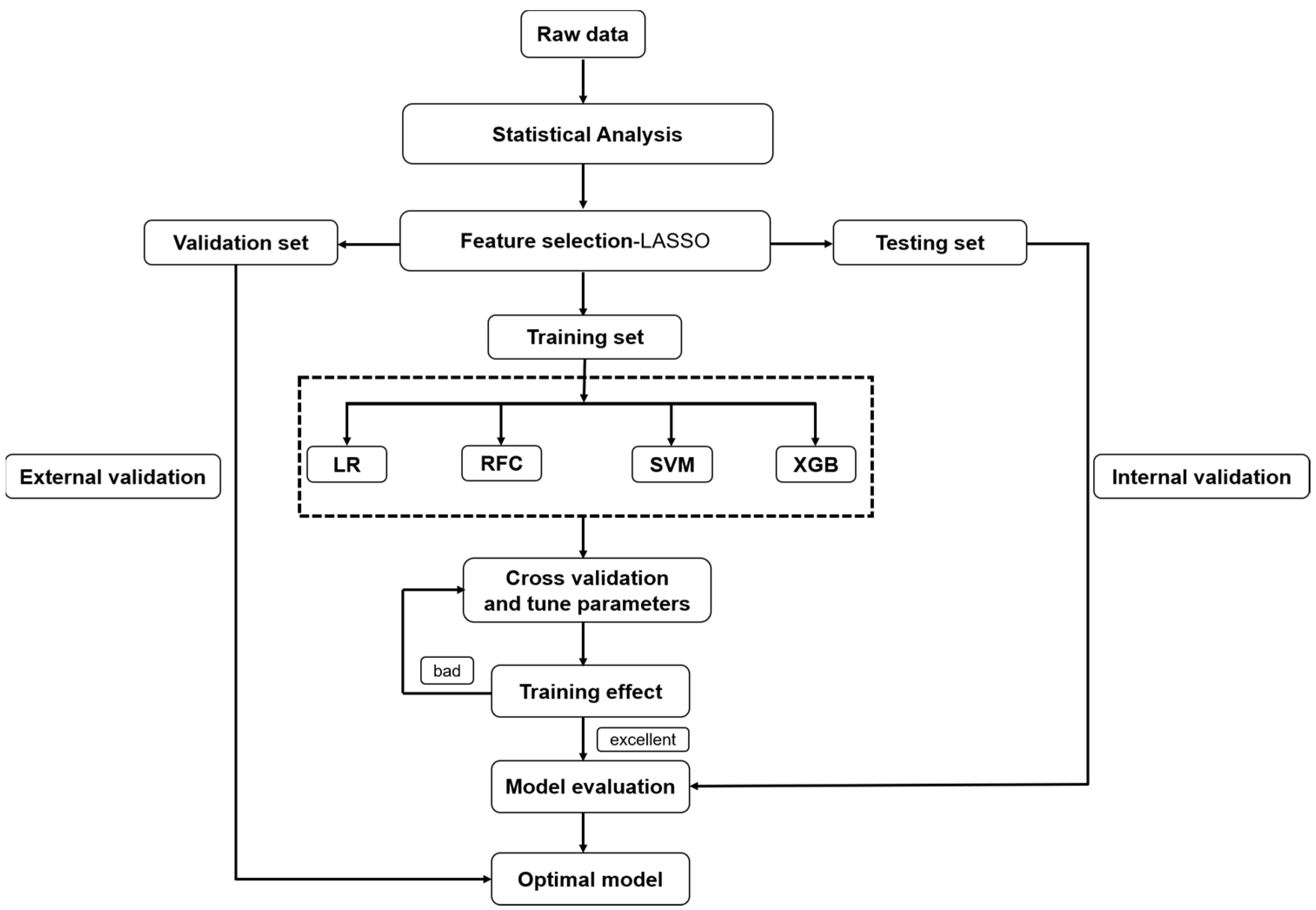
2.4. Data Processing and Feature Selection
2.5. Modeling Strategies
2.6. Model Evaluation
2.7. Explanation of the Model
3. Results
3.1. Study Population
3.2. Feature Selection
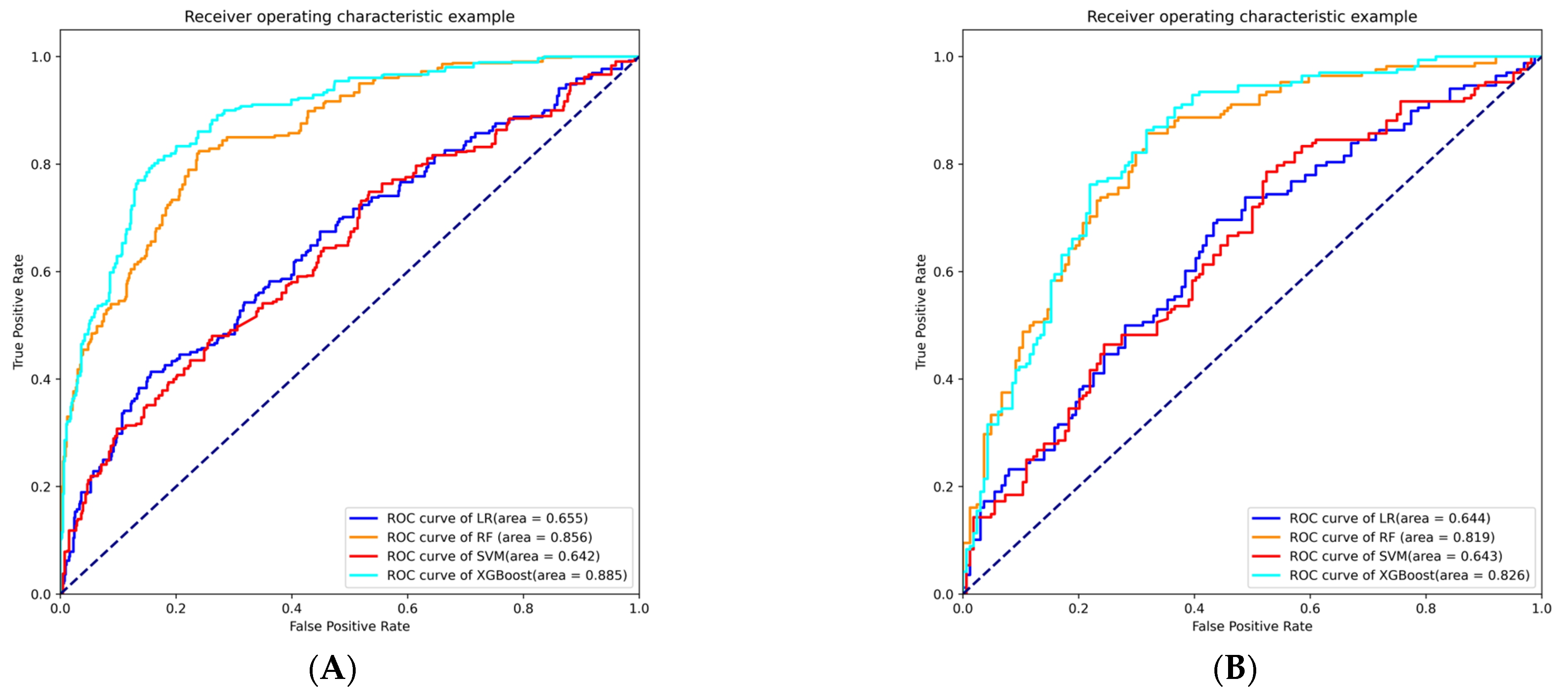
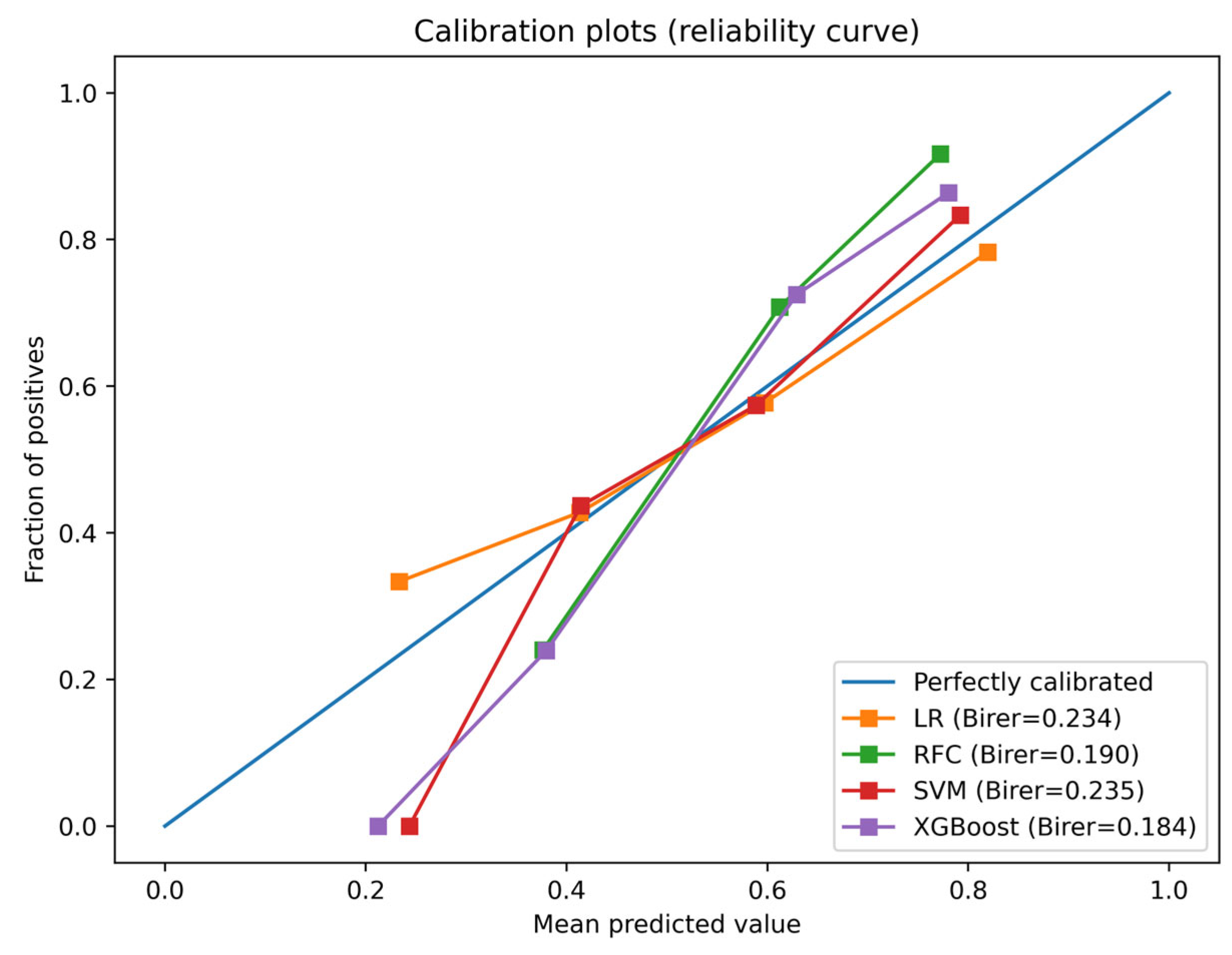
3.3. Model Building and Evaluation
3.4. Explanation of the Model at the Feature Level
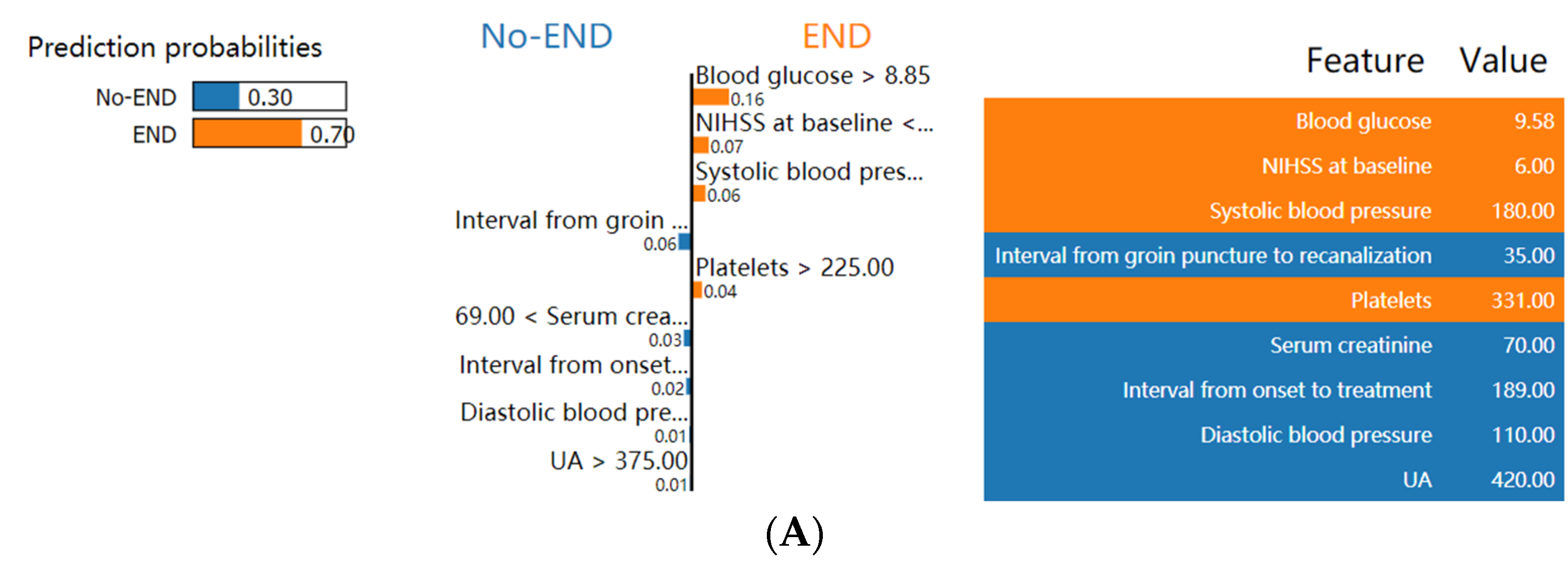
3.5. Explanation of the Model at the Individual Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Saver, J.L.; Altman, H. Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke 2012, 43, 1537–1541. [Google Scholar] [CrossRef]
- Mori, M.; Naganuma, M.; Okada, Y.; Hasegawa, Y.; Shiokawa, Y.; Nakagawara, J.; Furui, E.; Kimura, K.; Yamagami, H.; Kario, K.; et al. Early neurological deterioration within 24 hours after intravenous rt-PA therapy for stroke patients: The Stroke Acute Management with Urgent Risk Factor Assessment and Improvement rt-PA Registry. Cerebrovasc. Dis. 2012, 34, 140–146. [Google Scholar] [CrossRef]
- Dharmasaroja, P.A.; Muengtaweepongsa, S.; Dharmasaroja, P. Early outcome after intravenous thrombolysis in patients with acute ischemic stroke. Neurol. India 2011, 59, 351–354. [Google Scholar] [CrossRef]
- Davalos, A.; Toni, D.; Iweins, F.; Lesaffre, E.; Bastianello, S.; Castillo, J. Neurological deterioration in acute ischemic stroke: Potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke 1999, 30, 2631–2636. [Google Scholar] [CrossRef]
- Simonsen, C.Z.; Schmitz, M.L.; Madsen, M.H.; Mikkelsen, I.K.; Chandra, R.V.; Leslie-Mazwi, T.; Andersen, G. Early neurological deterioration after thrombolysis: Clinical and imaging predictors. Int. J. Stroke 2016, 11, 776–782. [Google Scholar] [CrossRef]
- Bourcier, R.; Goyal, M.; Muir, K.W.; Desal, H.; Dippel, D.W.J.; Majoie, C.; van Zwam, W.H.; Jovin, T.G.; Mitchell, P.J.; Demchuk, A.M.; et al. Risk factors of unexplained early neurological deterioration after treatment for ischemic stroke due to large vessel occlusion: A post hoc analysis of the HERMES study. J. Neurointerv. Surg. 2022, 15, 221–226. [Google Scholar] [CrossRef]
- Haeusler, K.G.; Gerischer, L.M.; Vatankhah, B.; Audebert, H.J.; Nolte, C.H. Impact of hospital admission during nonworking hours on patient outcomes after thrombolysis for stroke. Stroke 2011, 42, 2521–2525. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, X.; Gong, Y.; Liu, Y.; Wang, S.; Li, Z.; Chen, W.; Zhou, F.; Zhou, J.; Jiang, T.; et al. A novel nomogram to predict early neurological deterioration in patients with acute ischaemic stroke. Eur. J. Neurol. 2020, 27, 1996–2005. [Google Scholar] [CrossRef]
- Tanaka, R.; Ueno, Y.; Miyamoto, N.; Yamashiro, K.; Tanaka, Y.; Shimura, H.; Hattori, N.; Urabe, T. Impact of diabetes and prediabetes on the short-term prognosis in patients with acute ischemic stroke. J. Neurol. Sci. 2013, 332, 45–50. [Google Scholar] [CrossRef]
- Kim, B.J.; Park, J.M.; Kang, K.; Lee, S.J.; Ko, Y.; Kim, J.G.; Cha, J.K.; Kim, D.H.; Nah, H.W.; Han, M.K.; et al. ERRATUM: Table Correction: Case Characteristics, Hyperacute Treatment, and Outcome Information from the Clinical Research Center for Stroke-Fifth Division Registry in South Korea. J. Stroke 2015, 17, 377–378. [Google Scholar] [CrossRef]
- Sun, D.; Tong, X.; Huo, X.; Jia, B.; Raynald; Wang, A.; Ma, G.; Ma, N.; Gao, F.; Mo, D.; et al. Unexplained early neurological deterioration after endovascular treatment for acute large vessel occlusion: Incidence, predictors, and clinical impact: Data from ANGEL-ACT registry. J. Neurointerv. Surg. 2022, 14, 875–880. [Google Scholar] [CrossRef]
- Girot, J.B.; Richard, S.; Gariel, F.; Sibon, I.; Labreuche, J.; Kyheng, M.; Gory, B.; Dargazanli, C.; Maier, B.; Consoli, A.; et al. Predictors of Unexplained Early Neurological Deterioration After Endovascular Treatment for Acute Ischemic Stroke. Stroke 2020, 51, 2943–2950. [Google Scholar] [CrossRef]
- Bhole, R.; Nouer, S.S.; Tolley, E.A.; Turk, A.; Siddiqui, A.H.; Alexandrov, A.V.; Arthur, A.S.; Mocco, J.; COMPASS Investigators. Predictors of early neurologic deterioration (END) following stroke thrombectomy. J. Neurointerv. Surg. 2022. [Google Scholar] [CrossRef]
- Yao, Z.; Mao, C.; Ke, Z.; Xu, Y. An explainable machine learning model for predicting the outcome of ischemic stroke after mechanical thrombectomy. J. Neurointerv. Surg. 2022, jnis-2022-019598. [Google Scholar] [CrossRef]
- Jabal, M.S.; Joly, O.; Kallmes, D.; Harston, G.; Rabinstein, A.; Huynh, T.; Brinjikji, W. Interpretable Machine Learning Modeling for Ischemic Stroke Outcome Prediction. Front. Neurol. 2022, 13, 884693. [Google Scholar] [CrossRef]
- Mistry, E.A.; Yeatts, S.; de Havenon, A.; Mehta, T.; Arora, N.; De Los Rios La Rosa, F.; Starosciak, A.K.; Siegler, J.E., 3rd; Mistry, A.M.; Yaghi, S.; et al. Predicting 90-Day Outcome After Thrombectomy: Baseline-Adjusted 24-Hour NIHSS Is More Powerful Than NIHSS Score Change. Stroke 2021, 52, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, T.; Zhang, J.; Wang, X.; Cui, X.; Chen, N.; Zhou, J.; Jiang, F.; Zhu, J.; Zou, J. Dynamic Prediction of Mechanical Thrombectomy Outcome for Acute Ischemic Stroke Patients Using Machine Learning. Brain Sci. 2022, 12, 938. [Google Scholar] [CrossRef]
- Zorman, M.; Verlic, M. Explanatory approach for evaluation of machine learning-induced knowledge. J. Int. Med. Res. 2009, 37, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.H.; Kim, Y.; Lee, K.S.; Park, H.S. Development and Clinical Evaluation of a Web-Based Upper Limb Home Rehabilitation System Using a Smartwatch and Machine Learning Model for Chronic Stroke Survivors: Prospective Comparative Study. JMIR Mhealth Uhealth 2020, 8, e17216. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Ma, J.; Fisher, J.; Kreisberg, J.F.; Raphael, B.J.; Ideker, T. Visible Machine Learning for Biomedicine. Cell 2018, 173, 1562–1565. [Google Scholar] [CrossRef]
- Tjoa, E.; Guan, C. A Survey on Explainable Artificial Intelligence (XAI): Toward Medical XAI. IEEE Trans. Neural. Netw. Learn Syst. 2021, 32, 4793–4813. [Google Scholar] [CrossRef]
- von Kummer, R.; Broderick, J.P.; Campbell, B.C.; Demchuk, A.; Goyal, M.; Hill, M.D.; Treurniet, K.M.; Majoie, C.B.; Marquering, H.A.; Mazya, M.V.; et al. The Heidelberg Bleeding Classification: Classification of Bleeding Events After Ischemic Stroke and Reperfusion Therapy. Stroke 2015, 46, 2981–2986. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, K.; Wang, H.; Gong, P.; Jiang, T.; Xie, Y.; Sheng, L.; Liu, D.; Liu, X.; Xu, G. Nomogram to Predict Mortality of Endovascular Thrombectomy for Ischemic Stroke Despite Successful Recanalization. J. Am. Heart Assoc. 2020, 9, e014899. [Google Scholar] [CrossRef]
- Siegler, J.E.; Boehme, A.K.; Kumar, A.D.; Gillette, M.A.; Albright, K.C.; Martin-Schild, S. What change in the National Institutes of Health Stroke Scale should define neurologic deterioration in acute ischemic stroke? J. Stroke Cerebrovasc. Dis. 2013, 22, 675–682. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression shrinkage and selection via the lasso: A retrospective. J. R. Stat. Soc. Ser. B-Stat. Methodol. 2011, 73, 273–282. [Google Scholar] [CrossRef]
- Jelovsek, J.E.; Hill, A.J.; Chagin, K.M.; Kattan, M.W.; Barber, M.D. Predicting Risk of Urinary Incontinence and Adverse Events After Midurethral Sling Surgery in Women. Obstet. Gynecol. 2016, 127, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, R.; Bajorath, J. Interpretation of Compound Activity Predictions from Complex Machine Learning Models Using Local Approximations and Shapley Values. J. Med. Chem. 2020, 63, 8761–8777. [Google Scholar] [CrossRef]
- Thorsen-Meyer, H.C.; Nielsen, A.B.; Nielsen, A.P.; Kaas-Hansen, B.S.; Toft, P.; Schierbeck, J.; Strom, T.; Chmura, P.J.; Heimann, M.; Dybdahl, L.; et al. Dynamic and explainable machine learning prediction of mortality in patients in the intensive care unit: A retrospective study of high-frequency data in electronic patient records. Lancet Digit. Health 2020, 2, e179–e191. [Google Scholar] [CrossRef]
- Yu, Q.; Mao, X.; Fu, Z.; Luo, S.; Huang, Q.; Chen, Q.; Li, S.; Zhang, J.; Qiu, Y.; Wu, Y.; et al. Fasting blood glucose as a predictor of progressive infarction in men with acute ischemic stroke. J. Int. Med. Res. 2022, 50, 3000605221132416. [Google Scholar] [CrossRef] [PubMed]
- Seners, P.; Turc, G.; Oppenheim, C.; Baron, J.C. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: A systematic review with pathophysiological implications. J. Neurol. Neurosurg. Psychiatry 2015, 86, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, R.Y.; Cha, J.K.; Rha, H.W.; Kang, M.J.; Kim, D.H.; Park, H.S.; Choi, J.H.; Huh, J.T.; Lee, I.K. Pre-stroke glycemic control is associated with early neurologic deterioration in acute atrial fibrillation-related ischemic stroke. eNeurologicalSci 2017, 8, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Guo, W.; Tang, T.; Tao, L.; Gong, K.; Zhang, X. Relationship between high-sensitivity C-reactive protein and early neurological deterioration in stroke patients with and without atrial fibrillation. Heart Lung 2020, 49, 193–197. [Google Scholar] [CrossRef]
- Jamwal, S.; Sharma, S. Vascular endothelium dysfunction: A conservative target in metabolic disorders. Inflamm. Res. 2018, 67, 391–405. [Google Scholar] [CrossRef]
- Arboix, A.; Oliveres, M.; Massons, J.; Pujades, R.; Garcia-Eroles, L. Early differentiation of cardioembolic from atherothrombotic cerebral infarction: A multivariate analysis. Eur. J. Neurol. 1999, 6, 677–683. [Google Scholar] [CrossRef]
- Desilles, J.P.; Syvannarath, V.; Ollivier, V.; Journe, C.; Delbosc, S.; Ducroux, C.; Boisseau, W.; Louedec, L.; Di Meglio, L.; Loyau, S.; et al. Exacerbation of Thromboinflammation by Hyperglycemia Precipitates Cerebral Infarct Growth and Hemorrhagic Transformation. Stroke 2017, 48, 1932–1940. [Google Scholar] [CrossRef]
- Petersen, N.H.; Ortega-Gutierrez, S.; Wang, A.; Lopez, G.V.; Strander, S.; Kodali, S.; Silverman, A.; Zheng-Lin, B.; Dandapat, S.; Sansing, L.H.; et al. Decreases in Blood Pressure During Thrombectomy Are Associated with Larger Infarct Volumes and Worse Functional Outcome. Stroke 2019, 50, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Tong, X.; Sun, X.; Gao, F.; Mo, D.; Wang, Y.; Miao, Z. Early Neurological Deterioration Despite Recanalization in Basilar Artery Occlusion Treated by Endovascular Therapy. Front. Neurol. 2020, 11, 592003. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, W.; Zhang, Z.; Xiao, L.; Duan, Z.; Liu, D.; Xiong, Y.; Zhu, W.; Lu, G.; Liu, X. Asymmetrical cortical vessel sign on susceptibility-weighted imaging: A novel imaging marker for early neurological deterioration and unfavorable prognosis. Eur. J. Neurol. 2014, 21, 1411–1418. [Google Scholar] [CrossRef]
- Seo, W.K.; Seok, H.Y.; Kim, J.H.; Park, M.H.; Yu, S.W.; Oh, K.; Koh, S.B.; Park, K.W. C-reactive protein is a predictor of early neurologic deterioration in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2012, 21, 181–186. [Google Scholar] [CrossRef]
- Kwon, H.M.; Lee, Y.S.; Bae, H.J.; Kang, D.W. Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke 2014, 45, 871–873. [Google Scholar] [CrossRef]


| Variables | All Patients, n = 985 | with END, n = 157 | without END, n = 828 | p Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years, median (IQR) | 71.0(63.0–79.0) | 71.0(63.5–80.0) | 71.0(63.0–79.0) | 0.620 |
| Female, n (%) | 377(38.3) | 61(38.9) | 316(38.2) | 0.871 |
| Vascular risk factors, n (%) | ||||
| Hypertension | 677(68.7) | 117(74.5) | 560(67.6) | 0.088 |
| Diabetes mellitus | 232(23.6) | 46(29.3) | 186(22.5) | 0.064 |
| Hyperlipidemia | 17(1.7) | 3(1.9) | 14(1.7) | 0.846 |
| Coronary artery disease | 142(14.4) | 13(8.3) | 129(15.6) | 0.017 |
| Atrial fibrillation | 311(31.6) | 43(27.4) | 268(32.4) | 0.219 |
| Previous stroke or TIA | 215(21.8) | 30(19.1) | 185(22.3) | 0.368 |
| Smoking | 303(30.8) | 45(28.7) | 258(31.2) | 0.524 |
| Drinking | 232(23.6) | 28(17.8) | 204(24.6) | 0.065 |
| Clinical data, median (IQR) | ||||
| Systolic blood pressure, mmHg | 138.0(123.0–155.0) | 140.0(125.0–160.0) | 138.0(123.0–154.0) | 0.196 |
| Diastolic blood pressure, mmHg | 82.0(73.0–93.0) | 82.0(71.5–93.5) | 82.5(73.0–93.0) | 0.991 |
| NIHSS at baseline | 14.0(11.0–18.0) | 14.0(8.5–18.0) | 14.0(11.0–18.0) | 0.186 |
| Interval from onset to treatment, min | 270.0(190.0–410.0) | 300.0(189.5–500.0) | 270.0(190.0–404.3) | 0.426 |
| Interval from groin puncture to recanalization, min | 70.0(50.0–95.0) | 72.0(55.0–104.5) | 68.0(50.0–94.3) | 0.089 |
| Cause of stroke, n (%) | 0.067 | |||
| Atherosclerotic | 448(45.5) | 76(48.4) | 372(44.9) | |
| Cardioembolic | 433(44) | 58(36.9) | 375(45.3) | |
| Others | 104(10.6) | 23(14.6) | 81(9.8) | |
| Endovascular therapy, n (%) | ||||
| Intravenous thrombolysis | 379(38.5) | 64(40.8) | 315(38) | 0.521 |
| Tirofiban | 425(43.1) | 74(47.1) | 351(42.4) | 0.271 |
| sICH | 139(14.1) | 39(24.8) | 100(12.1) | 0.000 |
| Recanalization | 883(89.6) | 134(85.4) | 749(90.5) | 0.054 |
| Lesion location, n (%) | ||||
| Anterior circulation | 790(80.2) | 117(74.5) | 673(81.3) | 0.051 |
| Posterior circulation | 197(20) | 40(25.5) | 157(19) | 0.061 |
| Procedural modes | ||||
| Aspiration only, n (%) | 34(3.5) | 8(5.1) | 26(3.1) | 0.218 |
| Stent retriever only, n (%) | 740(75.1) | 104(66.2) | 636(76.8) | 0.005 |
| Stent retriever/aspiration with rescue therapy, n (%) | 228(23.1) | 47(29.9) | 181(21.9) | 0.028 |
| Passes of Stent retriever, median (IQR) | 2.0(1.0–3.0) | 2.0(1.0–3.0) | 2.0(1.0–3.0) | 0.830 |
| Laboratory data, median (IQR) | ||||
| Platelets, μmol/L | 180.0(145.0–220.0) | 185.0(145.5–228.5) | 180.0(145.0–219.0) | 0.433 |
| Serum creatinine, μmol/L | 69.0(58.0–83.5) | 69.7(55.8–90.5) | 69.0(58.0–83.0) | 0.825 |
| Blood glucose, mmol/L | 6.6(5.4–8.2) | 7.9(6.1–9.8) | 6.4(5.3–7.8) | 0.000 |
| Total cholesterol, mmol/L | 4.1(3.4–5.0) | 4.1(3.5–5.0) | 4.1(3.4–4.9) | 0.476 |
| Triglyceride, mmol/L | 1.0(0.8–1.5) | 1.1(0.8–1.5) | 1.0(0.7–1.4) | 0.074 |
| High-density lipoprotein, mmol/L | 1.1(0.9–1.3) | 1.1(0.9–1.3) | 1.1(0.9–1.3) | 0.391 |
| Low-density lipoprotein, mmol/L | 2.5(1.9–3.1) | 2.4(1.8–3.2) | 2.5(1.9–3.1) | 0.973 |
| UA, μmol/L | 314.0(241.5–383.0) | 321.0(246.0–382.5) | 313.0(241.0–383.0) | 0.604 |
| Glycated hemoglobin, mmol/L | 5.9(5.6–6.6) | 6.2(5.7–6.8) | 5.9(5.6–6.5) | 0.004 |
| Homocysteine, μmol/L | 13.1(10.9–15.7) | 13.1(10.4–15.7) | 13.1(11.0–15.7) | 0.697 |
| Model | AUC | Sensitivity | Specificity | Accuracy | Brier | Youden Index |
|---|---|---|---|---|---|---|
| LR | 0.644 | 0.387 | 0.793 | 0.587 | 0.234 | 0.565 |
| RF | 0.819 | 0.827 | 0.689 | 0.759 | 0.190 | 0.480 |
| SVM | 0.643 | 0.482 | 0.713 | 0.596 | 0.235 | 0.520 |
| XGBoost | 0.826 | 0.798 | 0.713 | 0.756 | 0.184 | 0.509 |
| Model | AUC | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|
| XGBoost | 0.846 | 0.750 | 0.836 | 0.815 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Hu, Y.; Pan, X.; Lou, S.; Zou, J.; Deng, Q.; Zhang, Q.; Zhou, J.; Zhu, J. Interpretable Machine Learning Model Predicting Early Neurological Deterioration in Ischemic Stroke Patients Treated with Mechanical Thrombectomy: A Retrospective Study. Brain Sci. 2023, 13, 557. https://doi.org/10.3390/brainsci13040557
Yang T, Hu Y, Pan X, Lou S, Zou J, Deng Q, Zhang Q, Zhou J, Zhu J. Interpretable Machine Learning Model Predicting Early Neurological Deterioration in Ischemic Stroke Patients Treated with Mechanical Thrombectomy: A Retrospective Study. Brain Sciences. 2023; 13(4):557. https://doi.org/10.3390/brainsci13040557
Chicago/Turabian StyleYang, Tongtong, Yixing Hu, Xiding Pan, Sheng Lou, Jianjun Zou, Qiwen Deng, Qingxiu Zhang, Junshan Zhou, and Junrong Zhu. 2023. "Interpretable Machine Learning Model Predicting Early Neurological Deterioration in Ischemic Stroke Patients Treated with Mechanical Thrombectomy: A Retrospective Study" Brain Sciences 13, no. 4: 557. https://doi.org/10.3390/brainsci13040557
APA StyleYang, T., Hu, Y., Pan, X., Lou, S., Zou, J., Deng, Q., Zhang, Q., Zhou, J., & Zhu, J. (2023). Interpretable Machine Learning Model Predicting Early Neurological Deterioration in Ischemic Stroke Patients Treated with Mechanical Thrombectomy: A Retrospective Study. Brain Sciences, 13(4), 557. https://doi.org/10.3390/brainsci13040557






