Abstract
Background: Currently, there is no effective treatment for amyotrophic lateral sclerosis (ALS), a devastating neurodegenerative disorder. Many biomarkers have been proposed, but because ALS is a clinically heterogeneous disease with an unclear etiology, biomarker discovery for ALS has been challenging due to the lack of specificity of these biomarkers. In recent years, the role of autophagy in the development and treatment of ALS has become a research hotspot. In our previous studies, we found that the expression of RabGGTase (low RABGGTB expression and no change in RABGGTA) is lower in the lumbar and thoracic regions of spinal cord motoneurons in SOD1G93A mice compared with WT (wild-type) mice groups, and upregulation of RABGGTB promoted prenylation modification of Rab7, which promoted autophagy to protect neurons by degrading SOD1. Given that RabGGTase is associated with autophagy and autophagy is associated with inflammation, and based on the above findings, since peripheral blood mononuclear cells are readily available from patients with ALS, we proposed to investigate the expression of RabGGTase in peripheral inflammatory cells. Methods: Information and venous blood were collected from 86 patients diagnosed with ALS between January 2021 and August 2023. Flow cytometry was used to detect the expression of RABGGTB in monocytes from peripheral blood samples collected from patients with ALS and healthy controls. Extracted peripheral blood mononuclear cells (PBMCs) were differentiated in vitro into macrophages, and then the expression of RABGGTB was detected by immunofluorescence. RABGGTB levels in patients with ALS were analyzed to determine their impact on disease progression. Results: Using flow cytometry in monocytes and immunofluorescence in macrophages, we found that RABGGTB expression in the ALS group was significantly higher than in the control group. Age, sex, original location, disease course, C-reactive protein (CRP), and interleukin-6 (IL-6) did not correlate with the ALS functional rating scale—revised (ALSFRS-R), whereas the RABGGTB level was significantly correlated with the ALSFRS-R. In addition, multivariate analysis revealed a significant correlation between RABGGTB and ALSFRS-R score. Further analysis revealed a significant correlation between RABGGTB expression levels and disease progression levels (ΔFS). Conclusions: The RABGGTB level was significantly increased in patients with ALS compared with healthy controls. An elevated RABGGTB level in patients with ALS is associated with the rate of progression in ALS, suggesting that elevated RABGGTB levels in patients with ALS may serve as an indicator for tracking ALS progression.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a chronic, progressive, and fatal neurodegenerative disease with a worldwide incidence of approximately 5/100,000–7/100,000. Pathological alterations occur as motor neurons in the anterior horn cells of the spinal cord, the brain stem, and the cerebral cortex undergo degenerative changes. Patients typically succumb to generalized muscle weakness and respiratory failure, with an average survival time of 3–5 years [1]. ALS is a rare, clinically heterogeneous disease that can be difficult to recognize, especially in the early stages. ALS is often misdiagnosed as more common illnesses due to the lack of specific markers in the diagnostic criteria for ALS, delaying diagnosis. Several biomarkers, including neurofilaments [2,3,4,5,6], interleukin-6 (IL-6) [7,8], C-reactive protein (CRP) [5,9], cholesterol [10], creatinine [11,12,13], uric acid [14,15,16,17], ferritin [18], TDP43 [19,20], and albumin [21], have been proposed as prognostic factors for ALS progression monitoring, but many factors, including age, BMI, etc., may be confounding factors that influence these biomarkers and should therefore be taken into account when evaluating these biomarkers in patients. Several studies [6,22] have suggested that neurofilament be used to monitor disease progression in ALS as well as for a differential diagnosis, but it lacks specificity, as it is a non-specific biomarker for a variety of neurological disorders including AD [23], MS [24], and frontotemporal lobe dementia [25]. A study from JAMA in 2017 found that ALS patients with elevated serum CRP levels progressed more rapidly than patients with lower CRP levels, with researchers concluding that serum CRP can be used as a prognostic biomarker for ALS [9]. CRP, a widely used non-specific biomarker of inflammation, has been found to be elevated in serum CRP in a number of gynecological oncological diseases and can be used as a prognostic indicator, including for cervical, ovarian, endometrial, and vulvar cancers [26,27]. This biomarker lacks specificity, as it is a non-specific biomarker for ALS, so biomarker discovery has been challenging for ALS. Although Riluzole [28] and Edaravone [29] have been approved by the Food and Drug Administration (FDA), there is currently no cure or effective treatment for ALS.
Rab protein [30] regulates cell signal transduction, cell growth, and differentiation as its primary function. Rab resides primarily in the plasma membrane and organelle membranes and is evolutionarily highly conserved. It regulates intracellular vesicle transport and is present in almost all eukaryotes, and is strictly regulated. Rab7 is a member of the Ras superfamily and plays a key role in the late stage of autophagy. Therefore, the dysfunction of Rab protein is linked to multiple human diseases, including cancer ([30,31,32,33,34], infection [30,35,36], cardiac disease [37], and neurodegeneration [38,39,40,41,42,43]. In recent years, an increasing number of researchers [44,45,46,47,48,49,50] have focused on the role of Rab protein in the development of ALS.
In our previous studies, we found that prenylation of Rab7 was inhibited in the ALS model, and RabGGTase mediated prenylation modification of Rab7. Autophagy obstacles have been confirmed to be one of the early pathological events of ALS [51,52], and autophagy plays an important role in inflammation [53]. In our previous studies, we found that the expression of RabGGTase (low RABGGTB expression and no change in RABGGTA) is lower in the lumbar and thoracic regions of spinal cord motoneurons in SOD1G93A mice compared with WT (wild-type) mice groups, and autophagy defects could be ameliorated by modulating RABGGTB in neurons [54]. However, it was difficult to obtain neurons in patients with ALS. RabGGTase is composed of Rab geranylgeranyltransferase subunit alpha (RABGGTA) and Rab geranylgeranyltransferase subunit beta (RABGGTB) [55], and our previous experiments determined that the RABGGTB expression level was decreased in spinal cord motoneurons in SOD1G93A mice, and the onset of SOD1G93A mice was significantly delayed and their survival time was prolonged by intrathecal injection of adeno-associated virus 9 (AAV9) carrying the human single chain RABGGTB gene. Given that RabGGTase is associated with autophagy and autophagy is associated with inflammation, important questions include how the expression of RabGGTase is in the peripheral inflammatory cells in patients with ALS, and whether it is associated with disease progression rate.
In recent years, several studies have focused on the role of RabGGTase in diseases. For instance, Taheri et al. compared the expression of genes coding for the different subunits of proteins implicated in protein prenylation between patients with multiple sclerosis and healthy subjects, and found that RABGGTB was significantly downregulated in the peripheral blood, suggesting dysregulation of the protein prenylation pathway in MS [56], whereas high RABGGTB expression has been reported in tumor-associated disease, and psoromic acid (PA) as a selective Rab-prenylation inhibitor has been proposed to be potentially therapeutic for cancer and osteoporosis [57]. RABGGTB is not used as a biomarker in these diseases. Several biomarkers have been proposed as prognostic factors for monitoring ALS progression, but these biomarkers lack specificity, as they are non-specific biomarkers for ALS, so biomarker discovery has been challenging for ALS.
However, to the best of our knowledge, we are the first to demonstrate that RABGGTB was found to be highly expressed in peripheral mononuclear macrophages of ALS patients compared with healthy controls, and we found that the expression of RABGGTB was significantly correlated with disease progression levels (ΔFS). Increased RABGGTB in patients with ALS could be used as a biomarker for prognostic assessment of ALS.
2. Materials and Methods
2.1. Subjects
The ethics committee of the Second Hospital of Hebei Medical University (No. 2022-R196) approved this research. We collected medical histories, physical examination records, laboratory tests, and electrophysiological examination records, among other data sources, of patients diagnosed with ALS at the Second Hospital of Hebei Medical University’s Department of Neurology between January 2021 and August 2023. All patients satisfied the revised ALS diagnostic criteria [58]. The department of physical examination collected data on the corresponding healthy controls, including gender, age, height, weight, past history, family history, and laboratory examination, among other data. Exclusion criteria included patients with acute or chronic inflammatory diseases, such as acute pneumonia and rheumatoid arthritis. The consent forms were signed by all participants. The ALS functional rating scale—revised (ALSFRS-R) was used to evaluate the severity of the disease [59], and the rate of disease progression was evaluated using the ALSFRS-R score to calculate the progression rate ratio as follows: (48 − ALSFRS-R score at the time of diagnosis)/time from onset to diagnosis. Patients were divided into 3 groups according to ΔFS: slow (ΔFS < 0.5), intermediate (ΔFS = 0.5–1.0), and rapid (ΔFS > 1.0) [60,61].
2.2. Serum Sample
Venous blood was drawn from the elbow of patients with ALS and healthy controls in the morning after an overnight fast, and samples were collected in EDTA vacutainers, and immediately centrifuged for 15 min at ∼2000× g at room temperature. After centrifugation, plasma was removed, aliquoted into polypropylene tubes, and used for biochemical analyses (BECKMAN, AU5800, Brea, CA, USA). All blood indicators, including IL-6 and CRP, were supplied by the laboratory division of the Second Hospital of Hebei Medical University; 0–7 pg/mL was the normal range for adult IL-6, and 0–6 mg/L was the normal range for CRP.
2.3. Flowcytometry
After completing routine examinations, blood samples from patients with ALS and healthy controls were collected. Using flow cytometry, RABGGTB expression levels in peripheral blood mononuclear cells (PBMCs) were determined (BD Biosciences, Franklin Lakes, NJ, USA). Primary antibodies included were monoclonal mouse anti-human CD14 (BD, 555399), monoclonal mouse anti-human CD16 (BD, 560717), Fc block (BD, 564219), and anti-RABGGTB antibodies (1:200, GeneTex, Irvine, CA, USA; GTX105874). The secondary antibody was goat anti-rabbit IgG (H+L) conjugated with fluorescein-5-isothiocyanate (FITC) (Proteintech, Rosemont, IL, USA; SA00003-2).
2.4. Isolation of PBMCs from Blood Samples
Human peripheral blood lymphocyte separation liquid (LTS1077) was added to PBMCs (Tianjin Haoyang Biological Products Science & Technology Co., Ltd., Tianjin, China; 601002), placed in a high-efficiency centrifuge tube, and centrifuged at 200× g for 2 min at room temperature. The peripheral blood samples were then added and centrifuged for an additional 30 min at 800× g. The intermediate mononuclear cell layer was carefully aspirated into a new centrifuge tube and centrifuged at 300× g for 13 min. After aspirating the supernatant, the pellet was washed multiple times with phosphate-buffered saline (PBS), and the PBMCs were resuspended in a cryopreserved solution containing 90% fetal bovine serum (FBS; CellMax, Beijing, China, SA211.02) and 10% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St Louis, MO, USA; D2650-100ML). The solution was then transferred to a 1.8 mL cryopreservation tube and stored at −80 °C overnight; it was then stored in liquid nitrogen for long-term preservation and examination at a later date.
2.5. In Vitro Culture of Macrophages
The previously prepared PBMCs were thawed in preheated Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco, Waltham, MA, USA; C11875500BT) containing 10% FBS. The supernatant was removed after centrifugation at 500× g for 5 min at room temperature, and the cell pellet was resuspended in RPMI-1640 culture medium with 1% penicillin-streptomycin (P/S) and 10% FBS. The culture medium was supplemented with macrophage colony-stimulating factor (M-CSF) (PeproTech, East Windsor, NJ, USA; 300-25-10), and the cells were seeded in 48-well plates for 7 days. The culture medium was replaced every 3 days [61]. On the seventh day of cell culture, the medium was withdrawn and fixed with 4% paraformaldehyde in PBS.
2.6. Immunofluorescence and Confocal Microscopy Analysis
Using a PBS solution containing 4% paraformaldehyde, the cells were fixed on 48-well plate slides. The cells were then permeated with PBS containing 0.3% Triton-X 100 for 15 min and blocked for 1 h at room temperature with PBS containing 10% sheep serum. After adding the primary antibody and allowing it to incubate overnight, the cells were washed three times with PBS and then incubated with the corresponding secondary antibody: donkey anti-rat IgG (H+L) highly cross-adsorbed secondary antibody (CD68(1:500, Abcam, Waltham, MA, USA, ab31630), F4/80(1:200, Abcam, Waltham, MA, USA, ab6640), and RABGGTB (1:500, GeneTeX, San-Antonio, TX, USA, GTX105874)), Alexa Fluor 488 (1:1000; Invitrogen, Carlsbad, CA, USA; A21208), Alexa Fluor 594-conjugated goat anti-mouse secondary antibody (1:1000; Thermo Fisher Scientific, Waltham, MA, USA; #A-11032), and goat anti-rabbit secondary antibody labeled with Alexa Fluor 647 (1:1000, Invitrogen, A21245). After 1 h of staining at room temperature with 4′,6-diamidino-2-phenylindole (DAPI), the cell nuclei were visible. The cells were then washed three times with PBS and observed using a confocal fluorescence microscope (ZEISS, Oberkochen, Germany; LSM900). The parameters of the microscope were set at the start of each individual imaging process and remained constant throughout.
2.7. Statistical Analysis
All enumeration data are presented as mean ± standard deviation (SD), and the Shapiro–Wilk test was used to examine the data distribution. For data with a normal distribution, the unpaired t-test was used to analyze the differences between two groups, whereas the Pearson’s correlation coefficient test was utilized to analyze the correlation. The Mann–Whitney U test was used for analysis of differences, Spearman’s rank correlation coefficient test was used for correlation analysis, and the multivariable regression model was adjusted for age, sex, and body mass index (BMI). All statistical analyses were conducted using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA) and SPSS26. p < 0.05 was considered statistically significant.
3. Results
3.1. General Information of the Participants
The data of 71 patients from 86 patients diagnosed with ALS at the Second Hospital of Hebei Medical University between January 2021 and August 2023 were collected (Figure 1). The healthy control group consisted of 54 subjects (male: 35, female: 19) with a mean age of 56 ± 8 years and BMI of 23.44 ± 1.839. The ALS group included a total of 71 patients (male: 47, female: 24), with a mean age of 58 ± 9 years. The distributions of age, gender, and BMI did not differ significantly (Table 1).
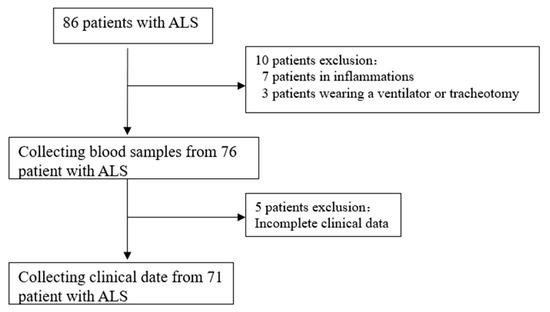
Figure 1.
ALS patient screening. ALS, amyotrophic lateral sclerosis.

Table 1.
Summary of donor information.
3.2. Elevated RABGGTB Levels Were Detected in the Monocytes from Patients with ALS
Flow cytometry was used to detect RABGGTB levels in monocytes from patients with ALS compared with healthy controls. Monocytes are important mononuclear blood cells that participate in the immune and inflammatory responses of humans. Cells were divided into three subpopulations based on the level of CD14 and CD16 surface expression: classical (CD14++CD16), intermediate (CD14++CD16+), and nonclassical (CD14+CD16+) monocyte subpopulations [62]. Comparing the expression levels of RABGGTB in classical-type monocytes between the groups, we discovered that RABGGTB was significantly upregulated in the peripheral blood of patients with ALS (Figure 2A–C).
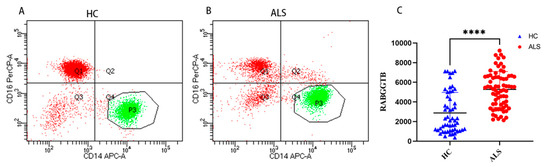
Figure 2.
RABGGTB expression in monocytes from healthy control and patients with ALS. (A) Analysis of peripheral blood classical-type monocytes by flow cytometry in healthy controls. (B) Analysis of peripheral blood classical-type monocytes by flow cytometry in patients with ALS. (C) Quantitative analysis of the RABGGTB concentration in peripheral blood monocytes of classical type. The statistical significance was determined using an unpaired t-test. ****, p < 0.0001. HC, healthy control; ALS, amyotrophic lateral sclerosis; RABGGTB, Rab geranylgeranyltransferase subunit beta.
3.3. Correlations between RABGGTB Levels in Monocytes with ALS Disease Severity or Progression Rates
The above findings suggest that RABGGTB expression may be associated with the clinical status of patients with ALS. We included indexes such as gender, age, BMI, site of disease onset, disease progression, CRP, IL-6, RABGGTB, and ALSFRS-R score. We first determined the relationship between disease severity and the aforementioned indicators. Gender, disease course, site of onset, BMI, CRP, and IL-6 did not correlate with ALSFRS-R score (Figure S1), whereas age and RABGGTB level were significantly correlated with ALSFRS-R score (p = 0.0418, p = 0.0441, respectively). Further multivariate analysis revealed a significant correlation between age and ALSFRS-R score (p = 0.0079). The results suggest that age and the expression of RABGGTB are associated with the severity of ALS (Figure 3A,B).
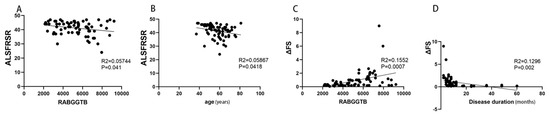
Figure 3.
Correlation between ALSFRS-R score or ΔFS and RABGGTB expression in monocytes from ALS patients. (A) Correlation between ALSFRS-R and the expression of RABGGTB in monocytes from ALS patients. (B) Correlation between ALSFRS-R and age in ALS. (C) Correlation between ΔFS and the expression of RABGGTB in monocytes from ALS patients. (D) Correlation between ΔFS and the duration of the disease in ALS.
Then we evaluated the correlation between the indicators and the progression rate of the disease. Age, gender, site of onset, BMI, CRP, and IL-6 were not significantly correlated with ΔFS (Figure S2), whereas RABGGTB expression level and disease course were significantly correlated with ΔFS (p = 0.002, p = 0.0007, respectively). The course of disease and RABGGTB were significantly correlated with ΔFS (p = 0.001 and p = 0.025, respectively). The results indicate that the expression of RABGGTB and the course of disease are related to the progression rate of the disease—the stronger the expression of RABGGTB, the shorter the disease course, and the faster the disease progressed (Figure 3C,D).
3.4. Elevated RABGGTB Levels Were Detected in Monocyte-Derived Macrophages Derived from Patients with ALS
As we know, most tissue macrophages are derived from peripheral blood monocytes; therefore, we induced monocyte differentiation into macrophages to further assess the expression of RABGGTB in macrophages. The data of 47 patients from 62 patients diagnosed with ALS at the Second Hospital of Hebei Medical University between January 2021 and October 2022 were collected (Figure 4). The healthy control group consisted of 34 subjects (male: 20, female: 14) with a mean age of 54 ± 7 years and BMI of 23.53 ± 2.172. The ALS group included a total of 47 patients (male: 32, female: 15), with a mean age of 58 ± 10 years and BMI of 23.28 ± 2.463. The distributions of age, gender, and BMI did not differ significantly (Table 2).
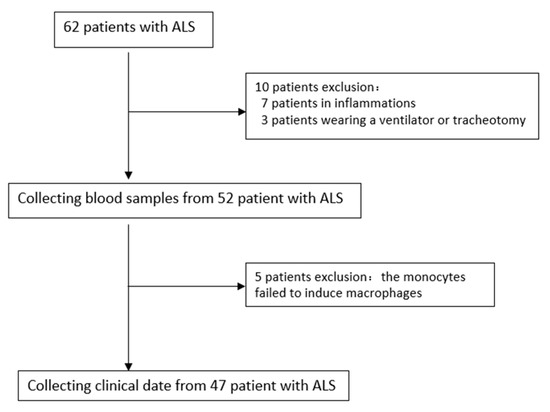
Figure 4.
Selection of patients with ALS in this study. ALS, amyotrophic lateral sclerosis.

Table 2.
Demographic parameters of healthy controls and ALS patients.
After M-CSF stimulation, monocytes extracted from peripheral blood differentiated into macrophages. The cells displayed typical macrophage morphology, with enlarged cell volume, round or oval shape, and pseudopodia exhibiting typical amoeboid morphology (Figure 5A), with CD68 and F4/80 macrophage markers expressed on the cell surface (Figure 5B). Immunofluorescence semiquantitative results revealed that the expression of RABGGTB in macrophages was significantly higher (p < 0.01) in the ALS group (17.340 ± 8.226) than in the control group (5.671 ± 2.932) (Figure 5C,D).
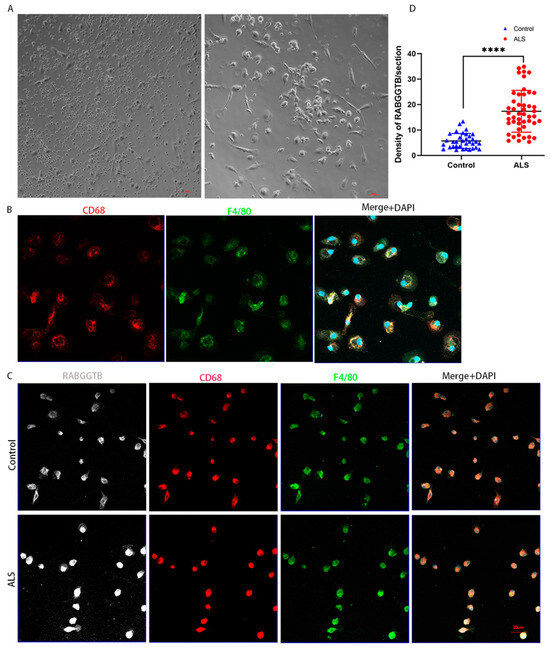
Figure 5.
RABGGTB expression in macrophages derived from healthy control and patients with ALS. (A) Phase-contrast microscopy images of macrophages differentiated with 20 ng/mL M-CSF for 4 days. Scale bars = 50 µm. (B) Phase-contrast microscopy images of macrophages differentiated with 20 ng/mL M-CSF for seven days. Scale bars = 50 µm. Immunofluorescence labeling of monocyte-derived macrophages for CD68 (red) and F4/80 (green). DAPI was used to stain nuclei (blue). Scale bars = 20 µm. (C) Immunofluorescence labeling for RABGGTB (gray/white), CD68 (red), and F4/80 (green) in monocyte-derived macrophages from patients with ALS and healthy controls. DAPI was used to stain nuclei (blue). Scale bars = 20 µm. (D) Quantitative analysis of RABGGTB levels in monocyte-derived macrophages from patients with ALS and healthy controls. The statistical significance was determined using an unpaired t-test. ****, p < 0.0001. ALS, amyotrophic lateral sclerosis; RABGGTB, Rab geranylgeranyltransferase subunit beta; M-CSF, macrophage colony-stimulating factor.
3.5. Correlations between RABGGTB Levels in Monocyte-Derived Macrophages with ALS Disease Severity or Progression Rates
The above findings suggest that RABGGTB expression may be associated with the clinical status of patients with ALS. We included indexes such as gender, age, BMI, site of disease onset, disease progression, CRP, IL-6, RABGGTB, and ALSFRS-R score. We first determined the relationship between disease severity and the aforementioned indicators. Age, gender, disease course, site of onset, BMI, CRP, and IL-6 did not correlate with ALSFRS-R score (Figure S3), whereas RABGGTB level was significantly correlated with ALSFRS-R score (p = 0.007). Further multivariate analysis revealed a significant correlation between RABGGTB and ALSFRS-R score (p = 0.041). The results suggest that the expression of RABGGTB is associated with the severity of ALS. The severity of the disease increased with the intensity of RABGGTB expression (Figure 6A).
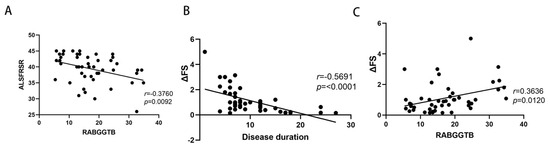
Figure 6.
Correlation between ALSFRS-R score or ΔFS and RABGGTB expression in macrophages derived from monocytes. (A) ALSFRS-R is the revised ALS functional rating scale. (B) Correlation between ΔFS and the duration of the disease in ALS. (C) Correlation between ΔFS and the expression of RABGGTB in monocyte-derived macrophages.
We also evaluated the correlation between the indicators and the progression rate of the disease. Age, gender, site of onset, BMI, CRP, and IL-6 were not significantly correlated with ΔFS (Figure S4), whereas RABGGTB expression level and disease course were significantly correlated with ΔFS (p < 0.01). The course of disease and RABGGTB were significantly correlated with ΔFS (p = 0.001 and p = 0.016, respectively). The results indicate that the expression of RABGGTB and the course of the disease are related to the progression rate of the disease—the stronger the expression of RABGGTB, the shorter the disease course, and the faster the disease progressed (Figure 6B,C).
4. Discussion
Amyotrophic lateral sclerosis (ALS) is a progressive fatal neurodegenerative disease, and the etiology and pathogenesis of ALS are unknown, leading to delayed diagnosis, so patients have a poor prognosis with an average survival time of 3–5 years [1]. There is growing evidence that monocytes, which are involved in the human immune response and inflammatory response, are implicated in ALS [63,64,65]. The expression levels of surface markers CD14 and CD16 were used to divide cells into three subgroups. Classical (CD14++CD16) monocytes participate in phagocytosis, immune response, and migration [66], which are essential for the body’s initial line of defense against innate immune defenses. Classical monocytes can differentiate into macrophages in tissues and mediate chronic diseases [67] such as obesity [68], Alzheimer’s disease [69], and cancer [70]. Nonclassical (CD14+CD16++) monocytes utilize the integrin lymphocyte function-associated antigen 1 (LFA-1) and the chemokine receptor CX3CR [71] to patrol the inner lining of vascular endothelium. Studies have demonstrated that nonclassical monocytes can differentiate into macrophages and participate in inflammatory responses under stimulation [72], and it has been suggested that nonclassical monocytes have a protective effect against chronic diseases and a positive correlation with disease burden [67]. There is more evidence demonstrating that monocytes in the peripheral circulation of patients with ALS promote inflammatory response [65]. According to our study, flow cytometry revealed that the RABGGTB expression levels of classical-type monocytes were significantly higher than those of controls, suggesting that RABGGTB may be linked to inflammation in classical monocytes.
To further demonstrate that the expression of RABGGTB was increased in patients with ALS, we extracted their monocytes. Using immunofluorescence, we detected the expression of RABGGTB in macrophages induced by monocytes from patients with ALS. Compared to the control group, the expression of RABGGTB was significantly higher in the ALS group. Macrophages [73]) play crucial roles in the onset and progression of ALS, in which their phenotypes and functions are altered. According to studies, M1 macrophages derived from monocytes in patients with ALS produce more proinflammatory cytokines than healthy controls [65]. Unfortunately, we did not further distinguish the phenotype of macrophages in this study; in subsequent research, we will examine the phenotype and function of macrophages.
We included gender, age, BMI, site of disease onset, disease progression, CRP, IL-6, and RABGGTB levels, and ALSFRS-R score based on the above results. In our analysis, the expression levels of RABGGTB were examined by flow cytometry and immunofluorescence, respectively, and the included indicators were used to perform correlation analyses. RABGGTB levels were not associated with sex, age at diagnosis, BMI, or disease onset site. ALSFRS-R score and ΔFS were also unrelated to gender, age at diagnosis, BMI, and site of onset. Multiple studies have confirmed that the gender disparity in onset of ALS is substantial [74,75]. There is evidence that estrogen has a protective effect in ALS, with ovariectomy hSOD1-G93A transgenic mice exhibiting earlier disease onset as well as diminished anti-inflammatory and anti-apoptotic abilities, suggesting that estrogen may play a significant role in protecting spinal motor neurons [76]. However, our analysis revealed no correlation, possibly because the majority of our patients were confined to the Hebei region, which may have contributed to these disparities. ALS is characterized by a gradual increase with age, with the incidence beginning to rise at 40 years and peaking between 60 and 70 years of age, followed by a steep decline [77]. Some age-specific studies compared the age distribution differences between continents and found that, with the exception of East Asia, the age-specific pattern of ALS incidence was consistent [77]. Multiple studies have determined that disease onset age is a significant factor in disease progression and prognosis [78]; the older the disease onset age is, the faster the disease progression rate and the shorter the survival [79]. In our analysis, we did not find a significant correlation between age and other variables. This may have been due to the limited sampling regions and small sample sizes.
Previous research has demonstrated that a higher BMI is associated with improved survival and that a high BMI and weight gain are associated with a lower risk of ALS [80]. RABGGTB levels were not found to be associated with BMI or disease onset site in our analysis. ALSFRS-R scores and ΔFS were also not associated with BMI or disease onset site. These differences may be attributable to sampling restrictions and the relatively small sample size, and we need to confirm our findings with larger samples.
A study published in JAMA found that patients with ALS with elevated serum CRP levels progressed more rapidly than those with lower CRP levels [9], suggesting that serum CRP could serve as a prognostic biomarker for ALS. A subsequent study analyzing the association between serum CRP and ALS concluded that CRP was not associated with ALS survival [5], suggesting that CRP is not a risk factor for ALS [81]. In our analysis, serum CRP was not associated with RABGGTB level, ALSFRS-R score, or ΔFS, indicating that serum CRP may be a useful prognostic biomarker for ALS; however, multicenter and multiregional validation are required. IL-6 is a multifunctional cytokine; IL-6 levels were significantly elevated in some patients, but not in the majority. Our analysis revealed that RABGGTB levels were not associated with serum IL-6, and based on our limited data, ALSFRS-R scores and ΔFS were also not associated with serum IL-6.
Decades of research on the disease course as a prognostic factor in ALS have revealed that the longer the delay between symptom onset and diagnosis [81], the better the prognosis. A disease with a shorter duration progresses more rapidly, necessitating more urgent treatment [79]. In our analysis, RABGGTB level and ALSFRS-R score were not associated with disease progression. However, ALSFRS-R score was associated with RABGGTB level, and ΔFS was associated with RABGGTB level and disease progression, regardless of age, gender, BMI, site of onset, or inflammation level. These findings suggest that the expression of RABGGTB is associated with the severity of ALS, and that patients with higher RABGGTB expression levels and a shorter disease course experience a more severe disease and aggressive progression. The significance of the RABGGTB level as a useful, feasible, and potential prognostic factor in patients with ALS is highlighted in this study.
RABGGTB as a potential indicator to assess the progression of ALS is not without limitations. The main limitation is that ALS is rare and heterogeneous in clinical manifestations, and the limited sample size means that our data are not fully representative of sporadic ALS (sALS), such as patients of different ethnicities or geographical regions. A second limitation is that the data collected come from sporadic ALS, and these data are not representative of familial ALS (fALS), such as those with mutations in the Cu/Zn-superoxide dismutase (SOD1) gene. The third limitation is that it is not clear what role RabGGTase plays in regulating inflammation and what effect inflammation has on RabGGTase. Therefore, we need a larger sample size to reliably comment on the value of RABGGTB as a prognostic biomarker. In subsequent experiments, we will investigate the role of RabGGTase in the regulation of inflammation and the effect of inflammation on RabGGTase. We will determine how RABGGTB responds in patients with different inflammatory conditions and whether the high expression is a primary phenomenon in macrophages or secondary to the inflammatory state of the cells.
5. Conclusions
In this study, we demonstrated for the first time that RABGGTB expression was greater in monocyte macrophages of patients with ALS than in healthy controls. Compared to controls, the expression of RABGGTB in monocytes and monocyte-differentiated macrophages derived from patients with ALS was correlated with progression rate—the stronger the expression of RABGGTB, the faster the disease progressed in patients with ALS, suggesting that RABGGTB may serve as an indicator for ALS progression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci13111531/s1, Figure S1: Correlation between ALSFRS-R score and BMI, serum CRP level, disease duration, serum IL-6 level, and age at diagnosis; Figure S2: Correlation between amyotrophic lateral sclerosis progression rate (ΔFS) and BMI, serum CRP, serum IL-6, and age at diagnosis; Figure S3: Correlation between ALSFRS-R score and BMI, serum CRP level, disease duration, serum IL-6 level, and age at diagnosis; Figure S4: Correlation between amyotrophic lateral sclerosis progression rate (ΔFS) and BMI, serum CRP, serum IL-6, and age at diagnosis.
Author Contributions
J.Y. participated in the experimental design, the implementation of the experiment, and the article writing; C.X. and J.H. participated in the implementation of the experiment; X.L. and H.D. participated in data analysis; H.D. and Q.L. participated in proofreading and review of the first draft; R.L. and Y.L. participated in the experimental design and project administration and obtained funding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Hebei Province H2021206310 and the Key Project of Technical Health Research and Achievement Transformation of Hebei Provincial Department of Health zh2018004 and Training Project for Professional Leaders of Hebei Provincial Department of Finance.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Second Hospital of Hebei Medical University (Shijia-zhuang, Hebei, P.R. China, protocol code: No. 2022-R196 and date of approval: 7 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the technical support from Chunyan Li’s Lab in the Key Laboratory of Neurology (Hebei Medical University), Ministry of Education.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, E.; Bonetto, V.; Soraru, G.; Martinelli, I.; Parchi, P.; Liguori, R.; Mandrioli, J. Neurofilaments in motor neuron disorders: Towards promising diagnostic and prognostic biomarkers. Mol. Neurodegener. 2020, 15, 58. [Google Scholar] [CrossRef]
- Vacchiano, V.; Mastrangelo, A.; Zenesini, C.; Masullo, M.; Quadalti, C.; Avoni, P.; Polischi, B.; Cherici, A.; Capellari, S.; Salvi, F.; et al. Plasma and CSF Neurofilament Light Chain in Amyotrophic Lateral Sclerosis: A Cross-Sectional and Longitudinal Study. Front. Aging Neurosci. 2021, 13, 753242. [Google Scholar] [CrossRef] [PubMed]
- Thouvenot, E.; Demattei, C.; Lehmann, S.; Maceski-Maleska, A.; Hirtz, C.; Juntas-Morales, R.; Pageot, N.; Esselin, F.; Alphandéry, S.; Vincent, T.; et al. Serum neurofilament light chain at time of diagnosis is an independent prognostic factor of survival in amyotrophic lateral sclerosis. Eur. J. Neurol. 2020, 27, 251–257. [Google Scholar] [CrossRef]
- De Schaepdryver, M.; Lunetta, C.; Tarlarini, C.; Mosca, L.; Chio, A.; Van Damme, P.; Poesen, K. Neurofilament light chain and C reactive protein explored as predictors of survival in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, A.; Pujol-Calderon, F.; Tjust, A.E.; Wuolikainen, A.; Höglund, K.; Forsberg, K.; Portelius, E.; Blennow, K.; Zetterberg, H.; Andersen, P.M. Neurofilaments can differentiate ALS subgroups and ALS from common diagnostic mimics. Sci. Rep. 2021, 11, 22128. [Google Scholar] [CrossRef]
- Pronto-Laborinho, A.; Pinto, S.; Gromicho, M.; Pereira, M.; Swash, M.; de Carvalho, M. Interleukin-6 and amyotrophic lateral sclerosis. J. Neurol. Sci. 2019, 398, 50–53. [Google Scholar] [CrossRef]
- Wosiski-Kuhn, M.; Caress, J.B.; Cartwright, M.S.; Hawkins, G.A.; Milligan, C. Interleukin 6 (IL6) level is a biomarker for functional disease progression within IL6R(358)Ala variant groups in amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 248–259. [Google Scholar] [CrossRef]
- Lunetta, C.; Lizio, A.; Maestri, E.; Sansone, V.A.; Mora, G.; Miller, R.G.; Appel, S.H.; Chiò, A. Serum C-Reactive Protein as a Prognostic Biomarker in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2017, 74, 660–667. [Google Scholar] [CrossRef]
- Dorst, J.; Kuhnlein, P.; Hendrich, C.; Kassubek, J.; Sperfeld, A.D.; Ludolph, A.C. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis. J. Neurol. 2011, 258, 613–617. [Google Scholar] [CrossRef]
- van Eijk, R.P.A.; Eijkemans, M.J.C.; Ferguson, T.A.; Nikolakopoulos, S.; Veldink, J.H.; Van Den Berg, L.H. Monitoring disease progression with plasma creatinine in amyotrophic lateral sclerosis clinical trials. J. Neurol. Neurosurg. Psychiatry 2018, 89, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; Garofalo, D.C.; Santella, R.M.; Sorenson, E.J.; Oskarsson, B.; Fernandes, J.A.M.; Andrews, H.; Hupf, J.; Gilmore, M.; Heitzman, D.; et al. Plasma creatinine and oxidative stress biomarkers in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 263–272. [Google Scholar] [CrossRef]
- Vucic, S. Plasma creatinine: A potential prognostic biomarker in amyotrophic lateral sclerosis? J. Neurol. Neurosurg. Psychiatry 2018, 89, 119. [Google Scholar] [CrossRef] [PubMed]
- Haji, S.; Sako, W.; Murakami, N.; Osaki, Y.; Furukawa, T.; Izumi, Y.; Kaji, R. The value of serum uric acid as a prognostic biomarker in amyotrophic lateral sclerosis: Evidence from a meta-analysis. Clin. Neurol. Neurosurg. 2021, 203, 106566. [Google Scholar] [CrossRef]
- Xu, L.Q.; Hu, W.; Guo, Q.F.; Xu, G.-R.; Wang, N.; Zhang, Q.-J. Serum Uric Acid Levels Predict Mortality Risk in Male Amyotrophic Lateral Sclerosis Patients. Front. Neurol. 2021, 12, 602663. [Google Scholar] [CrossRef]
- Paganoni, S.; Zhang, M.; Quiroz Zarate, A.; Jaffa, M.; Yu, H.; Cudkowicz, M.E.; Wills, A.-M. Uric acid levels predict survival in men with amyotrophic lateral sclerosis. J. Neurol. 2012, 259, 1923–1928. [Google Scholar] [CrossRef]
- Oh, S.I.; Baek, S.; Park, J.S.; Piao, L.; Oh, K.-W.; Kim, S.H. Prognostic Role of Serum Levels of Uric Acid in Amyotrophic Lateral Sclerosis. J. Clin. Neurol. 2015, 11, 376–382. [Google Scholar] [CrossRef]
- Ikeda, K.; Hirayama, T.; Takazawa, T.; Kawabe, K.; Iwasaki, Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: A cross-sectional study. Intern. Med. 2012, 51, 1501–1508. [Google Scholar] [CrossRef]
- Raghunathan, R.; Turajane, K.; Wong, L.C. Biomarkers in Neurodegenerative Diseases: Proteomics Spotlight on ALS and Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 9299. [Google Scholar] [CrossRef]
- Suk, T.R.; Rousseaux, M.W.C. The role of TDP-43 mislocalization in amyotrophic lateral sclerosis. Mol. Neurodegener. 2020, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chio, A.; Calvo, A.; Bovio, G.; Canosa, A.; Bertuzzo, D.; Galmozzi, F.; Cugnasco, P.; Clerico, M.; De Mercanti, S.; Bersano, E.; et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: A population-based study. JAMA Neurol. 2014, 71, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Benatar, M.; Wuu, J.; Andersen, P.M.; Lombardi, V.; Malaspina, A. Neurofilament light: A candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann. Neurol. 2018, 84, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, J.; Ye, F.; Xu, G.; Su, H.; Su, Y.; Zhang, X.; Alzheimer’s Disease Neuroimaging Initiative. Plasma neurofilament light chain levels in Alzheimer’s disease. Neurosci. Lett. 2017, 650, 60–64. [Google Scholar] [CrossRef]
- Dietmann, A.S.; Kruse, N.; Stork, L.; Gloth, M.; Brück, W.; Metz, I. Neurofilament light chains in serum as biomarkers of axonal damage in early MS lesions: A histological-serological correlative study. J. Neurol. 2022, 270, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Santos-Santos, M.; Illan-Gala, I.; Montal, V.; Estellés, T.; Barroeta, I.; Altuna, M.; Arranz, J.; Muñoz, L.; Belbin, O.; et al. Plasma glial fibrillary acidic protein and neurofilament light chain for the diagnostic and prognostic evaluation of frontotemporal dementia. Transl. Neurodegener. 2021, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Qian, H.; Di, G.; Zhou, R.; Dong, Y.; Chen, W.; Ren, Q. C-Reactive Protein as a Prognostic Biomarker for Gynecologic Cancers: A Meta-Analysis. Comput. Intell. Neurosci. 2022, 2022, 6833078. [Google Scholar] [CrossRef]
- Socha, M.W.; Malinowski, B.; Puk, O.; Wartęga, M.; Bernard, P.; Nowaczyk, M.; Wolski, B.; Wiciński, M. C-reactive protein as a diagnostic and prognostic factor of endometrial cancer. Crit. Rev. Oncol. Hematol. 2021, 164, 103419. [Google Scholar] [CrossRef]
- Bensimon, G.; Lacomblez, L.; Meininger, V. ALS/Riluzole Study Group. A controlled trial of riluzole in amyotrophic lateral sclerosis. N. Engl. Med. 1994, 330, 585–591. [Google Scholar] [CrossRef]
- Witzel, S.; Maier, A.; Steinbach, R.; Grosskreutz, J.; Koch, J.C.; Sarikidi, A.; Petri, S.; Günther, R.; Wolf, J.; Hermann, A.; et al. Safety and Effectiveness of Long-term Intravenous Administration of Edaravone for Treatment of Patients With Amyotrophic Lateral Sclerosis. JAMA Neurol. 2022, 79, 121–130. [Google Scholar] [CrossRef]
- Guadagno, N.A.; Progida, C. Rab GTPases: Switching to Human Diseases. Cells 2019, 8, 909. [Google Scholar] [CrossRef]
- Tzeng, H.T.; Wang, Y.C. Rab-mediated vesicle trafficking in cancer. J. Biomed. Sci. 2016, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, L.; Gao, Q.; Liu, Y.; Feng, X.; Ye, S.; Yang, Z. The Role of RAB GTPases and Its Potential in Predicting Immunotherapy Response and Prognosis in Colorectal Cancer. Front. Genet. 2022, 13, 828373. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Lee, H.Y. Rab25 and RCP in cancer progression. Arch. Pharm. Res. 2019, 42, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, H.; Chen, W.; Cheng, M.; Zou, L.; Yang, Q.; Chan, C.B.; Zhu, H.; Chen, C.; Nie, J.; et al. Rab13 Sustains Breast Cancer Stem Cells by Supporting Tumor-Stroma Cross-talk. Cancer Res. 2022, 82, 2124–2140. [Google Scholar] [CrossRef] [PubMed]
- Monroy-Merida, G.; Guzman-Beltran, S.; Hernandez, F.; Santos-Mendoza, T.; Bobadilla, K. High Glucose Concentrations Impair the Processing and Presentation of Mycobacterium tuberculosis Antigens In Vitro. Biomolecules 2021, 11, 1763. [Google Scholar] [CrossRef]
- Chung, I.Y.W.; Li, L.; Tyurin, O.; Gagarinova, A.; Wibawa, R.; Li, P.; Hartland, E.L.; Cygler, M. Structural and functional study of Legionella pneumophila effector RavA. Protein Sci. 2021, 30, 940–955. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, X.; Wu, X. The Rab GTPase in the heart: Pivotal roles in development and disease. Life Sci. 2022, 306, 120806. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.Y.; Yancey, J.; Luo, H.; Zhang, Y.W. Role of Rab GTPases in Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 828–838. [Google Scholar] [CrossRef]
- Xu, W.; Fang, F.; Ding, J.; Wu, C. Dysregulation of Rab5-mediated endocytic pathways in Alzheimer’s disease. Traffic 2018, 19, 253–262. [Google Scholar] [CrossRef]
- Lara Ordonez, A.J.; Fasiczka, R.; Naaldijk, Y.; Hilfiker, S. Rab GTPases in Parkinson’s disease: A primer. Essays Biochem. 2021, 65, 961–974. [Google Scholar]
- Gao, Y.; Wilson, G.R.; Stephenson, S.E.M.; Bozaoglu, K.; Farrer, M.J.; Lockhart, P.J. The emerging role of Rab GTPases in the pathogenesis of Parkinson’s disease. Mov. Disord. 2018, 33, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.M.; Shi, C.H.; Xu, Y.M. Rab GTPases: The Key Players in the Molecular Pathway of Parkinson’s Disease. Front. Cell Neurosci. 2017, 11, 81. [Google Scholar] [PubMed]
- Bonet-Ponce, L.; Cookson, M.R. The role of Rab GTPases in the pathobiology of Parkinson’ disease. Curr. Opin. Cell Biol. 2019, 59, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Farg, M.A.; Sundaramoorthy, V.; Sultana, J.M.; Yang, S.; Atkinson, R.A.; Levina, V.; Halloran, M.A.; Gleeson, P.A.; Blair, I.P.; Soo, K.Y.; et al. C9ORF72, implicated in amytrophic lateral sclerosis and frontotemporal dementia, regulates endosomal trafficking. Hum. Mol. Genet. 2014, 23, 3579–3595. [Google Scholar] [CrossRef]
- Parakh, S.; Perri, E.R.; Jagaraj, C.J.; Ragagnin, A.M.G.; Atkin, J.D. Rab-dependent cellular trafficking and amyotrophic lateral sclerosis. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 623–651. [Google Scholar] [CrossRef]
- Webster, C.P.; Smith, E.F.; Grierson, A.J.; De Vos, K.J. C9orf72 plays a central role in Rab GTPase-dependent regulation of autophagy. Small GTPases 2018, 9, 399–408. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X. Research progress on vesicular trafficking in amyotrophic lateral sclerosis. Zhejiang Da Xue Xue Bao Yi Xue Ban 2022, 51, 380–387. [Google Scholar]
- Jung, J.; Behrends, C. Multifaceted role of SMCR8 as autophagy regulator. Small GTPases 2020, 11, 53–61. [Google Scholar] [CrossRef]
- Burk, K.; Pasterkamp, R.J. Disrupted neuronal trafficking in amyotrophic lateral sclerosis. Acta Neuropathol. 2019, 137, 859–877. [Google Scholar]
- Qu, L.; Pan, C.; He, S.M.; Lang, B.; Gao, G.D.; Wang, X.L.; Wang, Y. The Ras Superfamily of Small GTPases in Non-neoplastic Cerebral Diseases. Front. Mol. Neurosci. 2019, 12, 121. [Google Scholar]
- Cozzi, M.; Ferrari, V. Autophagy Dysfunction in ALS: From Transport to Protein Degradation. J. Mol. Neurosci. 2022, 72, 1456–1481. [Google Scholar] [PubMed]
- Vic encio, E.; Beltran, S.; Labrador, L.; Manque, P.; Nassif, M.; Woehlbier, U. Implications of Selective Autophagy Dysfunction for ALS Pathology. Cells 2020, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar]
- Gao, T.; Huo, J.; Xin, C.; Yang, J.; Liu, Q.; Dong, H.; Li, R.; Liu, Y. Protective effects of intrathecal injection of AAV9-RabGGTB-GFP(+) in SOD1(G93A) mice. Front. Aging Neurosci. 2023, 15, 1092607. [Google Scholar]
- Shirakawa, R.; Goto-Ito, S.; Goto, K.; Wakayama, S.; Kubo, H.; Sakata, N.; Trinh, D.A.; Yamagata, A.; Sato, Y.; Masumoto, H.; et al. A SNARE geranylgeranyltransferase essential for the organization of the Golgi apparatus. EMBO J. 2020, 39, e104120. [Google Scholar] [CrossRef]
- Taheri, M.; Ghafouri-Fard, S.; Sayad, A.; Arsang-Jang, S.; Mazdeh, M.; Toghi, M.; Omrani, M.D. Assessment of Protein Prenylation Pathway in Multiple Sclerosis Patients. J. Mol. Neurosci. 2018, 64, 581–590. [Google Scholar]
- Deraeve, C.; Guo, Z.; Bon, R.S.; Blankenfeldt, W.; DiLucrezia, R.; Wolf, A.; Menninger, S.; Stigter, E.A.; Wetzel, S.; Choidas, A.; et al. Psoromic acid is a selective and covalent Rab-prenylation inhibitor targeting autoinhibited RabGGTase. J. Am. Chem. Soc. 2012, 134, 7384–7391. [Google Scholar] [PubMed]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Labra, J.; Menon, P.; Byth, K.; Morrison, S.; Vucic, S. Rate of disease progression: A prognostic biomarker in ALS. J. Neurol. Neurosurg. Psychiatry 2016, 87, 628–632. [Google Scholar] [CrossRef]
- Quek, H.; Cuni-Lopez, C.; Stewart, R.; Colletti, T.; Notaro, A.; Nguyen, T.H.; Sun, Y.; Guo, C.C.; Lupton, M.K.; Roberts, T.L.; et al. ALS monocyte-derived microglia-like cells reveal cytoplasmic TDP-43 accumulation, DNA damage, and cell-specific impairment of phagocytosis associated with disease progression. J. Neuroinflamm. 2022, 19, 58. [Google Scholar]
- Passlick, B.; Flieger, D.; Ziegler-Heitbrock, H.W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989, 74, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Schroth, J.; Lombardi, V.; Pucino, V.; Bobeva, Y.; Yip, P.K.; Schmierer, K.; Mauro, C.; Tree, T.; Henson, S.M.; et al. The Expression of Active CD11b Monocytes in Blood and Disease Progression in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2022, 23, 3370. [Google Scholar] [CrossRef] [PubMed]
- Zondler, L.; Muller, K.; Khalaji, S.; Bliederhäuser, C.; Ruf, W.P.; Grozdanov, V.; Thiemann, M.; Fundel-Clemes, K.; Freischmidt, A.; Holzmann, K.; et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol. 2016, 132, 391–411. [Google Scholar]
- Du, Y.; Zhao, W.; Thonhoff, J.R.; Wang, J.; Wen, S.; Appel, S.H. Increased activation ability of monocytes from ALS patients. Exp. Neurol. 2020, 328, 113259. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemund, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar]
- Narasimhan, P.B.; Marcovecchio, P.; Hamers, A.A.J.; Hedrick, C.C. Nonclassical Monocytes in Health and Disease. Annu. Rev. Immunol. 2019, 37, 439–456. [Google Scholar]
- Russo, L.; Lumeng, C.N. Properties and functions of adipose tissue macrophages in obesity. Immunology 2018, 155, 407–417. [Google Scholar] [CrossRef]
- Guedes, J.R.; Lao, T.; Cardoso, A.L.; El Khoury, J. Roles of Microglial and Monocyte Chemokines and Their Receptors in Regulating Alzheimer’s Disease-Associated Amyloid-beta and Tau Pathologies. Front. Neurol. 2018, 9, 549. [Google Scholar] [CrossRef]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Olingy, C.E.; San Emeterio, C.L.; Ogle, M.E.; Krieger, J.R.; Bruce, A.C.; Pfau, D.D.; Jordan, B.T.; Peirce, S.M.; Botchwey, E.A. Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci. Rep. 2017, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, W.; Yamasaki, R.; Hashimoto, Y.; Ko, S.; Kobayakawa, Y.; Isobe, N.; Matsushita, T.; Kira, J.-I. Clearance of peripheral nerve misfolded mutant protein by infiltrated macrophages correlates with motor neuron disease progression. Sci. Rep. 2021, 11, 16438. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.J.; Goutman, S.A.; Boss, J.; Kim, S.; Feldman, E.L. Amyotrophic Lateral Sclerosis Survival Associates With Neutrophils in a Sex-specific Manner. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e953. [Google Scholar] [CrossRef] [PubMed]
- Riar, A.K.; Burstein, S.R.; Palomo, G.M.; Arreguin, A.; Manfredi, G.; Germain, D. Sex specific activation of the ERalpha axis of the mitochondrial UPR (UPRmt) in the G93A-SOD1 mouse model of familial ALS. Hum. Mol. Genet. 2017, 26, 1318–1327. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Sun, C.; Zheng, Q.; Hao, P.; Zhai, J.; Liu, Y. Effects of Ovariectomy in an hSOD1-G93A Transgenic Mouse Model of Amyotrophic Lateral Sclerosis (ALS). Med. Sci. Monit. 2018, 24, 678–686. [Google Scholar] [CrossRef]
- Marin, B.; Fontana, A.; Arcuti, S.; Copetti, M.; Boumédiene, F.; Couratier, P.; Beghi, E.; Preux, P.M.; Logroscino, G. Age-specific ALS incidence: A dose-response meta-analysis. Eur. J. Epidemiol. 2018, 33, 621–634. [Google Scholar]
- Westeneng, H.J.; Debray, T.P.A.; Visser, A.E.; van Eijk, R.P.; Rooney, J.P.; Calvo, A.; Martin, S.; McDermott, C.J.; Thompson, A.G.; Pinto, S.; et al. Prognosis for patients with amyotrophic lateral sclerosis: Development and validation of a personalised prediction model. Lancet Neurol. 2018, 17, 423–433. [Google Scholar] [CrossRef]
- Chio, A.; Logroscino, G.; Hardiman, O.; Swingler, R.; Mitchell, D.; Beghi, E.; Traynor, B.G.; Eurals Consortium. Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009, 10, 310–323. [Google Scholar] [CrossRef]
- Nakken, O.; Meyer, H.E.; Stigum, H.; Holmøy, T. High BMI is associated with low ALS risk: A population-based study. Neurology 2019, 93, e424–e432. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, M.; Zhang, J.; Huang, X. Association Between C-Reactive Protein and Risk of Amyotrophic Lateral Sclerosis: A Mendelian Randomization Study. Front. Genet. 2022, 13, 919031. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).