Detecting Central Auditory Processing Disorders in Awake Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgery
2.3. Post-Surgery
2.4. Electrophysiology Recordings
2.5. Statistical Tests
3. Results
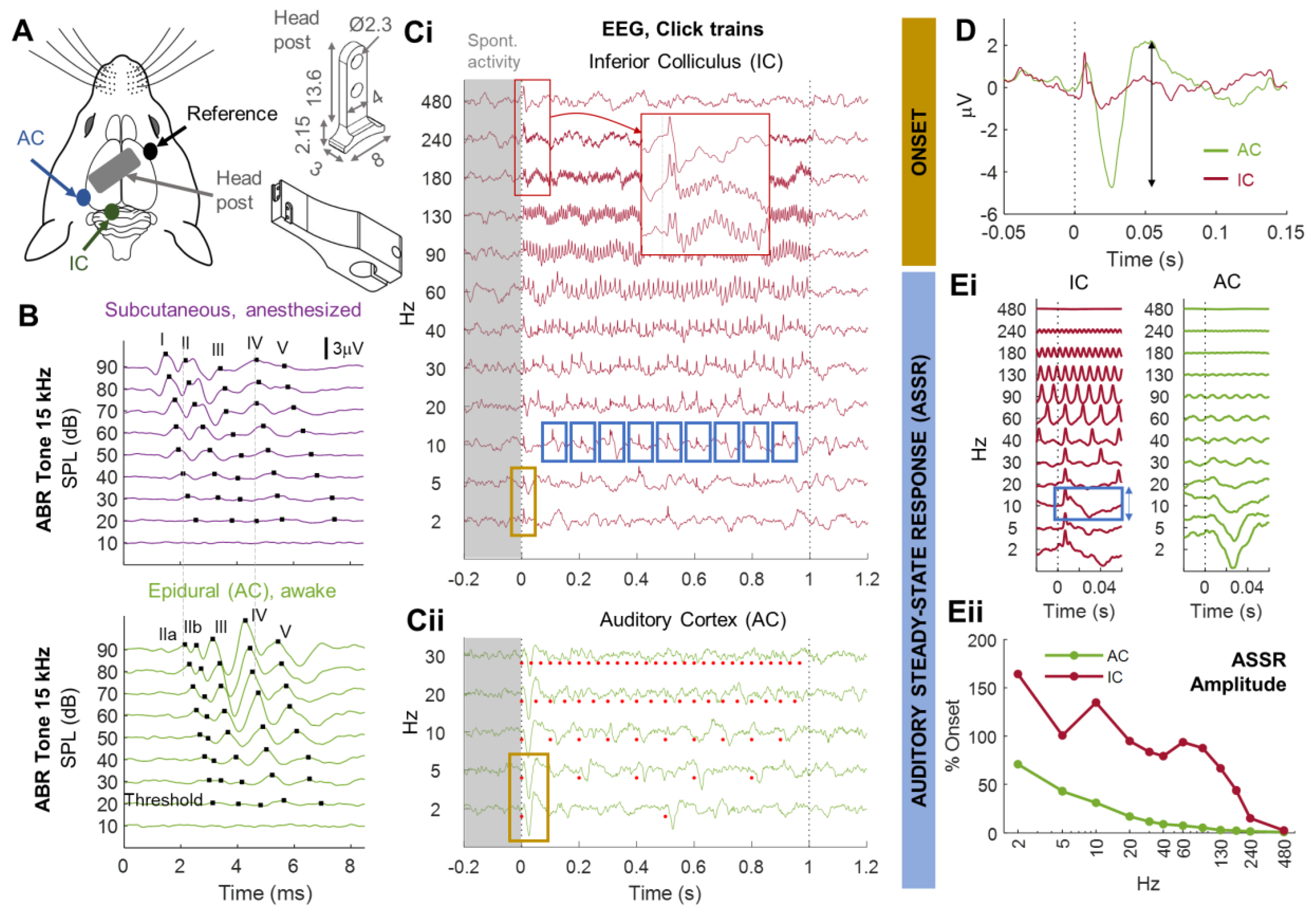
3.1. Monitoring Central Auditory Processing in Head-Fixed Awake Animals
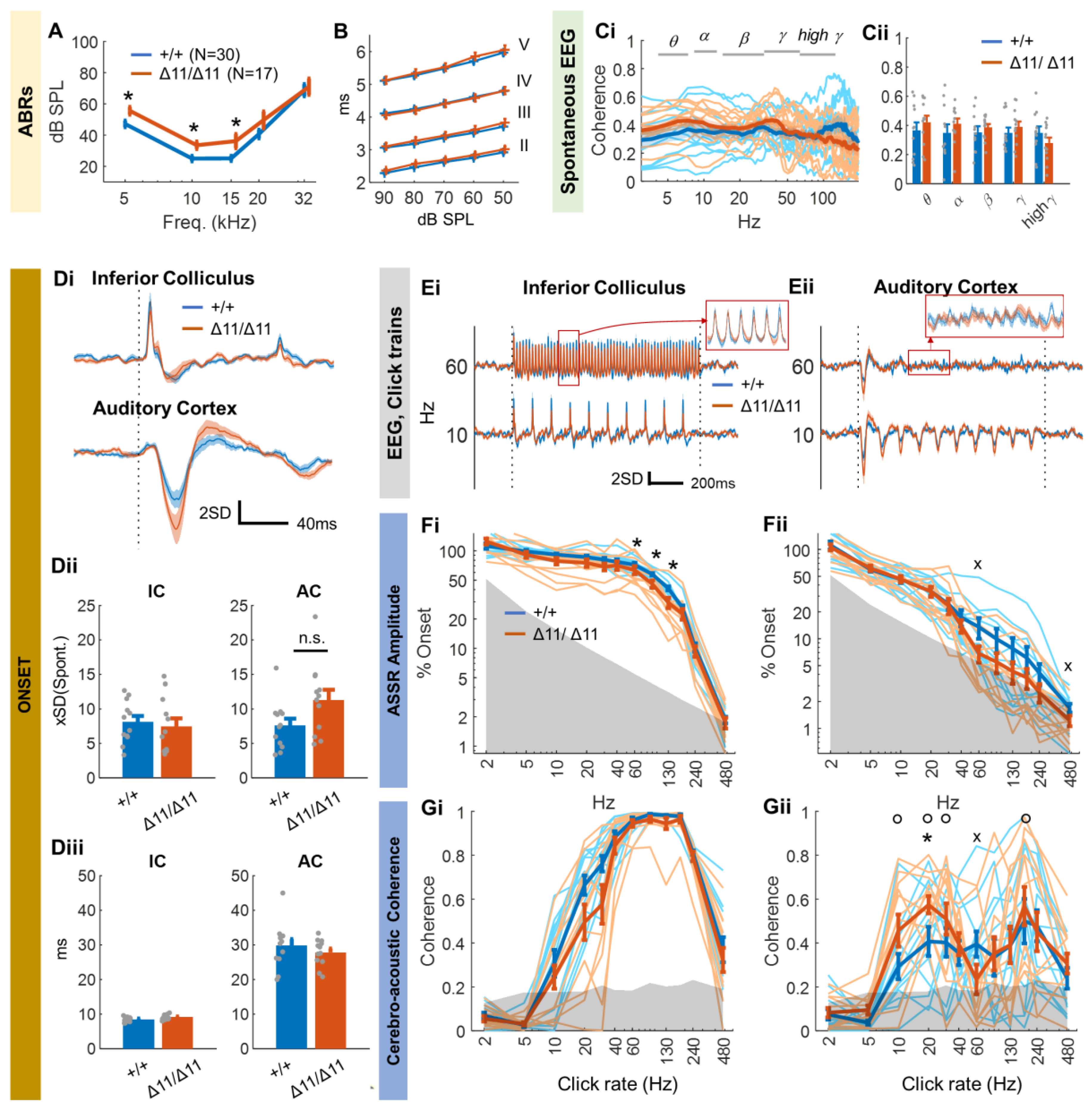
3.2. Central Auditory Processing of Shank3∆11/∆11 Animals
4. Discussion
4.1. Technical Advantages and Limitations
4.2. Shank3∆11/∆11 Mice as a Model of ASD
4.3. Spontaneous and Evoked EEG Signals in the Auditory Pathways of Shank3∆11/∆11 Mice
4.4. ASSR in Shank3∆11/∆11 Animals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Statistical Tests
| Reference Index | Test Description | Statistics | p Value |
| a | 2-factor ANOVA, frequency and genotype with interaction, genotype effect | F(1;209) = 14.8 | 2 × 10−4 |
| b | Post-hoc t-test wave II 5 kHz, ctrl vs. Shank3∆11/∆11 | 0.01 | |
| c | Post-hoc t-test wave II 10 kHz, ctrl vs. Shank3∆11/∆11 | 8 × 10−3 | |
| d | Post-hoc t-test wave II 15 kHz, ctrl vs. Shank3∆11/∆11 | 0.03 | |
| e | 3-factor ANOVA, wave, SPL and genotype with genotype * wave interaction | F(3;912) = 2.89 | 0.15 |
| f | 3-factor ANOVA frequency band, cerebral area and genotype with frequency band x genotype interaction | F(4;214) = 0.33 | 0.86 |
| g | 2-factor ANOVA frequency band and genotype with frequency band x genotype interaction | F(4;105) = 0.79 | 0.54 |
| h | t-test onset amplitude in the IC, ctrl vs. Shank3∆11/∆11 | 0.67 | |
| i | t-test onset amplitude in the AC, ctrl vs. Shank3∆11/∆11 | 0.054 | |
| j | t-test onset latency in the IC, ctrl vs. Shank3∆11/∆11 | 0.06 | |
| k | t-test onset latency in the AC, ctrl vs. Shank3∆11/∆11 | 0.38 | |
| l | 3-factor ANOVA click rate, cerebral area and genotype with stimulus x genotype interaction | F(11;443) = 2.2 | 0.01 |
| m | Post-hoc t-test, click rate 60 Hz, IC, ctrl vs. Shank3∆11/∆11 | 0.04 | |
| n | Post-hoc t-test, click rate 90 Hz, IC, ctrl vs. Shank3∆11/∆11 | 0.03 | |
| o | Post-hoc t-test, click rate 130 Hz, IC, ctrl vs. Shank3∆11/∆11 | 0.04 | |
| p | Proportion test on ASSR amplitude, click rate 60 Hz, AC, ctrl vs. Shank3∆11/∆11 | 0.04 | |
| q | t-test, click rate 60 Hz vs. 10, 20, 30, 180 Hz, AC, Shank3∆11/∆11 | p < 0.04 | |
| r | Proportion test on CAC, click rate 60 Hz, AC, ctrl vs. Shank3∆11/∆11 | 0.04 | |
| s | Post-hoc t-test, click rate 20 Hz, AC, ctrl vs. Shank3∆11/∆11 | 0.04 |
References
- Ingham, N.J. Evoked Potential Recordings of Auditory Brainstem Activity in the Mouse: An Optimized Method for the Assessment of Hearing Function of Mice. Bio Protoc. 2019, 9, e3447. [Google Scholar] [CrossRef]
- Rüttiger, L.; Zimmermann, U.; Knipper, M. Biomarkers for Hearing Dysfunction: Facts and Outlook. ORL 2017, 79, 93–111. [Google Scholar] [CrossRef]
- Felix, R.A.; Gourévitch, B.; Portfors, C.V. Subcortical Pathways: Towards a Better Understanding of Auditory Disorders. Hear. Res. 2018, 362, 48–60. [Google Scholar] [CrossRef]
- Bellis, T.J.; Bellis, J.D. Chapter 30—Central Auditory Processing Disorders in Children and Adults. In Handbook of Clinical Neurology; Aminoff, M.J., Boller, F., Swaab, D.F., Eds.; The Human Auditory System; Elsevier: Amsterdam, The Netherlands, 2015; Volume 129, pp. 537–556. [Google Scholar]
- Atcherson, S.R.; Nagaraj, N.K.; Kennett, S.E.W.; Levisee, M. Overview of Central Auditory Processing Deficits in Older Adults. Semin. Hear. 2015, 36, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Sardone, R.; Battista, P.; Panza, F.; Lozupone, M.; Griseta, C.; Castellana, F.; Capozzo, R.; Ruccia, M.; Resta, E.; Seripa, D.; et al. The Age-Related Central Auditory Processing Disorder: Silent Impairment of the Cognitive Ear. Front. Neurosci. 2019, 13, 619. [Google Scholar] [CrossRef]
- Bamiou, D.-E.; Musiek, F.E.; Luxon, L.M. Aetiology and Clinical Presentations of Auditory Processing Disorders—A Review. Arch. Dis. Child. 2001, 85, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R. Auditory Processing Disorder (APD). Ear Hear. 2018, 39, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Idrizbegovic, E.; Hederstierna, C.; Dahlquist, M.; Kämpfe Nordström, C.; Jelic, V.; Rosenhall, U. Central Auditory Function in Early Alzheimer’s Disease and in Mild Cognitive Impairment. Age Ageing 2011, 40, 249–254. [Google Scholar] [CrossRef]
- Gates, G.A.; Anderson, M.L.; Feeney, M.P.; McCurry, S.M.; Larson, E.B. Central Auditory Dysfunction in Older Persons with Memory Impairment or Alzheimer Dementia. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 771–777. [Google Scholar] [CrossRef]
- Ocak, E.; Eshraghi, R.S.; Danesh, A.; Mittal, R.; Eshraghi, A.A. Central Auditory Processing Disorders in Individuals with Autism Spectrum Disorders. Balk. Med. J. 2018, 35, 367–372. [Google Scholar] [CrossRef]
- Kozou, H.; Azouz, H.G.; Abdou, R.M.; Shaltout, A. Evaluation and Remediation of Central Auditory Processing Disorders in Children with Autism Spectrum Disorders. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 36–42. [Google Scholar] [CrossRef]
- Gonçalves, L.F.; Paiva, K.M.; Patatt, F.S.A.; Stolz, J.V.; Haas, P. Association between Autism Spectrum Disorder and Changes in the Central Auditory Processing in Children. Rev. Assoc. Med. Bras. 2021, 67, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Light, G.A.; Swerdlow, N.R.; Thomas, M.L.; Calkins, M.E.; Green, M.F.; Greenwood, T.A.; Gur, R.E.; Gur, R.C.; Lazzeroni, L.C.; Nuechterlein, K.H.; et al. Validation of Mismatch Negativity and P3a for Use in Multi-Site Studies of Schizophrenia: Characterization of Demographic, Clinical, Cognitive, and Functional Correlates in COGS-2. Schizophr. Res. 2015, 163, 63–72. [Google Scholar] [CrossRef]
- Moschopoulos, N.; Nimatoudis, I.; Kaprinis, S.; Sidiras, C.; Iliadou, V. Auditory Processing Disorder May Be Present in Schizophrenia and It Is Highly Correlated with Formal Thought Disorder. Psychiatry Res. 2020, 291, 113222. [Google Scholar] [CrossRef]
- Ferhat, A.-T.; Halbedl, S.; Schmeisser, M.J.; Kas, M.J.; Bourgeron, T.; Ey, E. Behavioural Phenotypes and Neural Circuit Dysfunctions in Mouse Models of Autism Spectrum Disorder. In Translational Anatomy and Cell Biology of Autism Spectrum Disorder; Schmeisser, M.J., Boeckers, T.M., Eds.; Advances in Anatomy, Embryology and Cell Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 85–101. ISBN 978-3-319-52498-6. [Google Scholar]
- Zerbi, V.; Pagani, M.; Markicevic, M.; Matteoli, M.; Pozzi, D.; Fagiolini, M.; Bozzi, Y.; Galbusera, A.; Scattoni, M.L.; Provenzano, G.; et al. Brain Mapping across 16 Autism Mouse Models Reveals a Spectrum of Functional Connectivity Subtypes. Mol. Psychiatry 2021, 26, 7610–7620. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Kobayashi, H.; Tatsumi, L.; Tomita, T. Mouse Models of Alzheimer’s Disease. Front. Mol. Neurosci. 2022, 15, 912995. [Google Scholar] [CrossRef]
- Götz, J.; Bodea, L.-G.; Goedert, M. Rodent Models for Alzheimer Disease. Nat. Rev. Neurosci. 2018, 19, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Winship, I.R.; Dursun, S.M.; Baker, G.B.; Balista, P.A.; Kandratavicius, L.; Maia-de-Oliveira, J.P.; Hallak, J.; Howland, J.G. An Overview of Animal Models Related to Schizophrenia. Can. J. Psychiatry 2019, 64, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Neijenhuis, K.; Campbell, N.G.; Cromb, M.; Luinge, M.R.; Moore, D.R.; Rosen, S.; de Wit, E. An Evidence-Based Perspective on “Misconceptions” Regarding Pediatric Auditory Processing Disorder. Front. Neurol. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Kraus, N. BioMAP: A Neurodiagnostic Tool for Auditory Processing Disorders. ASHA Leader 2005, 10, 5. [Google Scholar] [CrossRef]
- White-Schwoch, T.; Carr, K.W.; Thompson, E.C.; Anderson, S.; Nicol, T.; Bradlow, A.R.; Zecker, S.G.; Kraus, N. Auditory Processing in Noise: A Preschool Biomarker for Literacy. PLoS Biol. 2015, 13, e1002196. [Google Scholar] [CrossRef]
- Vander Werff, K.R.; Rieger, B. Brainstem Evoked Potential Indices of Subcortical Auditory Processing After Mild Traumatic Brain Injury. Ear Hear. 2017, 38, e200. [Google Scholar] [CrossRef]
- Kraus, N.; Thompson, E.C.; Krizman, J.; Cook, K.; White-Schwoch, T.; LaBella, C.R. Auditory Biological Marker of Concussion in Children. Sci. Rep. 2016, 6, 39009. [Google Scholar] [CrossRef] [PubMed]
- Grent-‘t-Jong, T.; Gajwani, R.; Gross, J.; Gumley, A.I.; Krishnadas, R.; Lawrie, S.M.; Schwannauer, M.; Schultze-Lutter, F.; Uhlhaas, P.J. 40-Hz Auditory Steady-State Responses Characterize Circuit Dysfunctions and Predict Clinical Outcomes in Clinical High-Risk for Psychosis Participants: A Magnetoencephalography Study. Biol. Psychiatry 2021, 90, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-W.; O’Donnell, B.F.; Youn, S.; Kwon, J.S. Gamma Oscillation in Schizophrenia. Psychiatry Investig. 2011, 8, 288–296. [Google Scholar] [CrossRef]
- Van Deursen, J.A.; Vuurman, E.F.P.M.; van Kranen-Mastenbroek, V.H.J.M.; Verhey, F.R.J.; Riedel, W.J. 40-Hz Steady State Response in Alzheimer’s Disease and Mild Cognitive Impairment. Neurobiol. Aging 2011, 32, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A.; Rippon, G.; Gooding-Williams, G.; Sowman, P.F.; Kessler, K. Reduced Auditory Steady State Responses in Autism Spectrum Disorder. Mol. Autism 2020, 11, 56. [Google Scholar] [CrossRef]
- Sugiyama, S.; Ohi, K.; Kuramitsu, A.; Takai, K.; Muto, Y.; Taniguchi, T.; Kinukawa, T.; Takeuchi, N.; Motomura, E.; Nishihara, M.; et al. The Auditory Steady-State Response: Electrophysiological Index for Sensory Processing Dysfunction in Psychiatric Disorders. Front. Psychiatry 2021, 12, 644541. [Google Scholar] [CrossRef]
- Gordon-Salant, S.; Fitzgibbons, P.J. Temporal Factors and Speech Recognition Performance in Young and Elderly Listeners. J. Speech Hear. Res. 1993, 36, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.L.; Gordon-Salant, S.; Fitzgibbons, P.J.; Yeni-Komshian, G. Frequency and Temporal Resolution in Elderly Listeners with Good and Poor Word Recognition. J. Speech Lang. Hear. Res. 2000, 43, 217–228. [Google Scholar] [CrossRef]
- Anderson, S.; Karawani, H. Objective Evidence of Temporal Processing Deficits in Older Adults. Hear. Res. 2020, 397, 108053. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Herrmann, B.; Bartlett, E.L. Aging Alters Envelope Representations of Speech-like Sounds in the Inferior Colliculus. Neurobiol. Aging 2019, 73, 30–40. [Google Scholar] [CrossRef]
- Parthasarathy, A.; Bartlett, E.L.; Kujawa, S.G. Age-Related Changes in Neural Coding of Envelope Cues: Peripheral Declines and Central Compensation. Neuroscience 2019, 407, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Chang, W.-P.; Gavin, W. Middle and Late Latency ERP Components Discriminate between Adults, Typical Children, and Children with Sensory Processing Disorders. Front. Integr. Neurosci. 2010, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, V.; Deiber, M.P.; Fischer, C. Middle Latency Auditory Evoked Potentials in Cortical Lesions. Critical of Interhemispheric Asymmetry. Arch. Neurol. 1989, 46, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M. Late Auditory Event-Related Potentials in Children with Cochlear Implants: A Review. Dev. Neuropsychol. 2009, 34, 701–720. [Google Scholar] [CrossRef]
- Litscher, G. Middle Latency Auditory Evoked Potentials in Intensive Care Patients and Normal Controls. Int. J. Neurosci. 1995, 83, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Supp, G.G.; Higgen, F.L.; Hipp, J.F.; Engel, A.K.; Siegel, M. Mid-Latency Auditory Evoked Potentials Differentially Predict Sedation and Drug Level Under Opioid and Hypnotic Agents. Front. Pharmacol. 2018, 9, 1427. [Google Scholar] [CrossRef]
- Winkler, I.; Denham, S.; Escera, C. Auditory Event-Related Potentials. In Encyclopedia of Computational Neuroscience; Jaeger, D., Jung, R., Eds.; Springer: New York, NY, USA, 2013; pp. 1–29. ISBN 978-1-4614-7320-6. [Google Scholar]
- Postal, O.; Bakay, W.; Dupont, T.; Buck, A.; Daoud, É.; Petit, C.; Michalski, N.; Gourévitch, B. Characterizing Subcutaneous Cortical Auditory Evoked Potentials in Mice. Hear. Res. 2022, 422, 108566. [Google Scholar] [CrossRef]
- Farley, B.J.; Morozova, E.; Dion, J.; Wang, B.; Harvey, B.D.; Gianni, D.; Wipke, B.; Cadavid, D.; Wittmann, M.; Hajos, M. Evoked Potentials as a Translatable Biomarker to Track Functional Remyelination. Mol. Cell. Neurosci. 2019, 99, 103393. [Google Scholar] [CrossRef] [PubMed]
- Metzger, K.L.; Maxwell, C.R.; Liang, Y.; Siegel, S.J. Effects of Nicotine Vary Across Two Auditory Evoked Potentials in the Mouse. Biol. Psychiatry 2007, 61, 23–30. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, J.A.; Conway, B.A. Classical and Controlled Auditory Mismatch Responses to Multiple Physical Deviances in Anaesthetised and Conscious Mice. Eur. J. Neurosci. 2021, 53, 1839–1854. [Google Scholar] [CrossRef] [PubMed]
- Siegel, S.J.; Connolly, P.; Liang, Y.; Lenox, R.H.; Gur, R.E.; Bilker, W.B.; Kanes, S.J.; Turetsky, B.I. Effects of Strain, Novelty, and NMDA Blockade on Auditory-Evoked Potentials in Mice. Neuropsychopharmacology 2003, 28, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Heinke, W.; Koelsch, S. The Effects of Anesthetics on Brain Activity and Cognitive Function. Curr. Opin. Anesthesiol. 2005, 18, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.; Grady, S.M.; Krause, B.M.; Uhlrich, D.J.; Manning, K.A.; Banks, M.I. Preferential Effect of Isoflurane on Top-down vs. Bottom-up Pathways in Sensory Cortex. Front. Syst. Neurosci. 2014, 8, 191. [Google Scholar] [CrossRef]
- Madler, C.; Keller, I.; Schwender, D.; Pöppel, E. Sensory Information Processing during General Anaesthesia: Effect of Isoflurane on Auditory Evoked Neuronal Oscillations. Br. J. Anaesth. 1991, 66, 81–87. [Google Scholar] [CrossRef]
- Manninen, P.H.; Lam, A.M.; Nicholas, J.F. The Effects of Isoflurane and Isoflurane-Nitrous Oxide Anesthesia on Brainstem Auditory Evoked Potentials in Humans. Anesth. Analg. 1985, 64, 43–47. [Google Scholar]
- Gaese, B.H.; Ostwald, J. Anesthesia Changes Frequency Tuning of Neurons in the Rat Primary Auditory Cortex. J. Neurophysiol. 2001, 86, 1062–1066. [Google Scholar] [CrossRef]
- Huetz, C.; Philibert, B.; Edeline, J.M. A Spike-Timing Code for Discriminating Conspecific Vocalizations in the Thalamocortical System of Anesthetized and Awake Guinea Pigs. J. Neurosci. 2009, 29, 334–350. [Google Scholar] [CrossRef]
- Noda, T.; Takahashi, H. Anesthetic Effects of Isoflurane on the Tonotopic Map and Neuronal Population Activity in the Rat Auditory Cortex. Eur. J. Neurosci. 2015, 42, 2298–2311. [Google Scholar] [CrossRef]
- Durand, C.M.; Betancur, C.; Boeckers, T.M.; Bockmann, J.; Chaste, P.; Fauchereau, F.; Nygren, G.; Rastam, M.; Gillberg, I.C.; Anckarsäter, H.; et al. Mutations in the Gene Encoding the Synaptic Scaffolding Protein SHANK3 Are Associated with Autism Spectrum Disorders. Nat. Genet. 2007, 39, 25–27. [Google Scholar] [CrossRef]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-Analysis of SHANK Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments. PLoS Genet. 2014, 10, e1004580. [Google Scholar] [CrossRef]
- De Chaumont, F.; Lemière, N.; Coqueran, S.; Bourgeron, T.; Ey, E. LMT USV Toolbox, a Novel Methodological Approach to Place Mouse Ultrasonic Vocalizations in Their Behavioral Contexts—A Study in Female and Male C57BL/6J Mice and in Shank3 Mutant Females. Front. Behav. Neurosci. 2021, 15, 735920. [Google Scholar] [CrossRef]
- De Chaumont, F.; Ey, E.; Torquet, N.; Lagache, T.; Dallongeville, S.; Imbert, A.; Legou, T.; Sourd, A.-M.L.; Faure, P.; Bourgeron, T.; et al. Real-Time Analysis of the Behaviour of Groups of Mice via a Depth-Sensing Camera and Machine Learning. Nat. Biomed. Eng. 2019, 3, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Bozdagi, O.; Sakurai, T.; Papapetrou, D.; Wang, X.; Dickstein, D.L.; Takahashi, N.; Kajiwara, Y.; Yang, M.; Katz, A.M.; Scattoni, M.L.; et al. Haploinsufficiency of the Autism-Associated Shank3 Gene Leads to Deficits in Synaptic Function, Social Interaction, and Social Communication. Mol. Autism 2010, 1, 15. [Google Scholar] [CrossRef]
- Peça, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 Mutant Mice Display Autistic-like Behaviours and Striatal Dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; McCoy, P.A.; Rodriguiz, R.M.; Pan, Y.; Je, H.S.; Roberts, A.C.; Kim, C.J.; Berrios, J.; Colvin, J.S.; Bousquet-Moore, D.; et al. Synaptic Dysfunction and Abnormal Behaviors in Mice Lacking Major Isoforms of Shank3. Hum. Mol. Genet. 2011, 20, 3093–3108. [Google Scholar] [CrossRef] [PubMed]
- Ferhat, A.-T.; Verpy, E.; Biton, A.; Forget, B.; De Chaumont, F.; Mueller, F.; Le Sourd, A.-M.; Coqueran, S.; Schmitt, J.; Rochefort, C.; et al. Excessive Self-Grooming, Gene Dysregulation and Imbalance between the Striosome and Matrix Compartments in the Striatum of Shank3 Mutant Mice. Front. Mol. Neurosci. 2023, 16, 1139118. [Google Scholar] [CrossRef]
- Schmeisser, M.J.; Ey, E.; Wegener, S.; Bockmann, J.; Stempel, A.V.; Kuebler, A.; Janssen, A.-L.; Udvardi, P.T.; Shiban, E.; Spilker, C.; et al. Autistic-like Behaviours and Hyperactivity in Mice Lacking ProSAP1/Shank2. Nature 2012, 486, 256–260. [Google Scholar] [CrossRef]
- Keithley, E.M.; Canto, C.; Zheng, Q.Y.; Fischel-Ghodsian, N.; Johnson, K.R. Age-Related Hearing Loss and the Ahl Locus in Mice. Hear. Res. 2004, 188, 21–28. [Google Scholar] [CrossRef]
- Arnal, L.H.; Kleinschmidt, A.; Spinelli, L.; Giraud, A.-L.; Mégevand, P. The Rough Sound of Salience Enhances Aversion through Neural Synchronisation. Nat. Commun. 2019, 10, 3671. [Google Scholar] [CrossRef] [PubMed]
- Peelle, J.E.; Gross, J.; Davis, M.H. Phase-Locked Responses to Speech in Human Auditory Cortex Are Enhanced During Comprehension. Cereb. Cortex 2013, 23, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.X.; Schreiner, C.E.; Rees, A. Neural Processing of Amplitude-Modulated Sounds. Physiol. Rev. 2004, 84, 541–577. [Google Scholar] [CrossRef] [PubMed]
- Van Hemmen, J.L. Vector Strength after Goldberg, Brown, and von Mises: Biological and Mathematical Perspectives. Biol. Cybern. 2013, 107, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.M.; Timi, P.; Hong, L.E.; O’Donnell, P. Effects of NMDA and GABA-A Receptor Antagonism on Auditory Steady-State Synchronization in Awake Behaving Rats. Int. J. Neuropsychopharmacol. 2015, 18, pyu118. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, S.; Huang, D.; Wu, X.; Zhang, Y. Role of Inter-Trial Phase Coherence in Atypical Auditory Evoked Potentials to Speech and Nonspeech Stimuli in Children with Autism. Clin. Neurophysiol. 2018, 129, 1374–1382. [Google Scholar] [CrossRef]
- Van Diepen, R.M.; Mazaheri, A. The Caveats of Observing Inter-Trial Phase-Coherence in Cognitive Neuroscience. Sci. Rep. 2018, 8, 2990. [Google Scholar] [CrossRef]
- Lovelace, J.W.; Ethell, I.M.; Binder, D.K.; Razak, K.A. Translation-Relevant EEG Phenotypes in a Mouse Model of Fragile X Syndrome. Neurobiol. Dis. 2018, 115, 39–48. [Google Scholar] [CrossRef]
- Nakao, K.; Nakazawa, K. Brain State-Dependent Abnormal LFP Activity in the Auditory Cortex of a Schizophrenia Mouse Model. Front. Neurosci. 2014, 8, 168. [Google Scholar] [CrossRef]
- Hirano, Y.; Oribe, N.; Kanba, S.; Onitsuka, T.; Nestor, P.G.; Spencer, K.M. Spontaneous Gamma Activity in Schizophrenia. JAMA Psychiatry 2015, 72, 813–821. [Google Scholar] [CrossRef]
- Land, R.; Burghard, A.; Kral, A. The Contribution of Inferior Colliculus Activity to the Auditory Brainstem Response (ABR) in Mice. Hear. Res. 2016, 341, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Scimemi, P.; Santarelli, R.; Selmo, A.; Mammano, F. Auditory Brainstem Responses to Clicks and Tone Bursts in C57 BL/6J Mice. Acta Otorhinolaryngol. Ital. 2014, 34, 264–271. [Google Scholar] [PubMed]
- Shahriari, Y.; Krusienski, D.; Dadi, Y.S.; Seo, M.; Shin, H.-S.; Choi, J.H. Impaired Auditory Evoked Potentials and Oscillations in Frontal and Auditory Cortex of a Schizophrenia Mouse Model. World J. Biol. Psychiatry 2016, 17, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Wang, S.; Wang, X.; Chen, J.; Qin, L. Laminar Profile of Auditory Steady-State Response in the Auditory Cortex of Awake Mice. Front. Syst. Neurosci. 2021, 15, 636395. [Google Scholar] [CrossRef]
- Sivarao, D.V.; Chen, P.; Senapati, A.; Yang, Y.; Fernandes, A.; Benitex, Y.; Whiterock, V.; Li, Y.-W.; Ahlijanian, M.K. 40 Hz Auditory Steady-State Response Is a Pharmacodynamic Biomarker for Cortical NMDA Receptors. Neuropsychopharmacol 2016, 41, 2232–2240. [Google Scholar] [CrossRef]
- Robertson, C.E.; Baron-Cohen, S. Sensory Perception in Autism. Nat. Rev. Neurosci. 2017, 18, 671–684. [Google Scholar] [CrossRef]
- Vicidomini, C.; Ponzoni, L.; Lim, D.; Schmeisser, M.J.; Reim, D.; Morello, N.; Orellana, D.; Tozzi, A.; Durante, V.; Scalmani, P.; et al. Pharmacological Enhancement of mGlu5 Receptors Rescues Behavioral Deficits in SHANK3 Knock-out Mice. Mol. Psychiatry 2017, 22, 689–702. [Google Scholar] [CrossRef]
- Guilmatre, A.; Huguet, G.; Delorme, R.; Bourgeron, T. The Emerging Role of SHANK Genes in Neuropsychiatric Disorders. Dev. Neurobiol. 2014, 74, 113–122. [Google Scholar] [CrossRef]
- Vitrac, A.; Leblond, C.S.; Rolland, T.; Cliquet, F.; Mathieu, A.; Maruani, A.; Delorme, R.; Schön, M.; Grabrucker, A.M.; van Ravenswaaij-Arts, C.; et al. Dissecting the 22q13 Region to Explore the Genetic and Phenotypic Diversity of Patients with Phelan-McDermid Syndrome. Eur. J. Med. Genet. 2023, 66, 104732. [Google Scholar] [CrossRef]
- Wang, A.T.; Lim, T.; Jamison, J.; Bush, L.; Soorya, L.V.; Tavassoli, T.; Siper, P.M.; Buxbaum, J.D.; Kolevzon, A. Neural Selectivity for Communicative Auditory Signals in Phelan-McDermid Syndrome. J. Neurodev. Disord. 2016, 8, 5. [Google Scholar] [CrossRef]
- Drapeau, E.; Riad, M.; Kajiwara, Y.; Buxbaum, J.D. Behavioral Phenotyping of an Improved Mouse Model of Phelan–McDermid Syndrome with a Complete Deletion of the Shank3 Gene. eNeuro 2018, 5, 3. [Google Scholar] [CrossRef]
- Amal, H.; Barak, B.; Bhat, V.; Gong, G.; Joughin, B.A.; Wang, X.; Wishnok, J.S.; Feng, G.; Tannenbaum, S.R. Shank3 Mutation in a Mouse Model of Autism Leads to Changes in the S-Nitroso-Proteome and Affects Key Proteins Involved in Vesicle Release and Synaptic Function. Mol. Psychiatry 2020, 25, 1835–1848. [Google Scholar] [CrossRef]
- Holder, J.L.; Quach, M.M. The Spectrum of Epilepsy and Electroencephalographic Abnormalities Due to SHANK3 Loss of Function Mutations. Epilepsia 2016, 57, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Figura, M.G.; Coppola, A.; Bottitta, M.; Calabrese, G.; Grillo, L.; Luciano, D.; Gaudio, L.D.; Torniero, C.; Striano, S.; Elia, M. Seizures and EEG Pattern in the 22q13.3 Deletion Syndrome: Clinical Report of Six Italian Cases. Seizure Eur. J. Epilepsy 2014, 23, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Edgar, J.C.; Ehrlichman, R.S.; Mehta, M.; Roberts, T.P.L.; Siegel, S.J. Validating γ Oscillations and Delayed Auditory Responses as Translational Biomarkers of Autism. Biol. Psychiatry 2010, 68, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.W.; Rojas, D.C.; Reite, M.L.; Teale, P.D.; Rogers, S.J. Children and Adolescents with Autism Exhibit Reduced MEG Steady-State Gamma Responses. Biol. Psychiatry 2007, 62, 192–197. [Google Scholar] [CrossRef]
- Edgar, J.C.; Fisk, C.L.; Liu, S.; Pandey, J.; Herrington, J.D.; Schultz, R.T.; Roberts, T.P.L. Translating Adult Electrophysiology Findings to Younger Patient Populations: Difficulty Measuring 40-Hz Auditory Steady-State Responses in Typically Developing Children and Children with Autism Spectrum Disorder. Dev. Neurosci. 2016, 38, 1–14. [Google Scholar] [CrossRef]
- Simon, D.M.; Wallace, M.T. Dysfunction of Sensory Oscillations in Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 2016, 68, 848–861. [Google Scholar] [CrossRef]
- Roberts, T.P.L.; Khan, S.Y.; Rey, M.; Monroe, J.F.; Cannon, K.; Blaskey, L.; Woldoff, S.; Qasmieh, S.; Gandal, M.; Schmidt, G.L.; et al. MEG Detection of Delayed Auditory Evoked Responses in Autism Spectrum Disorders: Towards an Imaging Biomarker for Autism. Autism Res. 2010, 3, 8–18. [Google Scholar] [CrossRef]
- Cui, T.; Wang, P.P.; Liu, S.; Zhang, X. P300 Amplitude and Latency in Autism Spectrum Disorder: A Meta-Analysis. Eur. Child. Adolesc. Psychiatry 2017, 26, 177–190. [Google Scholar] [CrossRef]
- Miron, O.; Beam, A.L.; Kohane, I.S. Auditory Brainstem Response in Infants and Children with Autism Spectrum Disorder: A Meta-Analysis of Wave V. Autism Res. 2018, 11, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Wilde, M.; Constantin, L.; Thorne, P.R.; Montgomery, J.M.; Scott, E.K.; Cheyne, J.E. Auditory Processing in Rodent Models of Autism: A Systematic Review. J. Neurodev. Disord. 2022, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Dhamne, S.C.; Silverman, J.L.; Super, C.E.; Lammers, S.H.T.; Hameed, M.Q.; Modi, M.E.; Copping, N.A.; Pride, M.C.; Smith, D.G.; Rotenberg, A.; et al. Replicable in Vivo Physiological and Behavioral Phenotypes of the Shank3B Null Mutant Mouse Model of Autism. Mol. Autism 2017, 8, 26. [Google Scholar] [CrossRef]
- Delling, J.P.; Boeckers, T.M. Comparison of SHANK3 Deficiency in Animal Models: Phenotypes, Treatment Strategies, and Translational Implications. J. Neurodev. Disord. 2021, 13, 55. [Google Scholar] [CrossRef]
- Engineer, C.T.; Rahebi, K.C.; Borland, M.S.; Buell, E.P.; Im, K.W.; Wilson, L.G.; Sharma, P.; Vanneste, S.; Harony-Nicolas, H.; Buxbaum, J.D.; et al. Shank3-Deficient Rats Exhibit Degraded Cortical Responses to Sound. Autism Res. 2018, 11, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.C.; Teale, P.D.; Maharajh, K.; Kronberg, E.; Youngpeter, K.; Wilson, L.B.; Wallace, A.; Hepburn, S. Transient and Steady-State Auditory Gamma-Band Responses in First-Degree Relatives of People with Autism Spectrum Disorder. Mol. Autism 2011, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Neklyudova, A.K.; Portnova, G.V.; Rebreikina, A.B.; Voinova, V.Y.; Vorsanova, S.G.; Iourov, I.Y.; Sysoeva, O.V. 40-Hz Auditory Steady-State Response (ASSR) as a Biomarker of Genetic Defects in the SHANK3 Gene: A Case Report of 15-Year-Old Girl with a Rare Partial SHANK3 Duplication. Int. J. Mol. Sci. 2021, 22, 1898. [Google Scholar] [CrossRef]
- Arutiunian, V.; Arcara, G.; Buyanova, I.; Davydova, E.; Pereverzeva, D.; Sorokin, A.; Tyushkevich, S.; Mamokhina, U.; Danilina, K.; Dragoy, O. Neuromagnetic 40 Hz Auditory Steady-State Response in the Left Auditory Cortex Is Related to Language Comprehension in Children with Autism Spectrum Disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 122, 110690. [Google Scholar] [CrossRef]
- Ono, Y.; Kudoh, K.; Ikeda, T.; Takahashi, T.; Yoshimura, Y.; Minabe, Y.; Kikuchi, M. Auditory Steady-State Response at 20 Hz and 40 Hz in Young Typically Developing Children and Children with Autism Spectrum Disorder. Psychiatry Clin. Neurosci. 2020, 74, 354–361. [Google Scholar] [CrossRef]
- Snijders, T.M.; Milivojevic, B.; Kemner, C. Atypical Excitation–Inhibition Balance in Autism Captured by the Gamma Response to Contextual Modulation. NeuroImage Clin. 2013, 3, 65–72. [Google Scholar] [CrossRef]
- Whittington, M.A.; Traub, R.D.; Jefferys, J.G.R. Synchronized Oscillations in Interneuron Networks Driven by Metabotropic Glutamate Receptor Activation. Nature 1995, 373, 612–615. [Google Scholar] [CrossRef] [PubMed]
- Whittington, M.A.; Traub, R.D.; Kopell, N.; Ermentrout, B.; Buhl, E.H. Inhibition-Based Rhythms: Experimental and Mathematical Observations on Network Dynamics. Int. J. Psychophysiol. 2000, 38, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Carlén, M.; Meletis, K.; Siegle, J.H.; Cardin, J.A.; Futai, K.; Vierling-Claassen, D.; Rühlmann, C.; Jones, S.R.; Deisseroth, K.; Sheng, M.; et al. A Critical Role for NMDA Receptors in Parvalbumin Interneurons for Gamma Rhythm Induction and Behavior. Mol. Psychiatry 2012, 17, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Traub, R.D.; Jefferys, J.G.; Whittington, M.A. Simulation of Gamma Rhythms in Networks of Interneurons and Pyramidal Cells. J. Comput. Neurosci. 1997, 4, 141–150. [Google Scholar] [CrossRef]
- Nguyen, Q.-A.; Rubchinsky, L.L. Temporal Patterns of Synchrony in a Pyramidal-Interneuron Gamma (PING) Network. Chaos Interdiscip. J. Nonlinear Sci. 2021, 31, 043134. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Li, X.; Zhao, X.; Ye, Q.; Lin, Y.; Tao, H.W.; Rasch, M.J.; Zhang, X. Distinct Inhibitory Circuits Orchestrate Cortical Beta and Gamma Band Oscillations. Neuron 2017, 96, 1403–1418.e6. [Google Scholar] [CrossRef]
- Gourévitch, B.; Martin, C.; Postal, O.; Eggermont, J.J. Oscillations in the Auditory System and Their Possible Role. Neurosci. Biobehav. Rev. 2020, 113, 507–528. [Google Scholar] [CrossRef]
- Experiment Detail: Allen Brain Atlas: Mouse Brain. Available online: http://mouse.brain-map.org/experiment/show/69816840 (accessed on 29 June 2023).
- Golden, C.E.M.; Wang, V.X.; Harony-Nicolas, H.; Hof, P.R.; Buxbaum, J.D. Reduced Brain Volume and White Matter Alterations in Shank3-Deficient Rats. Autism Res. 2021, 14, 1837–1842. [Google Scholar] [CrossRef]
- Bracic, G.; Hegmann, K.; Engel, J.; Kurt, S. Impaired Subcortical Processing of Amplitude-Modulated Tones in Mice Deficient for Cacna2d3, a Risk Gene for Autism Spectrum Disorders in Humans. eNeuro 2022, 9, 2. [Google Scholar] [CrossRef]
- Van Looij, M.A.; Liem, S.S.; van der Burg, H.; van der Wees, J.; De Zeeuw, C.I.; van Zanten, B.G. Impact of Conventional Anesthesia on Auditory Brainstem Responses in Mice. Hear. Res. 2004, 193, 75–82. [Google Scholar] [CrossRef]
- Rojas, M.J.; Navas, J.A.; Rector, D.M. Evoked Response Potential Markers for Anesthetic and Behavioral States. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 291, R189–R196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Antunes, L.M.; Golledge, H.D.; Roughan, J.V.; Flecknell, P.A. Comparison of Electroencephalogram Activity and Auditory Evoked Responses during Isoflurane and Halothane Anaesthesia in the Rat. Vet. Anaesth. Analg. 2003, 30, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bey, A.L.; Katz, B.M.; Badea, A.; Kim, N.; David, L.K.; Duffney, L.J.; Kumar, S.; Mague, S.D.; Hulbert, S.W.; et al. Altered mGluR5-Homer Scaffolds and Corticostriatal Connectivity in a Shank3 Complete Knockout Model of Autism. Nat. Commun. 2016, 7, 11459. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dejean, C.; Dupont, T.; Verpy, E.; Gonçalves, N.; Coqueran, S.; Michalski, N.; Pucheu, S.; Bourgeron, T.; Gourévitch, B. Detecting Central Auditory Processing Disorders in Awake Mice. Brain Sci. 2023, 13, 1539. https://doi.org/10.3390/brainsci13111539
Dejean C, Dupont T, Verpy E, Gonçalves N, Coqueran S, Michalski N, Pucheu S, Bourgeron T, Gourévitch B. Detecting Central Auditory Processing Disorders in Awake Mice. Brain Sciences. 2023; 13(11):1539. https://doi.org/10.3390/brainsci13111539
Chicago/Turabian StyleDejean, Camille, Typhaine Dupont, Elisabeth Verpy, Noémi Gonçalves, Sabrina Coqueran, Nicolas Michalski, Sylvie Pucheu, Thomas Bourgeron, and Boris Gourévitch. 2023. "Detecting Central Auditory Processing Disorders in Awake Mice" Brain Sciences 13, no. 11: 1539. https://doi.org/10.3390/brainsci13111539
APA StyleDejean, C., Dupont, T., Verpy, E., Gonçalves, N., Coqueran, S., Michalski, N., Pucheu, S., Bourgeron, T., & Gourévitch, B. (2023). Detecting Central Auditory Processing Disorders in Awake Mice. Brain Sciences, 13(11), 1539. https://doi.org/10.3390/brainsci13111539





