Neurocognitive Adaptations for Spatial Orientation and Navigation in Astronauts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Acquisition
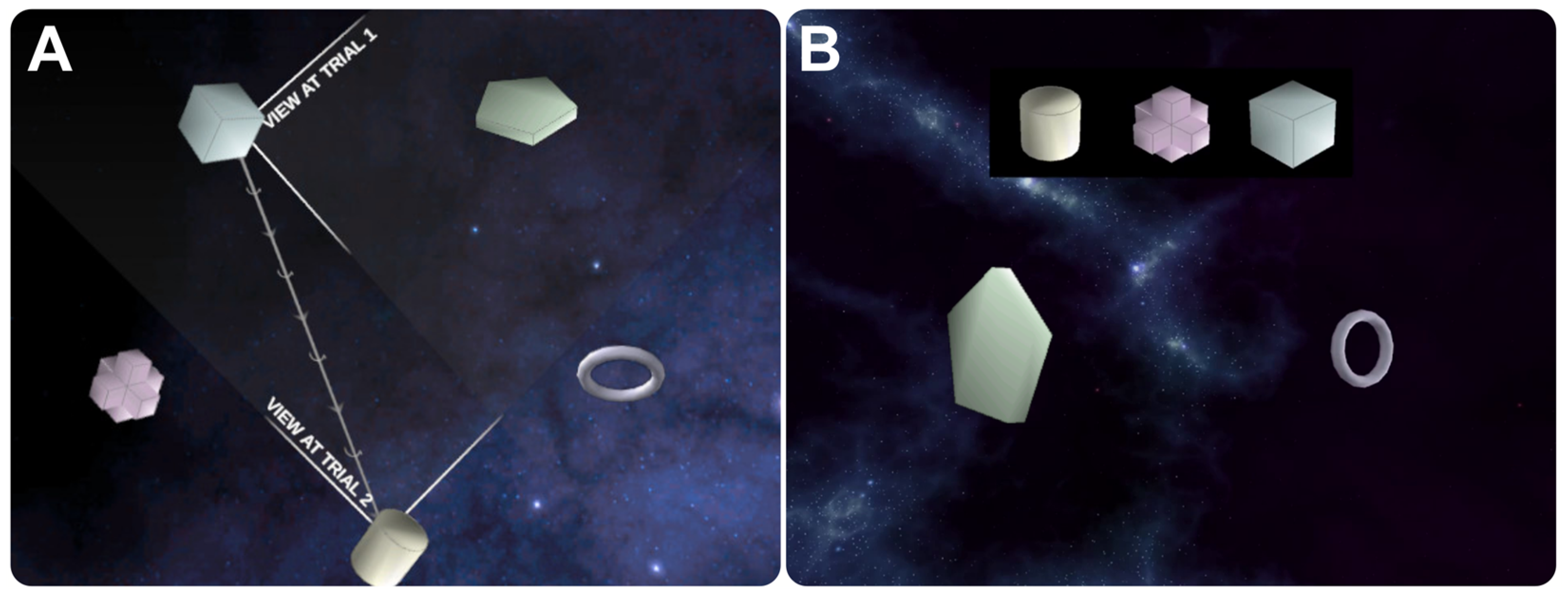
2.3. MRI Task Design
2.4. MRI Processing
- Using the Statistical Parametric Mapping (SPM12, Wellcome Centre for Human Neuroimaging, London, England, UK, 2018) software version 7487, we independently brain-extracted the MPRAGE and FLAIR data using the unified segmentation module. Default parameters were used, except bias-field-removed images were output, sampling distance was set to 2.5 mm, and n Gaussians for tissue classes 1 and 2 (i.e., grey and white matter) were set to 2.
- We generated brain masks for both MPRAGE and FLAIR images by combining the grey matter, white matter, and cerebrospinal fluid segmentation results from (1) using fslmaths, ‘FSL’ version 6.0.6, (FMRIB Analysis Group, Oxford University, Oxford, UK, 2022). We binarized the combined data, smoothed it with a 2 mm sigma Gaussian kernel, and re-binarized it at a threshold of 0.75.
- We used the antsRegistration.sh script from ANTs version 2.4.1 to non-linearly warp the bias-field-corrected and non-brain-extracted MPRAGE image from (1) to the bias-field-corrected and non-brain-extracted FLAIR image from (1). This registration included a rigid-body mutual information registration, followed by a heavily regularized SyN deformation [33] to ensure the resulting warps were spatially smooth. This was performed to correct for deformations between the MPRAGE and FLAIR images resulting from their differing receiver bandwidths. The FLAIR image was selected to define the resultant space because its higher bandwidth results in less geometric distortion. Both registrations were computed using masks from (2).
- We then performed a fine multimodal segmentation [34] and normalization using SPM12’s unified segmentation [35] with the bias-field-corrected FLAIR from (1) and the warped and bias-field-corrected MPRAGE from (3). Default parameters were used, except additionally, we output forward warps to Montreal Neurological Institute (MNI) space, using a sampling distance of 1 mm and setting the n Gaussians to 2, 2, 3, 4 and 5, for tissue classes 1 through 5, respectively.
- We motion-corrected the functional MRI data from each run in SPM12 using the Realign module. Default parameters were used except estimation quality was set to 0.95, separation was set to 3 mm, and smoothing was set to 4 mm full width at half maximum (FWHM), using a 3rd-degree B-spline for estimation interpolation. These data, as well as a mean image, were resliced using a 6th-degree B-spline.
- We performed slice-time correction on the motion-corrected outputs from (5) using SPM12 with the reference slice set at the spatial center of the acquisition volume.
- We coregistered the mean volume from (5) to the bias-field-corrected FLAIR image in SPM12’s coregistration module. We used default parameters, except set the estimation separation to (3 mm, 1 mm). We carried slice-time-corrected outputs from (6) along the computed transformation.
- We moved the coregistered slice-time-corrected fMRI data from (7) and the native space grey matter, white matter, and CSF tissue map from (4) to 2 mm isotropic MNI space using SPM12’s Normalize module with warps computed from (4). Data from (7) were interpolated with a 7th-degree B-spline, and the tissue maps for classes grey matter, white matter, and CSF from (4) were interpolated using trilinear interpolation.
- We computed additional first-level fMRI regressors with an in-house Python script. The resulting set of first-level fMRI regressors included six motion regressors, their temporal derivatives, framewise displacement, scrubbing regressors for global signal spikes calculated from (8) that exceed Z-scores of 3, and 5 aCompCor [36] regressors each from eroded WM and CSF masks from (8).
- We smoothed the fMRI data from (8) in SPM12 using an 8 mm FWHM Gaussian kernel.
2.5. fMRI Contrasts
2.6. fMRI Correlational Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, J.L.; Bülthoff, H.H. Multimodal Integration during Self-Motion in Virtual Reality. In The Neural Bases of Multisensory Processes; Murray, M.M., Wallace, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Mergner, T.; Rosemeier, T. Interaction of Vestibular, Somatosensory and Visual Signals for Postural Control and Motion Perception under Terrestrial and Microgravity Conditions—A Conceptual Model. Brain Res. Rev. 1998, 28, 118–135. [Google Scholar] [CrossRef]
- Poletti, M.; Burr, D.C.; Rucci, M. Optimal Multimodal Integration in Spatial Localization. J. Neurosci. 2013, 33, 14259–14268. [Google Scholar] [CrossRef]
- Taube, J.S. The Head Direction Signal: Origins and Sensory-Motor Integration. Annu. Rev. Neurosci. 2007, 30, 181–207. [Google Scholar] [CrossRef]
- Hubbard, T.L. Cognitive Representation of Linear Motion: Possible Direction and Gravity Effects in Judged Displacement. Mem. Cogn. 1990, 18, 299–309. [Google Scholar] [CrossRef]
- Jörges, B.; López-Moliner, J. Gravity as a Strong Prior: Implications for Perception and Action. Front. Hum. Neurosci. 2017, 11, 203. [Google Scholar] [CrossRef]
- Howard, I.P.; Hu, G. Visually Induced Reorientation Illusions. Perception 2001, 30, 583–600. [Google Scholar] [CrossRef]
- Mast, F.W.; Oman, C.M. Top-Down Processing and Visual Reorientation Illusions in a Virtual Reality Environment. Swiss J. Psychol. 2004, 63, 143–149. [Google Scholar] [CrossRef]
- Oman, C.M. Spatial Orientation and Navigation in Microgravity. In Spatial Processing in Navigation, Imagery and Perception; Mast, F., Jäncke, L., Eds.; Springer: Boston, MA, USA, 2007; pp. 209–247. ISBN 978-0-387-71977-1. [Google Scholar]
- Oman, C.M.; Lichtenberg, B.K.; Money, K.E.; McCoy, R.K. MIT/Canadian Vestibular Experiments on the Spacelab-1 Mission: Space Motion Sickness: Symptoms, Stimuli, and Predictability. Exp. Brain Res. 1986, 64, 316–334. [Google Scholar] [CrossRef]
- Lackner, J.R.; DiZio, P. Space Motion Sickness. Exp. Brain Res. 2006, 175, 377–399. [Google Scholar] [CrossRef]
- Carriot, J.; Jamali, M.; Cullen, K.E. Rapid Adaptation of Multisensory Integration in Vestibular Pathways. Front. Syst. Neurosci. 2015, 9, 59. [Google Scholar] [CrossRef]
- Hupfeld, K.E.; McGregor, H.R.; Koppelmans, V.; Beltran, N.E.; Kofman, I.S.; De Dios, Y.E.; Riascos, R.F.; Reuter-Lorenz, P.A.; Wood, S.J.; Bloomberg, J.J.; et al. Brain and Behavioral Evidence for Reweighting of Vestibular Inputs with Long-Duration Spaceflight. Cereb. Cortex 2022, 32, 755–769. [Google Scholar] [CrossRef]
- Reschke, M.F.; Clément, G. Vestibular and Sensorimotor Dysfunction during Space Flight. Curr. Pathobiol. Rep. 2018, 6, 177–183. [Google Scholar] [CrossRef]
- Evans, T.; Bicanski, A.; Bush, D.; Burgess, N. How Environment and Self-Motion Combine in Neural Representations of Space: Environment and Self-Motion in Neural Representations of Space. J. Physiol. 2016, 594, 6535–6546. [Google Scholar] [CrossRef]
- Frissen, I.; Campos, J.L.; Souman, J.L.; Ernst, M.O. Integration of Vestibular and Proprioceptive Signals for Spatial Updating. Exp. Brain Res. 2011, 212, 163–176. [Google Scholar] [CrossRef]
- Yoder, R.M.; Taube, J.S. The Vestibular Contribution to the Head Direction Signal and Navigation. Front. Integr. Neurosci. 2014, 8, 32. [Google Scholar] [CrossRef]
- Pfeiffer, C.; Serino, A.; Blanke, O. The Vestibular System: A Spatial Reference for Bodily Self-Consciousness. Front. Integr. Neurosci. 2014, 8, 31. [Google Scholar] [CrossRef]
- Fyhn, M.; Molden, S.; Witter, M.P.; Moser, E.I.; Moser, M.-B. Spatial Representation in the Entorhinal Cortex. Science 2004, 305, 1258–1264. [Google Scholar] [CrossRef]
- Alexander, A.S.; Place, R.; Starrett, M.J.; Chrastil, E.R.; Nitz, D.A. Rethinking Retrosplenial Cortex: Perspectives and Predictions. Neuron 2023, 111, 150–175. [Google Scholar] [CrossRef]
- Burles, F.; Slone, E.; Iaria, G. Dorso-Medial and Ventro-Lateral Functional Specialization of the Human Retrosplenial Complex in Spatial Updating and Orienting. Brain Struct. Funct. 2017, 222, 1481–1493. [Google Scholar] [CrossRef]
- Wolbers, T.; Hegarty, M. What Determines Our Navigational Abilities? Trends Cogn. Sci. 2010, 14, 138–146. [Google Scholar] [CrossRef]
- Burles, F.; Umiltá, A.; McFarlane, L.H.; Potocki, K.; Iaria, G. Ventral—Dorsal Functional Contribution of the Posterior Cingulate Cortex in Human Spatial Orientation: A Meta-Analysis. Front. Hum. Neurosci. 2018, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Cona, G.; Scarpazza, C. Where Is the “Where” in the Brain? A Meta-Analysis of Neuroimaging Studies on Spatial Cognition. Hum. Brain Mapp. 2019, 40, 1867–1886. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, A.D.; Hill, P.F. Spatial Navigation and Memory: A Review of the Similarities and Differences Relevant to Brain Models and Age. Neuron 2023, 111, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Schautzer, F.; Hamilton, D.A.; Brüning, R.; Markowitsch, H.J.; Kalla, R.; Darlington, C.; Smith, P.; Strupp, M. Vestibular Loss Causes Hippocampal Atrophy and Impaired Spatial Memory in Humans. Brain 2005, 128, 2732–2741. [Google Scholar] [CrossRef]
- Lackner, J.R.; DiZio, P. Vestibular, Proprioceptive, and Haptic Contributions to Spatial Orientation. Annu. Rev. Psychol. 2005, 56, 115–147. [Google Scholar] [CrossRef]
- Smith, P.F.; Darlington, C.L.; Zheng, Y. Move It or Lose It-Is Stimulation of the Vestibular System Necessary for Normal Spatial Memory? Hippocampus 2009, 20, 36–43. [Google Scholar] [CrossRef]
- Taube, J.S.; Valerio, S.; Yoder, R.M. Is Navigation in Virtual Reality with fMRI Really Navigation? J. Cogn. Neurosci. 2013, 25, 1008–1019. [Google Scholar] [CrossRef]
- Byrne, P.; Becker, S.; Burgess, N. Remembering the Past and Imagining the Future: A Neural Model of Spatial Memory and Imagery. Psychol. Rev. 2007, 114, 340–375. [Google Scholar] [CrossRef]
- Tutorial: The Spatial Configuration Task; Youtube 2021. Available online: https://youtu.be/4gjRgqunI_M (accessed on 10 October 2023).
- Tutorial: The Spatial Configuration Task—fMRI Addendum; Youtube 2021. Available online: https://youtu.be/zeeT6zS86zE (accessed on 10 October 2023).
- Avants, B.; Epstein, C.; Grossman, M.; Gee, J. Symmetric Diffeomorphic Image Registration with Cross-Correlation: Evaluating Automated Labeling of Elderly and Neurodegenerative Brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef]
- Viviani, R.; Stöcker, T.; Stingl, J.C. Multimodal FLAIR/MPRAGE Segmentation of Cerebral Cortex and Cortical Myelin. NeuroImage 2017, 152, 130–141. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified Segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A Component Based Noise Correction Method (CompCor) for BOLD and Perfusion Based fMRI. NeuroImage 2007, 37, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Cremers, H.R.; Wager, T.D.; Yarkoni, T. The Relation between Statistical Power and Inference in fMRI. PLoS ONE 2017, 12, e0184923. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.O.; Paul, E.J.; Miller, M.B.; Barbey, A.K. Small Sample Sizes Reduce the Replicability of Task-Based fMRI Studies. Commun. Biol. 2018, 1, 62. [Google Scholar] [CrossRef] [PubMed]
- Burles, F.; Williams, R.; Berger, L.; Pike, G.B.; Lebel, C.; Iaria, G. The Unresolved Methodological Challenge of Detecting Neuroplastic Changes in Astronauts. Life 2023, 13, 500. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The Precuneus: A Review of Its Functional Anatomy and Behavioural Correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef]
- Ghaem, O.; Mellet, E.; Crivello, F.; Tzourio, N.; Mazoyer, B.; Berthoz, A.; Denis, M. Mental Navigation along Memorized Routes Activates the Hippocampus, Precuneus, and Insula. NeuroReport 1997, 8, 739–744. [Google Scholar] [CrossRef]
- Mitchell, A.S.; Czajkowski, R.; Zhang, N.; Jeffery, K.; Nelson, A.J.D. Retrosplenial Cortex and Its Role in Spatial Cognition. Brain Neurosci. Adv. 2018, 13, 2. [Google Scholar] [CrossRef]
- Stahn, A.C.; Kühn, S. Brains in Space: The Importance of Understanding the Impact of Long-Duration Spaceflight on Spatial Cognition and Its Neural Circuitry. Cogn. Process. 2021, 22, 105–114. [Google Scholar] [CrossRef]
- Hagmann, P.; Cammoun, L.; Gigandet, X.; Meuli, R.; Honey, C.J.; Wedeen, V.J.; Sporns, O. Mapping the Structural Core of Human Cerebral Cortex. PLoS Biol. 2008, 6, e159. [Google Scholar] [CrossRef]
- Jitsuishi, T.; Yamaguchi, A. Characteristic Cortico-Cortical Connection Profile of Human Precuneus Revealed by Probabilistic Tractography. Sci. Rep. 2023, 13, 1936. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, D.J.; Saleem, K.S.; Baker, C.I.; Mishkin, M. A New Neural Framework for Visuospatial Processing. Nat. Rev. Neurosci. 2011, 12, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Ciaramelli, E.; Grady, C.L.; Moscovitch, M. Top-down and Bottom-up Attention to Memory: A Hypothesis (AtoM) on the Role of the Posterior Parietal Cortex in Memory Retrieval. Neuropsychologia 2008, 46, 1828–1851. [Google Scholar] [CrossRef] [PubMed]
- Seghier, M.L. The Angular Gyrus: Multiple Functions and Multiple Subdivisions. Neuroscience 2013, 19, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Harel, A.; Kravitz, D.J.; Baker, C.I. Deconstructing Visual Scenes in Cortex: Gradients of Object and Spatial Layout Information. Cereb. Cortex 2013, 23, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Roy-O’Reilly, M.; Mulavara, A.; Williams, T. A Review of Alterations to the Brain during Spaceflight and the Potential Relevance to Crew in Long-Duration Space Exploration. NPJ Microgravity 2021, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.F. The Vestibular System and Cognition. Curr. Opin. Neurol. 2017, 30, 84–89. [Google Scholar] [CrossRef]
- Smith, P.F.; Zheng, Y. From Ear to Uncertainty: Vestibular Contributions to Cognitive Function. Front. Integr. Neurosci. 2013, 7, 84. [Google Scholar] [CrossRef]
- Deshpande, N.; Patla, A.E. Dynamic Visual–Vestibular Integration during Goal Directed Human Locomotion. Exp. Brain Res. 2005, 166, 237–247. [Google Scholar] [CrossRef]
- Chen, Z.; Rong, L.; Xiao, L.; Wang, Q.; Liu, Y.; Lin, C.; Wang, J.; Liu, H.; Wei, X. Altered Brain Function in Patients with Vestibular Migraine: A Study on Resting State Functional Connectivity. Neuroradiology 2023, 65, 579–590. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Zhou, W.; Qian, T.; Sun, W.; Zhao, G. Electroclinical Characteristics of Seizures Arising from the Precuneus Based on Stereoelectroencephalography (SEEG). BMC Neurol. 2018, 18, 110. [Google Scholar] [CrossRef]
- Zhe, X.; Zhang, X.; Chen, L.; Zhang, L.; Tang, M.; Zhang, D.; Li, L.; Lei, X.; Jin, C. Altered Gray Matter Volume and Functional Connectivity in Patients with Vestibular Migraine. Front. Neurosci. 2021, 15, 683802. [Google Scholar] [CrossRef] [PubMed]
- Al-Ramadhani, R.R.; Shivamurthy, V.K.N.; Elkins, K.; Gedela, S.; Pedersen, N.P.; Kheder, A. The Precuneal Cortex: Anatomy and Seizure Semiology. Epileptic Disord. 2021, 23, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Beer, A.L.; Becker, M.; Frank, S.M.; Greenlee, M.W. Vestibular and Visual Brain Areas in the Medial Cortex of the Human Brain. J. Neurophysiol. 2023, 129, 948–962. [Google Scholar] [CrossRef] [PubMed]
- Black, F.O.; Paloski, W.H.; Doxey-Gasway, D.D.; Reschke, M.F. Vestibular Plasticity Following Orbital Spaceflight: Recovery from Postflight Postural Instability. Acta Oto-Laryngol. 1995, 115, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Heer, M.; Paloski, W.H. Space Motion Sickness: Incidence, Etiology, and Countermeasures. Auton. Neurosci. 2006, 129, 77–79. [Google Scholar] [CrossRef]
- Ertl, M.; Boegle, R. Investigating the Vestibular System Using Modern Imaging Techniques—A Review on the Available Stimulation and Imaging Methods. J. Neurosci. Methods 2019, 326, 108363. [Google Scholar] [CrossRef]
- Makowski, C.; Lepage, M.; Evans, A.C. Head Motion: The Dirty Little Secret of Neuroimaging in Psychiatry. JPN 2019, 44, 62–68. [Google Scholar] [CrossRef]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to Detect, Characterize, and Remove Motion Artifact in Resting State fMRI. NeuroImage 2014, 84, 320–341. [Google Scholar] [CrossRef]
- Oman, C.M.; Shebilske, W.L.; Tubré, T.C.; Beall, A.C.; Natapoff, A. Three Dimensional Spatial Memory and Learning in Real and Virtual Environments. Spat. Cogn. Comput. 2002, 2, 355–372. [Google Scholar] [CrossRef]
- Hebscher, M.; Levine, B.; Gilboa, A. The Precuneus and Hippocampus Contribute to Individual Differences in the Unfolding of Spatial Representations during Episodic Autobiographical Memory. Neuropsychologia 2018, 110, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.G.; Riemer, M.; Brandt, L.; Wolbers, T. Repetitive Transcranial Magnetic Stimulation Reveals a Causal Role of the Human Precuneus in Spatial Updating. Sci. Rep. 2018, 8, 10171. [Google Scholar] [CrossRef] [PubMed]
- Schott, B.H.; Wüstenberg, T.; Lücke, E.; Pohl, I.-M.; Richter, A.; Seidenbecher, C.I.; Pollmann, S.; Kizilirmak, J.M.; Richardson-Klavehn, A. Gradual Acquisition of Visuospatial Associative Memory Representations via the Dorsal Precuneus. Hum. Brain Mapp. 2019, 40, 1554–1570. [Google Scholar] [CrossRef]
- Tidwell, J.B.; Taylor, J.A.; Collins, H.R.; Chamberlin, J.H.; Barisano, G.; Sepehrband, F.; Turner, M.D.; Gauthier, G.; Mulder, E.R.; Gerlach, D.A.; et al. Longitudinal Changes in Cerebral Perfusion, Perivascular Space Volume, and Ventricular Volume in a Healthy Cohort Undergoing a Spaceflight Analog. Am. J. Neuroradiol. 2023, 44, 1026–1031. [Google Scholar] [CrossRef]
- Tays, G.D.; Hupfeld, K.E.; McGregor, H.R.; Salazar, A.P.; De Dios, Y.E.; Beltran, N.E.; Reuter-Lorenz, P.A.; Kofman, I.S.; Wood, S.J.; Bloomberg, J.J.; et al. The Effects of Long Duration Spaceflight on Sensorimotor Control and Cognition. Front. Neural Circuits 2021, 15, 723504. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Palumbo, R.; Di Domenico, A.; Mammarella, N. Simulating Extreme Environmental Conditions via Mental Imagery: The Case of Microgravity and Weight Estimation. Front. Psychol. 2022, 13, 913162. [Google Scholar] [CrossRef]
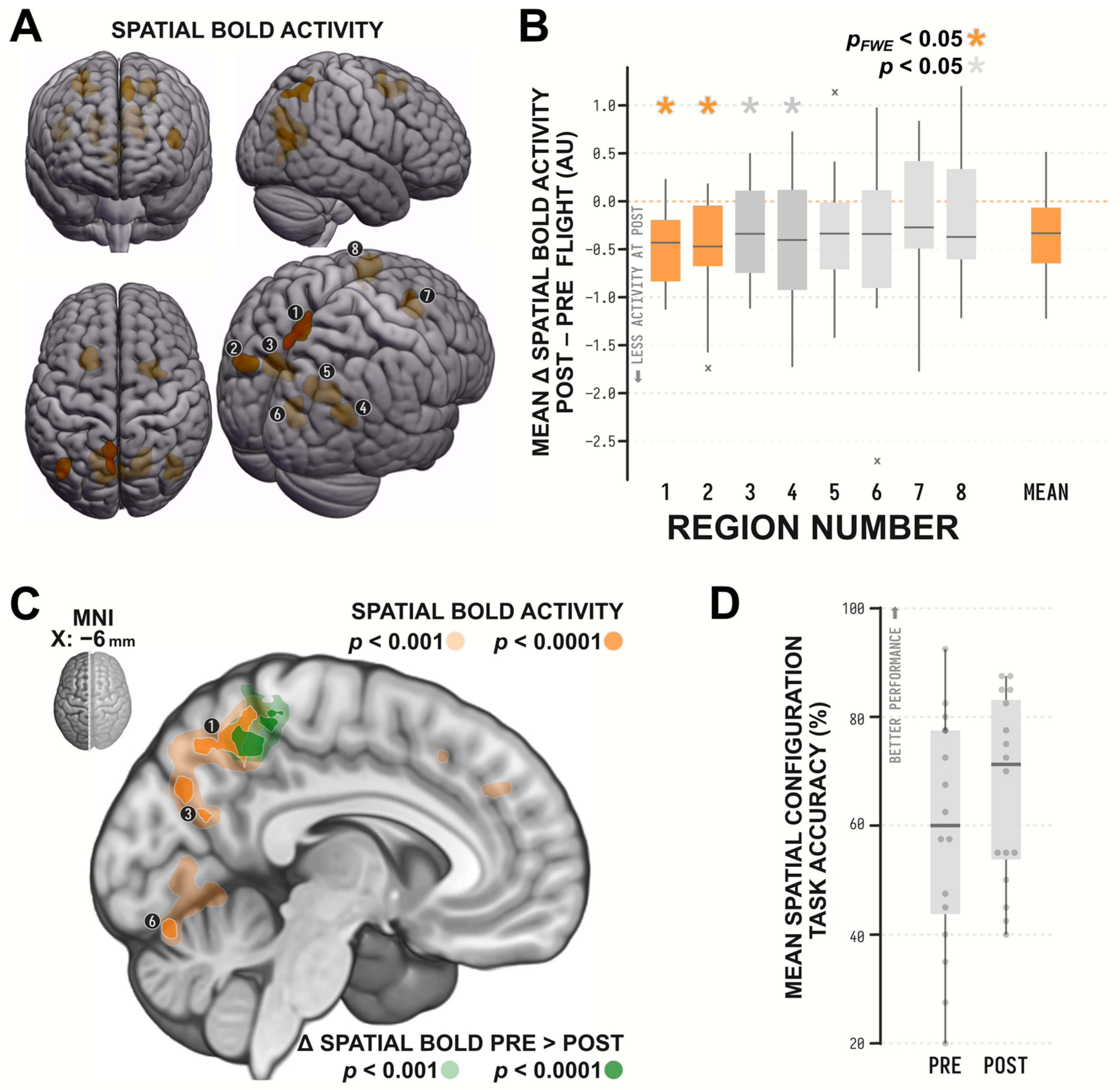
| Cluster | Peak | |||||||
|---|---|---|---|---|---|---|---|---|
| ROI # | Label | KE | pFWE | t15 | pFWE | x | y | z |
| 1 | Precuneus | 134 | 0.001 | 5.83 | 0.448 | −8 | −56 | 54 |
| 5.61 | 0.553 | −6 | −66 | 50 | ||||
| 5.37 | 0.673 | −8 | −52 | 44 | ||||
| 2 | Left Angular Gyrus | 128 | 0.001 | 6.45 | 0.229 | −42 | −64 | 12 |
| 5.69 | 0.515 | −44 | −74 | 18 | ||||
| 3 | Left Dorsal Retrosplenial Complex or Posterior Cingulate Cortex | 376 | <0.001 | 8.01 | 0.036 | −16 | −58 | 20 |
| 7.26 | 0.087 | −10 | −74 | 34 | ||||
| 5.80 | 0.462 | −4 | −70 | 26 | ||||
| 4 | Right Angular Gyrus | 198 | <0.001 | 7.49 | 0.066 | 42 | −64 | 20 |
| 5.86 | 0.435 | 40 | −72 | 36 | ||||
| 5.78 | 0.451 | 44 | −76 | 26 | ||||
| 5 | Right Dorsal Retrosplenial Complex or Posterior Cingulate Cortex | 287 | <0.001 | 9.10 | 0.010 | 18 | −66 | 26 |
| 5.90 | 0.416 | 20 | −56 | 14 | ||||
| 6 | Lingual Gyrus | 117 | 0.001 | 5.86 | 0.433 | 0 | −70 | 6 |
| 5.66 | 0.526 | 12 | −72 | 2 | ||||
| 7 | Right Frontal Eye Fields | 188 | <0.001 | 6.21 | 0.301 | 26 | 10 | 50 |
| 6.08 | 0.347 | 36 | 4 | 58 | ||||
| 5.78 | 0.469 | 16 | 12 | 54 | ||||
| 8 | Left Premotor or Supplementary Motor Cortex, BA6 | 217 | <0.001 | 7.13 | 0.103 | −20 | 22 | 56 |
| 6.75 | 0.160 | −18 | 12 | 54 | ||||
| 5.70 | 0.511 | −20 | 22 | 38 | ||||
| Effect of Spaceflight on Spatial BOLD | Correlation with Change in Spatial Performance | Effect Accounting for Changes in GM Concentration | Effect Accounting for Changes in GM Concentration and Performance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI # | t15 | p | sig | r14 | p | sig | t15 | p | sig | t14 | p | sig |
| 1 | −4.959 | <0.001 | *** | −0.317 | 0.232 | −4.542 | <0.001 | *** | −3.518 | 0.003 | *** | |
| 2 | −3.427 | 0.004 | *** | −0.307 | 0.248 | −3.291 | 0.005 | *** | −2.371 | 0.033 | * | |
| 3 | −2.366 | 0.032 | * | −0.079 | 0.770 | −2.145 | 0.049 | * | −1.698 | 0.112 | ||
| 4 | −2.243 | 0.040 | * | −0.368 | 0.161 | −2.164 | 0.047 | * | −1.296 | 0.216 | ||
| 5 | −2.020 | 0.062 | −0.151 | 0.576 | −1.760 | 0.099 | −1.256 | 0.230 | ||||
| 6 | −1.974 | 0.067 | −0.118 | 0.662 | −1.935 | 0.072 | −1.466 | 0.165 | ||||
| 7 | −1.081 | 0.297 | 0.129 | 0.634 | −0.809 | 0.431 | −1.004 | 0.332 | ||||
| 8 | −1.045 | 0.313 | 0.171 | 0.527 | −0.690 | 0.501 | −0.983 | 0.342 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burles, F.; Iaria, G. Neurocognitive Adaptations for Spatial Orientation and Navigation in Astronauts. Brain Sci. 2023, 13, 1592. https://doi.org/10.3390/brainsci13111592
Burles F, Iaria G. Neurocognitive Adaptations for Spatial Orientation and Navigation in Astronauts. Brain Sciences. 2023; 13(11):1592. https://doi.org/10.3390/brainsci13111592
Chicago/Turabian StyleBurles, Ford, and Giuseppe Iaria. 2023. "Neurocognitive Adaptations for Spatial Orientation and Navigation in Astronauts" Brain Sciences 13, no. 11: 1592. https://doi.org/10.3390/brainsci13111592
APA StyleBurles, F., & Iaria, G. (2023). Neurocognitive Adaptations for Spatial Orientation and Navigation in Astronauts. Brain Sciences, 13(11), 1592. https://doi.org/10.3390/brainsci13111592







