Paraventricular Mast Cell-Derived Histamine Activates CRH Neurons to Mediate Adult Visceral Hypersensitivity Induced by Neonatal Maternal Separation
Abstract
:1. Introduction
2. Methods and Materials
2.1. Animals
2.2. Reagents
2.3. NMS Induction
2.4. Assessment of Visceral Sensitivity
2.5. Intra-PVN Microinfusion
2.6. Western Blot Analysis
2.7. Immunofluorescence Staining
2.8. Mast Cell Staining by Toluidine Blue
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Statistical Analysis
3. Results
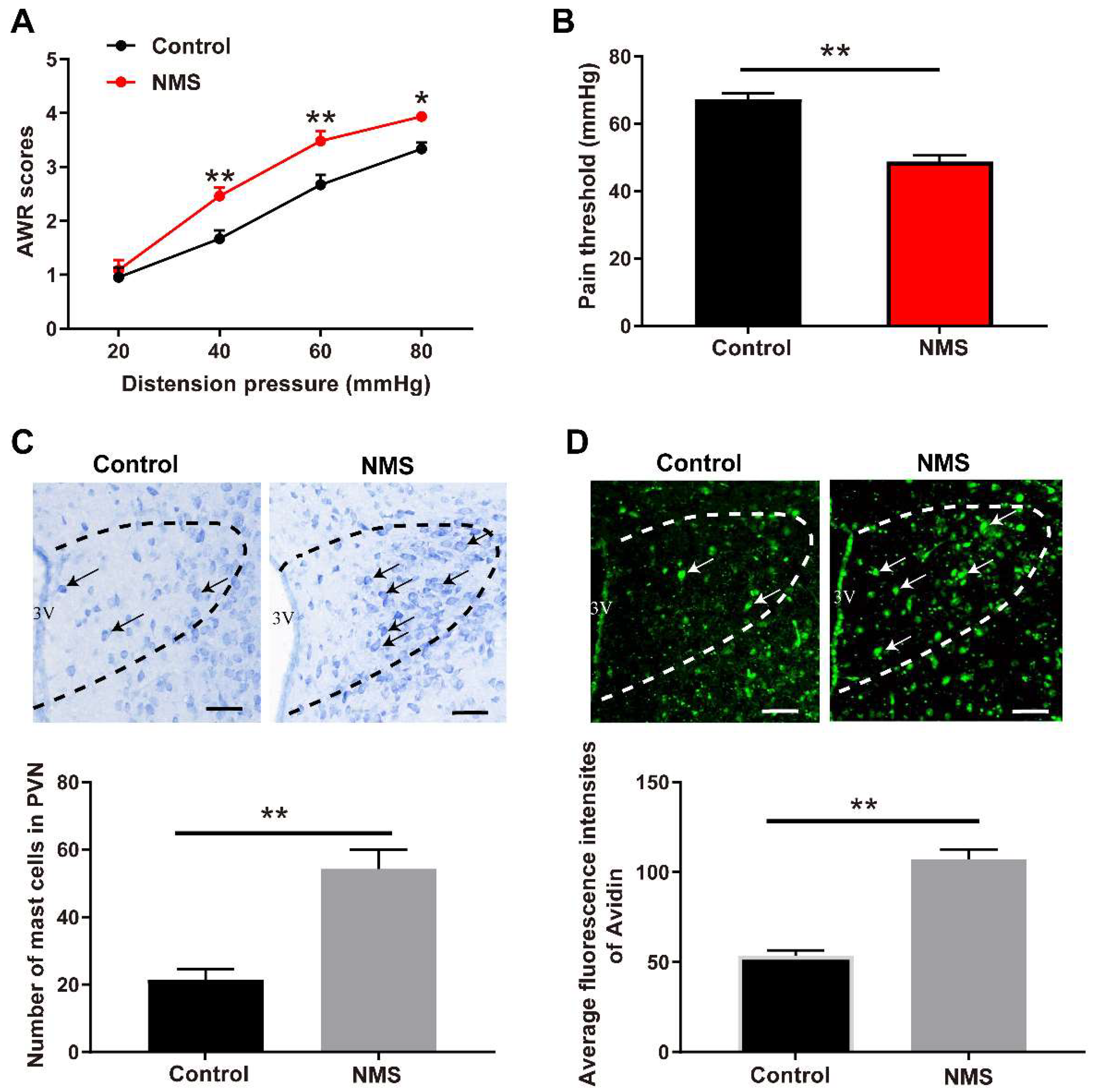
3.1. NMS-Induced Visceral Hypersensitivity in Adulthood Was Accompanied with Mast Cell Activation in PVN
3.2. Pharmacological Inhibition of Mast Cells Alleviates NMS-Induced Adult Visceral Hypersensitivity
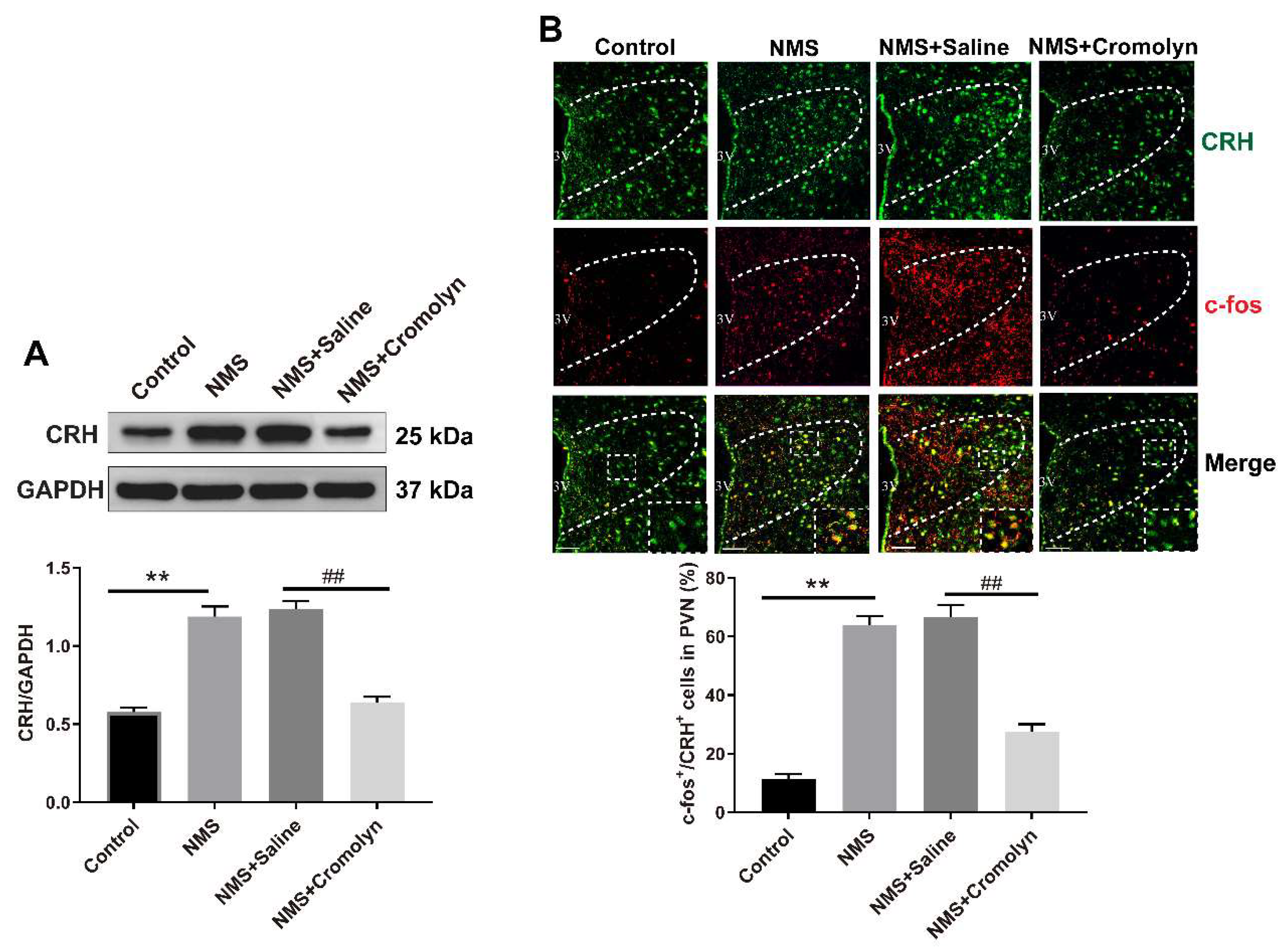
3.3. Inhibition of Mast Cells Alleviates the NMS-Induced CRH Neuronal Activation in PVN
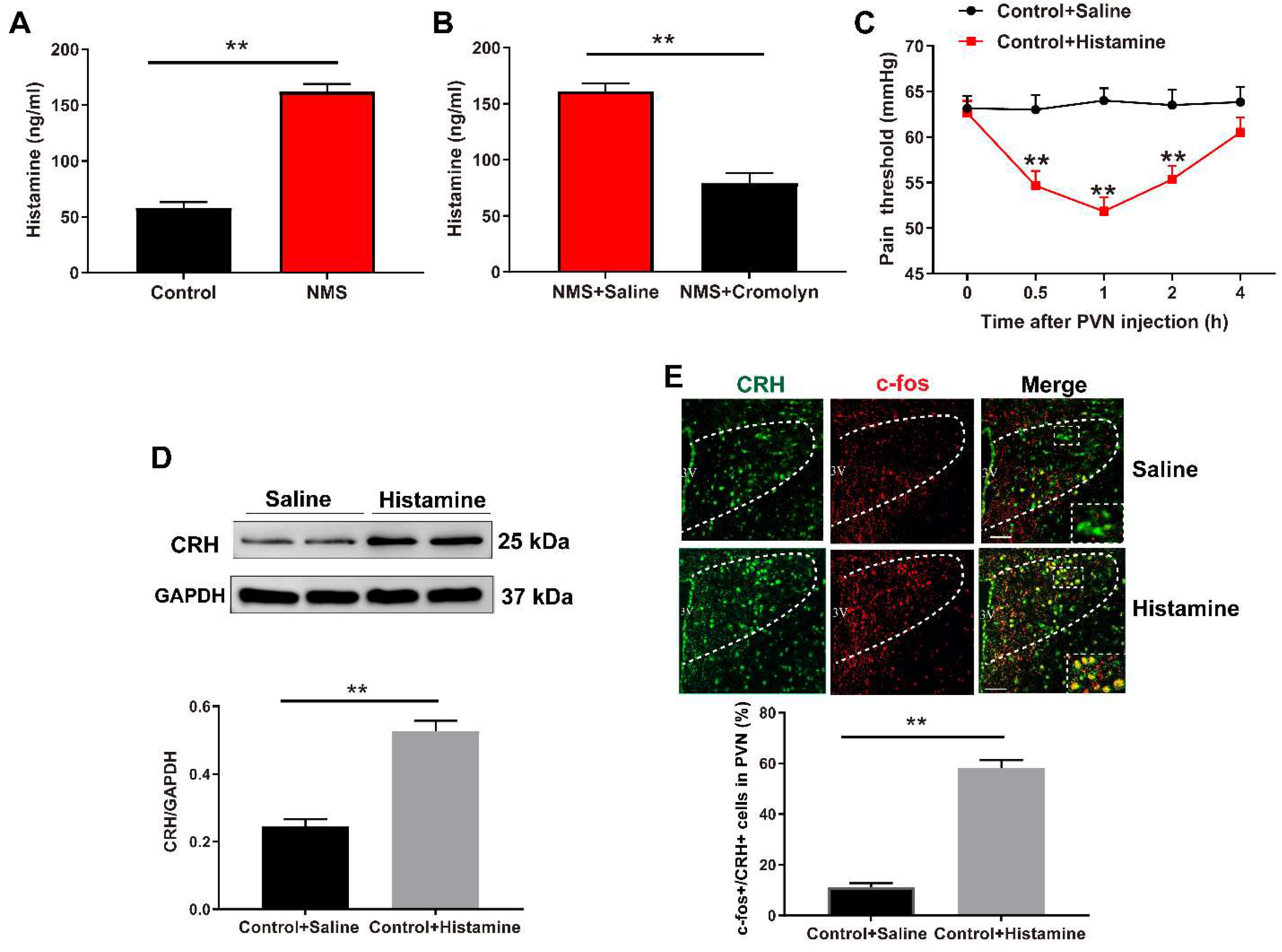
3.4. Mast Cell-Derived Histamine Activates CRH Neurons in PVN to Mediate Visceral Hypersensitivity
3.5. Histamine H2 Receptor (H2R) Mediates NMS-Induced Visceral Hypersensitivity
3.6. H2R Mediates the NMS-Induced Activation of PKA–CREB Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Grundy, L.; Erickson, A.; Brierley, S.M. Visceral Pain. Annu. Rev. Physiol. 2019, 81, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.; Rajilic-Stojanovic, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Prim. 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Midenfjord, I.; Borg, A.; Tornblom, H.; Simren, M. Cumulative Effect of Psychological Alterations on Gastrointestinal Symptom Severity in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 769–779. [Google Scholar] [CrossRef]
- Fuentes, I.M.; Christianson, J.A. The Influence of Early Life Experience on Visceral Pain. Front. Syst. Neurosci. 2018, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Gao, J.H.; Hua, R.; Peng, X.H.; Wang, H.; Zhang, Y.M. Predisposition of Neonatal Maternal Separation to Visceral Hypersensitivity via Downregulation of Small-Conductance Calcium-Activated Potassium Channel Subtype 2 (SK2) in Mice. Neural. Plast. 2020, 2020, 8876230. [Google Scholar] [CrossRef]
- Fan, F.; Tang, Y.; Dai, H.; Cao, Y.; Sun, P.; Chen, Y.; Chen, A.; Lin, C. Blockade of BDNF signalling attenuates chronic visceral hypersensitivity in an IBS-like rat model. Eur. J. Pain 2020, 24, 839–850. [Google Scholar] [CrossRef]
- Huang, S.T.; Wu, K.; Guo, M.M.; Shao, S.; Hua, R.; Zhang, Y.M. Glutamatergic and GABAergic anteroventral BNST projections to PVN CRH neurons regulate maternal separation-induced visceral pain. Neuropsychopharmacology 2023, 48, 1778–1788. [Google Scholar] [CrossRef]
- Burke, N.N.; Finn, D.P.; McGuire, B.E.; Roche, M. Psychological stress in early life as a predisposing factor for the development of chronic pain: Clinical and preclinical evidence and neurobiological mechanisms. J. Neurosci. Res. 2017, 95, 1257–1270. [Google Scholar] [CrossRef]
- Li, Y.C.; Wang, Q.; Li, M.G.; Hu, S.F.; Xu, G.Y. A paraventricular hypothalamic nucleus input to ventral of lateral septal nucleus controls chronic visceral pain. Pain 2023, 164, 625–637. [Google Scholar] [CrossRef]
- Song, Y.; Meng, Q.X.; Wu, K.; Hua, R.; Song, Z.J.; Song, Y.; Qin, X.; Cao, J.L.; Zhang, Y.M. Disinhibition of PVN-projecting GABAergic neurons in AV region in BNST participates in visceral hypersensitivity in rats. Psychoneuroendocrinology 2020, 117, 104690. [Google Scholar] [CrossRef] [PubMed]
- Daviu, N.; Fuzesi, T.; Rosenegger, D.G.; Rasiah, N.P.; Sterley, T.L.; Peringod, G.; Bains, J.S. Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat. Neurosci. 2020, 23, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Rasiah, N.P.; Loewen, S.P.; Bains, J.S. Windows into stress: A glimpse at emerging roles for CRH(PVN) neurons. Physiol. Rev. 2023, 103, 1667–1691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yu, L.; Chen, Z.Y.; Zhu, J.S.; Hua, R.; Qin, X.; Cao, J.L.; Zhang, Y.M. Activation of corticotropin-releasing factor neurons and microglia in paraventricular nucleus precipitates visceral hypersensitivity induced by colorectal distension in rats. Brain Behav. Immun. 2016, 55, 93–104. [Google Scholar] [CrossRef]
- Haykin, H.; Rolls, A. The neuroimmune response during stress: A physiological perspective. Immunity 2021, 54, 1933–1934. [Google Scholar] [CrossRef]
- Kempuraj, D.; Mentor, S.; Thangavel, R.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Dubova, I.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Mast Cells in Stress, Pain, Blood-Brain Barrier, Neuroinflammation and Alzheimer’s Disease. Front. Cell. Neurosci. 2019, 13, 54. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Zhang, X.; Zhang, X.; Qian, Y.; Ding, H.; Zhang, S. Stabilization of Brain Mast Cells Alleviates LPS-Induced Neuroinflammation by Inhibiting Microglia Activation. Front. Cell. Neurosci. 2019, 13, 191. [Google Scholar] [CrossRef]
- Flores, J.A.; Ramirez-Ponce, M.P.; Montes, M.A.; Balseiro-Gomez, S.; Acosta, J.; Alvarez de Toledo, G.; Ales, E. Proteoglycans involved in bidirectional communication between mast cells and hippocampal neurons. J. Neuroinflamm. 2019, 16, 107. [Google Scholar] [CrossRef]
- Hendriksen, E.; van Bergeijk, D.; Oosting, R.S.; Redegeld, F.A. Mast cells in neuroinflammation and brain disorders. Neurosci. Biobehav. Rev. 2017, 79, 119–133. [Google Scholar] [CrossRef]
- Forsythe, P. Mast Cells in Neuroimmune Interactions. Trends Neurosci. 2019, 42, 43–55. [Google Scholar] [CrossRef]
- McClain, J.L.; Mazzotta, E.A.; Maradiaga, N.; Duque-Wilckens, N.; Grants, I.; Robison, A.J.; Christofi, F.L.; Moeser, A.J.; Gulbransen, B.D. Histamine-dependent interactions between mast cells, glia, and neurons are altered following early-life adversity in mice and humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G655–G668. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, T.; Zhang, Y.; Dong, H.; Jin, W. Mast cells in the paraventricular nucleus participate in visceral hypersensitivity induced by neonatal maternal separation. Behav. Brain Res. 2021, 402, 113113. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Chen, Z. Central histaminergic signalling, neural excitability and epilepsy. Br. J. Pharmacol. 2022, 179, 3–22. [Google Scholar] [CrossRef]
- Manz, K.M.; Brady, L.J.; Calipari, E.S.; Grueter, B.A. Accumbal Histamine Signaling Engages Discrete Interneuron Microcircuits. Biol. Psychiatry 2023, 93, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Prusator, D.K.; Andrews, A.; Greenwood-Van Meerveld, B. Neurobiology of early life stress and visceral pain: Translational relevance from animal models to patient care. Neurogastroenterol. Motil. 2016, 28, 1290–1305. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Knickmeyer, R.C.; Gao, W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018, 19, 123–137. [Google Scholar] [CrossRef]
- Miguel, P.M.; Pereira, L.O.; Silveira, P.P.; Meaney, M.J. Early environmental influences on the development of children’s brain structure and function. Dev. Med. Child Neurol. 2019, 61, 1127–1133. [Google Scholar] [CrossRef]
- Sun, Q.; Weng, R.X.; Li, J.H.; Li, Y.C.; Xu, J.T.; Li, R.; Lu, X.; Xu, G.Y. Rab27a-mediated exosome secretion in anterior cingulate cortex contributes to colorectal visceral pain in adult mice with neonatal maternal deprivation. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 325, G356–G367. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, L.; Liu, B.; Sun, P.; Chen, Z.; Huang, Y.; Ai-Qin, C.; Chen, Y.; Lin, C. Spinal P2X4 Receptors Involved in Visceral Hypersensitivity of Neonatal Maternal Separation Rats. Purinergic Signal. 2023, 19, 113–122. [Google Scholar] [CrossRef]
- Mayer, E.A.; Labus, J.; Aziz, Q.; Tracey, I.; Kilpatrick, L.; Elsenbruch, S.; Schweinhardt, P.; Van Oudenhove, L.; Borsook, D. Role of brain imaging in disorders of brain-gut interaction: A Rome Working Team Report. Gut 2019, 68, 1701–1715. [Google Scholar] [CrossRef]
- Ji, N.N.; Du, L.; Wang, Y.; Wu, K.; Chen, Z.Y.; Hua, R.; Zhang, Y.M. Small-Conductance Ca2+-Activated K+ Channels 2 in the Hypothalamic Paraventricular Nucleus Precipitates Visceral Hypersensitivity Induced by Neonatal Colorectal Distension in Rats. Front. Pharmacol. 2020, 11, 605618. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, A.; Bair-Marshall, C.; Xu, H.; Jee, H.J.; Zhu, E.; Sun, M.; Zhang, Q. Oxytocin promotes prefrontal population activity via the PVN-PFC pathway to regulate pain. Neuron 2023, 111, 1795–1811. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, Q.; Nan, S.; Wang, H.; Ma, N.; Kiani, F.A.; Ding, M.; Chen, J. Electroacupuncture Relieves Visceral Hypersensitivity via Balancing PAR2 and PAR4 in the Descending Pain Modulatory System of Goats. Brain Sci. 2023, 13, 922. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Wang, Y.; Sha, H.; Zhou, L.; Chen, Y.; Zhou, Q.; Dong, H.; Qian, Y. The Mast Cell Is an Early Activator of Lipopolysaccharide-Induced Neuroinflammation and Blood-Brain Barrier Dysfunction in the Hippocampus. Mediat. Inflamm. 2020, 2020, 8098439. [Google Scholar] [CrossRef]
- Skaper, S.D. Mast Cell—Glia Dialogue in Chronic Pain and Neuropathic Pain: Blood-Brain Barrier Implications. CNS Neurol. Disord. Drug Targets 2016, 15, 1072–1107. [Google Scholar] [CrossRef]
- Grabauskas, G.; Wu, X.; Gao, J.; Li, J.Y.; Turgeon, D.K.; Owyang, C. Prostaglandin E2, Produced by Mast Cells in Colon Tissues From Patients With Irritable Bowel Syndrome, Contributes to Visceral Hypersensitivity in Mice. Gastroenterology 2020, 158, 2195–2207. [Google Scholar] [CrossRef]
- Gupta, K.; Harvima, I.T. Mast cell-neural interactions contribute to pain and itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef]
- Nozu, T.; Okumura, T. Corticotropin-releasing factor receptor type 1 and type 2 interaction in irritable bowel syndrome. J. Gastroenterol. 2015, 50, 819–830. [Google Scholar] [CrossRef]
- Bhuiyan, P.; Sun, Z.; Chen, Y.; Qian, Y. Peripheral surgery triggers mast cells activation: Focusing on neuroinflammation. Behav. Brain Res. 2023, 452, 114593. [Google Scholar] [CrossRef]
- Hasler, W.L.; Grabauskas, G.; Singh, P.; Owyang, C. Mast cell mediation of visceral sensation and permeability in irritable bowel syndrome. Neurogastroenterol. Motil. 2022, 34, e14339. [Google Scholar] [CrossRef] [PubMed]
- Valle-Dorado, M.G.; Santana-Gomez, C.E.; Orozco-Suarez, S.A.; Rocha, L. The mast cell stabilizer sodium cromoglycate reduces histamine release and status epilepticus-induced neuronal damage in the rat hippocampus. Neuropharmacology 2015, 92, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, J.J.; Yung, W.H.; Chan, Y.S.; Chow, B.K. Excitatory effect of histamine on neuronal activity of rat globus pallidus by activation of H2 receptors in vitro. Neurosci. Res. 2005, 53, 288–297. [Google Scholar] [CrossRef]
- Kilanowska, A.; Ziolkowska, A.; Stasiak, P.; Gibas-Dorna, M. cAMP-Dependent Signaling and Ovarian Cancer. Cells 2022, 11, 3835. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Zhang, X.W.; Yu, L.; Hua, R.; Zhao, X.P.; Qin, X.; Zhang, Y.M. Spinal toll-like receptor 4-mediated signalling pathway contributes to visceral hypersensitivity induced by neonatal colonic irritation in rats. Eur. J. Pain 2015, 19, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Sun, Y.; Ju, P.J.; Wei, J.B.; Li, Q.J.; Winston, J.H. Estrogen augmented visceral pain and colonic neuron modulation in a double-hit model of prenatal and adult stress. World J. Gastroenterol. 2021, 27, 5060–5075. [Google Scholar] [CrossRef]
- Hubbard, C.S.; Karpowicz, J.M.; Furman, A.J.; da Silva, J.T.; Seminowicz, D.A.; Traub, R.J. Estrogen-dependent visceral hypersensitivity following stress in rats: An fMRI study. Mol. Pain 2016, 12, 1744806916654145. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhou, T.; Li, Y.; Li, T.; Ding, Z.; Liu, L. Paraventricular Mast Cell-Derived Histamine Activates CRH Neurons to Mediate Adult Visceral Hypersensitivity Induced by Neonatal Maternal Separation. Brain Sci. 2023, 13, 1595. https://doi.org/10.3390/brainsci13111595
Chen Z, Zhou T, Li Y, Li T, Ding Z, Liu L. Paraventricular Mast Cell-Derived Histamine Activates CRH Neurons to Mediate Adult Visceral Hypersensitivity Induced by Neonatal Maternal Separation. Brain Sciences. 2023; 13(11):1595. https://doi.org/10.3390/brainsci13111595
Chicago/Turabian StyleChen, Ziyang, Tiantian Zhou, Yunfan Li, Tingting Li, Zhengnian Ding, and Li Liu. 2023. "Paraventricular Mast Cell-Derived Histamine Activates CRH Neurons to Mediate Adult Visceral Hypersensitivity Induced by Neonatal Maternal Separation" Brain Sciences 13, no. 11: 1595. https://doi.org/10.3390/brainsci13111595
APA StyleChen, Z., Zhou, T., Li, Y., Li, T., Ding, Z., & Liu, L. (2023). Paraventricular Mast Cell-Derived Histamine Activates CRH Neurons to Mediate Adult Visceral Hypersensitivity Induced by Neonatal Maternal Separation. Brain Sciences, 13(11), 1595. https://doi.org/10.3390/brainsci13111595




