Role of Vagus Nerve Stimulation in Refractory and Super Refractory Status Epilepticus: A Pediatric Case Series
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trinka, E.; Höfler, J.; Leitinger, M.; Brigo, F. Pharmacotherapy for Status Epilepticus. Drugs 2015, 75, 1499–1521. [Google Scholar] [CrossRef] [PubMed]
- Trinka, E.; Cock, H.; Hesdorffer, D.; Rossetti, A.O.; Scheffer, I.E.; Shinnar, S.; Shorvon, S.; Lowenstein, D.H. A definition and classification of status epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015, 56, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Vossler, D.G.; Bainbridge, J.L.; Boggs, J.G.; Novotny, E.J.; Loddenkemper, T.; Faught, E.; Amengual-Gual, M.; Fischer, S.N.; Gloss, D.S.; Olson, D.M.; et al. Treatment of Refractory Convulsive Status Epilepticus: A Comprehensive Review by the American Epilepsy Society Treatments Committee. Epilepsy Curr. 2020, 20, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Shorvon, S.; Ferlisi, M. The treatment of super-refractory status epilepticus: A critical review of available therapies and a clinical treatment protocol. Brain 2011, 134 Pt 10, 2802–2818. [Google Scholar] [CrossRef] [PubMed]
- Kravljanac, R.; Djuric, M.; Jankovic, B.; Pekmezovic, T. Etiology, clinical course and response to the treatment of status epilepticus in children: A 16-year single- center experience based on 602 episodes of status epilepticus. Eur. J. Paediatr. Neurol. 2015, 19, 584–590. [Google Scholar] [CrossRef]
- Sahin, M.; Menache, C.C.; Holmes, G.L.; Riviello, J.J. Outcome of severe refractory status epilepticus in children. Epilepsia 2001, 42, 1461–1467. [Google Scholar] [CrossRef]
- Vasquez, A.; Farias-Moeller, R.; Tatum, W. Pediatric refractory and super-refractory status epilepticus. Seizure 2019, 68, 62–71. [Google Scholar] [CrossRef]
- Specchio, N.; Pietrafusa, N. New-onset refractory status epilepticus and febrile infection-related epilepsy syndrome. Dev. Med. Child Neurol. 2020, 62, 897–905. [Google Scholar] [CrossRef]
- Arya, R.; Rotenberg, A. Dietary, immunological, surgical, and other emerging treatments for pediatric refractory status epilepticus. Seizure 2019, 68, 89–96. [Google Scholar] [CrossRef]
- Grioni, D.; Landi, A.; Fiori, L.; Sganzerla, E.P. Does emergent implantation of a vagal nerve stimulator stop refractory status epilepticus in children? Seizure 2018, 61, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Specchio, N.; Ferretti, A.; Pietrafusa, N.; Trivisano, M.; Calabrese, C.; Carfì Pavia, G.; De Benedictis, A.; Marras, C.E.; de Palma, L.; Vigevano, F. Refractory Status Epilepticus in Genetic Epilepsy-Is Vagus Nerve Stimulation an Option? Front. Neurol. 2020, 11, 443. [Google Scholar] [CrossRef]
- Luo, T.; Wang, Y.; Lu, G.; Zhou, Y.; Wang, Y. Vagus nerve stimulation for super-refractory status epilepticus in febrile infection-related epilepsy syndrome: A pediatric case report and literature review. Child’s Nerv. Syst. 2022, 38, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, C.M.; Furlanis, G.M.; Toldo, I.; Guarrera, B.; Luisi, C.; Pettenazzo, A.; Nosadini, M.; Boniver, C.; Sartori, S.; Landi, A. Myoclonic super-refractory status epilepticus with favourable evolution in a teenager with FIRES: Is the association of vagus nerve stimulation and cannabidiol effective? Brain Dev. 2023, 45, 293–299. [Google Scholar] [CrossRef]
- Ochoa, J.G.; Dougherty, M.; Papanastassiou, A.; Gidal, B.; Mohamed, I.; Vossler, D.G. Treatment of Super-Refractory Status Epilepticus: A Review. Epilepsy Curr. 2021, 21, 1535759721999670. [Google Scholar] [CrossRef] [PubMed]
- Dibué-Adjei, M.; Brigo, F.; Yamamoto, T.; Vonck, K.; Trinka, E. Vagus nerve stimulation in refractory and super-refractory status epilepticus—A systematic review. Brain Stimul. 2019, 12, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Furlanis, G.M.; Fascetti Leon, F.; Bresolin, N.; Favaro, J.; Baro, V.; D’Amico, A.; Denaro, L.; Sartori, S.; Landi, A. Aesthetic transaxillary subpectoral placement of vagus nerve stimulator in children and young adults: A technical note. Epilepsy Behav. 2023, 147, 109419. [Google Scholar] [CrossRef]
- McHugh, J.C.; Singh, H.W.; Phillips, J.; Murphy, K.; Doherty, C.P.; Delanty, N. Outcome measurement after vagal nerve stimulation therapy: Proposal of a new classification. Epilepsia 2007, 48, 375–378. [Google Scholar] [CrossRef]
- Rodgers, W.P.; Durnford, A.J.; Kirkham, F.J.; Whitney, A.; Mullee, M.A.; Gray, W.P. Interrater reliability of Engel, International League Against Epilepsy, and McHugh seizure outcome classifications following vagus nerve stimulator implantation. J. Neurosurg. Pediatr. 2012, 10, 226–229. [Google Scholar] [CrossRef]
- Kurukumbi, M.; Leiphart, J.; Asif, A.; Wang, J. Vagus nerve stimulation (VNS) in super refractory new onset refractory status epilepticus (NORSE). Case Rep. Neurol. Med. 2019, 2019, 7852017. [Google Scholar] [CrossRef]
- Yamazoe, T.; Okanishi, T.; Yamamoto, A.; Yamada, T.; Nishimura, M.; Fujimoto, A.; Enoki, H.; Yokota, T.; Sato, K.; Yamamoto, T. New-onset refractory status epilepticus treated with vagus nerve stimulation: A case report. Seizure 2017, 47, 1–4. [Google Scholar] [CrossRef]
- Majoie, H.J.M.; Rijkers, K.; Berfelo, M.W.; Hulsman, J.A.R.J.; Myint, A.; Schwarz, M.; Vles, J.S.H. Vagus nerve stimulation in refractory epilepsy: Effects on pro- and anti-inflammatory cytokines in peripheral blood. Neuroimmunomodulation 2011, 18, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, C.W.; Ragozzino, F.J.; Lindberg, J.E.; Peterson, B.; Lugo, J.M.; McLaughlin, R.J.; Peters, J.H. Cannabidiol activation of vagal afferent neurons requires TRPA1. J. Neurophysiol. 2020, 124, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Heck, C.; Grafton, S.; Apuzzo, M.L.; Couldwell, W.T.; Chen, T.; Diaz, D.J.; Zelman, V.; Smith, T.; DeGiorgio, C. Vagus nerve stimulation activates central nervous system structures in epileptic patients during PET H2(15)O blood flow imaging. Neurosurgery 1996, 39, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.R.; Bakay, R.A.E.; Pennell, P.B.; Epstein, C.M.; Votaw, J.R. Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. Prolonged effects at high and low levels of stimulation. Epilepsia 2004, 45, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Van Baalen, A. Febrile infection-related epilepsy syndrome in childhood: A clinical review and practical approach. Seizure 2023, 111, 215–222. [Google Scholar] [CrossRef]
- Iimura, Y.; Suzuki, H.; Mitsuhashi, T.; Ueda, T.; Nishioka, K.; Horikoshi, K.; Nomura, K.; Sugano, H.; Kondo, A. Effect of vagus nerve stimulation against generalized seizure and status epilepticus recurrence. Front. Neurol. 2023, 14, 1258854. [Google Scholar] [CrossRef]
- Sierra-Marcos, A.; Maestro, I.; Rodríguez-Osorio, X.; Miró, J.; Donaire, A.; Aparicio, J.; Rumiá, J.; Forcadas, M.; Garamendi, I.; Pardo, J.; et al. Successful outcome of episodes of status epilepticus after vagus nerve stimulation: A multicenter study. Eur. J. Neurol. 2012, 19, 1219–1223. [Google Scholar] [CrossRef]
- Wickstrom, R.; Taraschenko, O.; Dilena, R.; Payne, E.T.; Specchio, N.; Nabbout, R.; Koh, S.; Gaspard, N.; Hirsch, L.J.; The International NORSE Consensus Group. International consensus recommendations for management of New Onset Refractory Status Epilepticus (NORSE) incl. Febrile Infection-Related Epilepsy Syndrome (FIRES): Statements and Supporting Evidence. Epilepsia 2022, 63, 2840–2864. [Google Scholar] [CrossRef]
- Mantoan Ritter, L.; Selway, R. Perspective: Vagal nerve stimulation in the treatment of new-onset refractory status epilepticus. Front. Neurol. 2023, 14, 1172898. [Google Scholar] [CrossRef]
- Carron, R.; Roncon, P.; Lagarde, S.; Dibué, M.; Zanello, M.; Bartolomei, F. Latest Views on the Mechanisms of Action of Surgically Implanted Cervical Vagal Nerve Stimulation in Epilepsy. Neuromodulation 2023, 26, 498–506. [Google Scholar] [CrossRef]
- Marrosu, F.; Santoni, F.; Puligheddu, M.; Barberini, L.; Maleci, A.; Ennas, F.; Mascia, M.; Zanetti, G.; Tuveri, A.; Biggio, G. Increase in 20–50 Hz (gamma frequencies) power spectrum and synchronization after chronic vagal nerve stimulation. Clin. Neurophysiol. 2005, 116, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.D.; Steffan, P.J.; Srikanth, P.; Wiedrick, J.; Spencer, D.C.; Datta, P.; Joseph, N.M.; Wernovsky, M.; Becker, D.A. Electrocorticography analysis in patients with dual neurostimulators supports desynchronization as a mechanism of action for acute vagal nerve stimulator stimulation. J. Clin. Neurophysiol. 2023, 40, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hallböök, T.; Lundgren, J.; Blennow, G.; Strömblad, L.G.; Rosén, I. Long term effects on epileptiform activity with vagus nerve stimulation in children. Seizure 2005, 14, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Kuba, R.; Guzaninová, M.; Brázdil, M.; Novák, Z.; Chrastina, J.; Rektor, I. Effect of vagal nerve stimulation on interictal epileptiform discharges: A scalp EEG study. Epilepsia 2002, 43, 1181–1188. [Google Scholar] [CrossRef]
- Mollet, L.; Grimonprez, A.; Raedt, R.; Delbeke, J.; El Tahry, R.I.E.M.; De Herdt, V.; Meurs, A.; Wadman, W.; Boon, P.; Vonck, K. Intensity-dependent modulatory effects of vagus nerve stimulation on cortical excitability. Acta Neurol. Scand. 2013, 128, 391–396. [Google Scholar] [CrossRef]
- Fraschini, M.; Puligheddu, M.; Demuru, M.; Polizzi, L.; Maleci, A.; Tamburini, G.; Congia, S.; Bortolato, M.; Marrosu, F. VNS induced desynchronization in gamma bands correlates with positive clinical outcome in temporal lobe pharmacoresistant epilepsy. Neurosci. Lett. 2013, 536, 14–18. [Google Scholar] [CrossRef]
- Bartolomei, F.; Bonini, F.; Vidal, E.; Trébuchon, A.; Lagarde, S.; Lambert, I.; McGonigal, A.; Scavarda, D.; Carron, R.; Benar, C.G. How does vagal nerve stimulation (VNS) change EEG brain functional connectivity? Epilepsy Res. 2016, 126, 141–146. [Google Scholar] [CrossRef]
- Sangare, A.; Marchi, A.; Pruvost-Robieux, E.; Soufflet, C.; Crepon, B.; Ramdani, C.; Chassoux, F.; Turak, B.; Landre, E.; Gavaret, M. The effectiveness of vagus nerve stimulation in drug-resistant epilepsy correlates with vagus nerve stimulationinduced electroencephalography desynchronization. Brain Connect. 2020, 10, 566–577. [Google Scholar] [CrossRef]
- Ravan, M.; Sabesan, S.; D’Cruz, O. On quantitative biomarkers of VNS therapy using EEG and ECG signals. IEEE Trans. Biomed. Eng. 2017, 64, 419–428. [Google Scholar] [CrossRef]
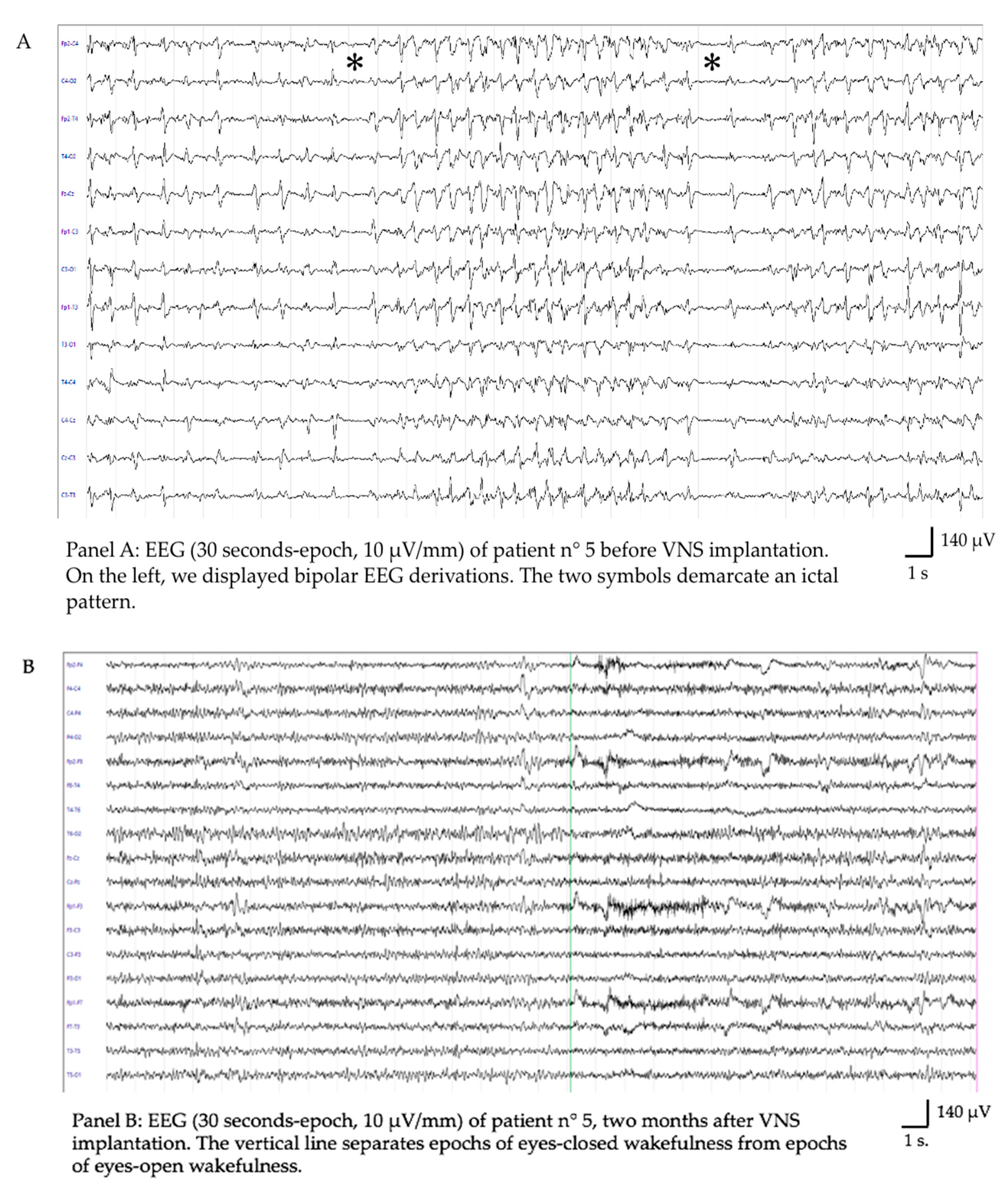
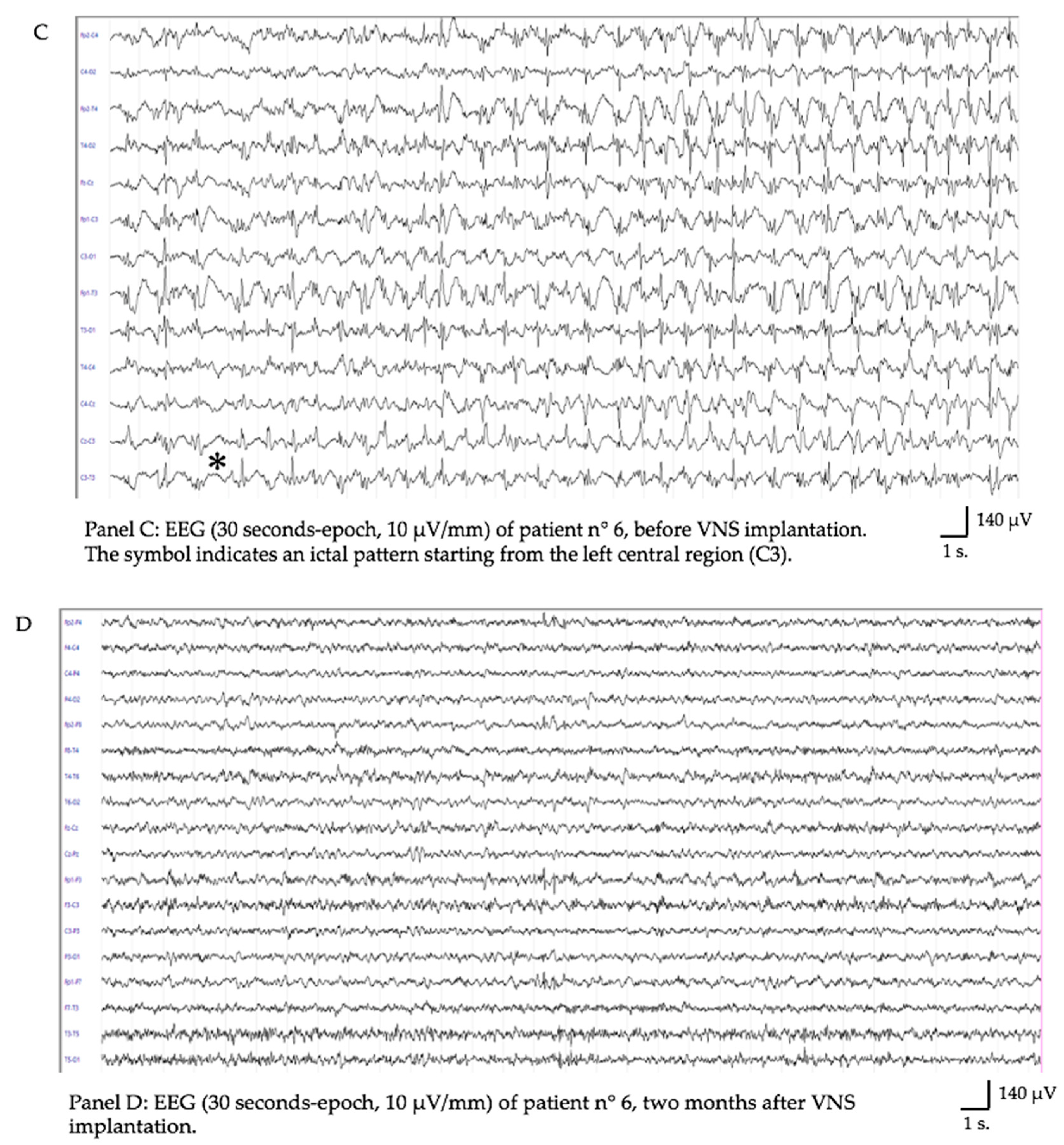
| Sex | Diagnosis | Age | Time Onset-to-implant (days) | RSE Stop | Latency to SE Resolution (days) | Stimulation Parameters (at SE Resolution) | Follow Up | McHugh Score | Level of Function | Outcome | Adverse Events due to VNS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pt1 | F | Left Hemimegalencephaly | 16 mo | 18 | Yes | 4 | 1 mA, 500 usec, 30 Hz, DC 10% | 9 ys | IA | Unable to speak Tetraparesis | Alive | No |
| Pt1 | M | NonKetotic Hyperglycinemia | 16 mo | 5 | Yes | 5 | 1 mA, 500 usec, 30 Hz, DC 10% | 8 ys | IA | n/a | Deceased for metabolic cause | No |
| Pt3 | F | Microdeletion of 1q43q44 | 17 mo | 16 | Yes | 3 | 1 mA, 250 usec, 30 Hz, DC 10% | 7 ys | IA | Unable to walk Profound mental retardation | Alive | No |
| Pt4 | M | Migrating Epilepsy | 7 mo | 90 | No | n/a | n/a | 1 mo | V | n/a | Deceased during RSE | No |
| Pt5 | F | FIRES | 14 ys | 43 | Yes | 28 | 2.25 mA, 250 usec, 30 Hz, DC 16% | 1 y | IA | Mild neuropsych impairment | Alive | Tachycardia Coughing |
| Pt6 | M | FIRES | 6 ys | 26 | Yes | 28 | 2.00 mA, 250 usec, 20 Hz, DC 15% | 1 y | IA | Behavioral problems | Alive | No |
| Pt7 | F | FIRES | 6 ys | 25 | No | n/a | n/a | 4 mo | IIIB | n/a | Alive | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlanis, G.M.; Favaro, J.; Bresolin, N.; Grioni, D.; Baro, V.; D’Amico, A.; Sartori, S.; Denaro, L.; Landi, A. Role of Vagus Nerve Stimulation in Refractory and Super Refractory Status Epilepticus: A Pediatric Case Series. Brain Sci. 2023, 13, 1589. https://doi.org/10.3390/brainsci13111589
Furlanis GM, Favaro J, Bresolin N, Grioni D, Baro V, D’Amico A, Sartori S, Denaro L, Landi A. Role of Vagus Nerve Stimulation in Refractory and Super Refractory Status Epilepticus: A Pediatric Case Series. Brain Sciences. 2023; 13(11):1589. https://doi.org/10.3390/brainsci13111589
Chicago/Turabian StyleFurlanis, Giulia Melinda, Jacopo Favaro, Nicola Bresolin, Daniele Grioni, Valentina Baro, Alberto D’Amico, Stefano Sartori, Luca Denaro, and Andrea Landi. 2023. "Role of Vagus Nerve Stimulation in Refractory and Super Refractory Status Epilepticus: A Pediatric Case Series" Brain Sciences 13, no. 11: 1589. https://doi.org/10.3390/brainsci13111589
APA StyleFurlanis, G. M., Favaro, J., Bresolin, N., Grioni, D., Baro, V., D’Amico, A., Sartori, S., Denaro, L., & Landi, A. (2023). Role of Vagus Nerve Stimulation in Refractory and Super Refractory Status Epilepticus: A Pediatric Case Series. Brain Sciences, 13(11), 1589. https://doi.org/10.3390/brainsci13111589








