Neuromodulation Techniques in Children with Super-Refractory Status Epilepticus
Abstract
:1. Introduction
1.1. Non-Invasive Neuromodulation Techniques
1.2. Invasive Neuromodulation Techniques
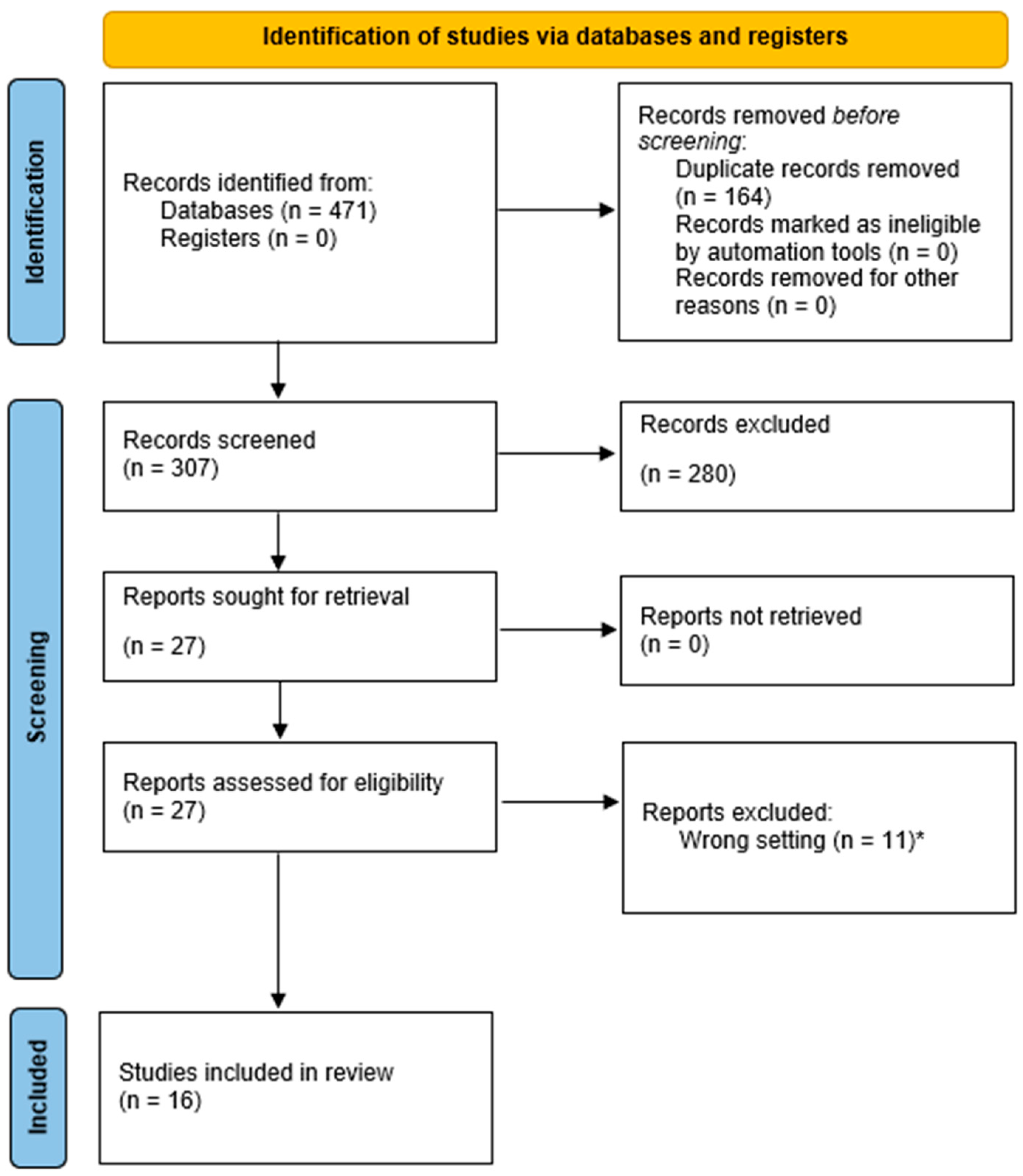
2. Materials and Methods
3. Results
3.1. Electroconvulsive Technique (ECT)
3.2. Vagus Nerve Stimulation (VNS)
3.3. Deep Brain Stimulation (DBS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trinka, E.; Cock, H.; Hesdorffer, D.; Rossetti, A.O.; Scheffer, I.E.; Shinnar, S.; Shorvon, S.; Lowenstein, D.H. A definition and classification of status epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015, 56, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Rincon, F. Status Epilepticus: Epidemiology and Public Health Needs. J. Clin. Med. 2016, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, D.H.; Bleck, T.; Macdonald, R.L. It’s time to revise the definition of status epilepticus. Epilepsia 1999, 40, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Al-Mufti, F.; Claassen, J. Neurocritical care: Status epilepticus review. Crit. Care Clin. 2014, 30, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.L.; Gratopp, A.; Prager, C.; Elger, C.E.; Kaindl, A.M. Treatment of pediatric convulsive status epilepticus. Front. Neurol. 2023, 14, 1175370. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, M.; Imamura, M.; Counsell, C.; Aucott, L.; Manson, P.; Booth, C.; Scotland, G.; Brazzelli, M. Management of the first stage of convulsive status epilepticus in adults: A systematic review of current randomised evidence. J. Neurol. 2022, 269, 3420–3429. [Google Scholar] [CrossRef]
- Cornwall, C.D.; Krøigård, T.; Kristensen, J.S.S.; Callesen, H.E.; Beier, C.P. Outcomes and Treatment Approaches for Super-Refractory Status Epilepticus: A Systematic Review and Meta-Analysis. JAMA Neurol. 2023, 80, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.; Trinka, E. Nonconvulsive status epilepticus and coma. Epilepsia 2010, 51, 177–190. [Google Scholar] [CrossRef]
- Trinka, E.; Brigo, F.; Shorvon, S. Recent advances in status epilepticus. Curr. Opin. Neurol. 2016, 29, 189–198. [Google Scholar] [CrossRef]
- Trinka, E.; Kälviäinen, R. 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure 2017, 44, 65–73. [Google Scholar] [CrossRef]
- NICE. Epilepsies: Diagnosis and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2019. [Google Scholar]
- Shorvon, S.; Ferlisi, M. The treatment of super-refractory status epilepticus: A critical review of available therapies and a clinical treatment protocol. Brain A J. Neurol. 2011, 134, 2802–2818. [Google Scholar] [CrossRef] [PubMed]
- Trinka, E.; Höfler, J.; Leitinger, M.; Brigo, F. Pharmacotherapy for Status Epilepticus. Drugs 2015, 75, 1499–1521. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, M.; Trinka, E.; Giovannini, G.; Zimmermann, G.; Florea, C.; Rohracher, A.; Kalss, G.; Neuray, C.; Kreidenhuber, R.; Höfler, J.; et al. Epidemiology of status epilepticus in adults: A population-based study on incidence, causes, and outcomes. Epilepsia 2019, 60, 53–62. [Google Scholar] [CrossRef]
- Arya, R.; Rotenberg, A. Dietary, immunological, surgical, and other emerging treatments for pediatric refractory status epilepticus. Seizure 2019, 68, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.; Farias-Moeller, R.; Sanchez-Fernandez, I.; Abend, N.S.; Amengual-Gual, M.; Anderson, A.; Arya, R.; Brenton, J.N.; Carpenter, J.L.; Chapman, K.; et al. Super-Refractory Status Epilepticus in Children: A Retrospective Cohort Study. Pediatr. Crit. Care Med. 2021, 22, e613–e625. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Matuszczak, M.; Teitelbaum, J.; Kazina, C.J.; Gillman, L.M. Plasmapheresis for refractory status epilepticus, part I: A scoping systematic review of the adult literature. Seizure 2016, 43, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Matuszczak, M.; Teitelbaum, J.; Kazina, C.J.; Gillman, L.M. Plasmapheresis for refractory status epilepticus Part II: A scoping systematic review of the pediatric literature. Seizure 2016, 43, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Legriel, S.; Lemiale, V.; Schenck, M.; Chelly, J.; Laurent, V.; Daviaud, F.; Srairi, M.; Hamdi, A.; Geri, G.; Rossignol, T.; et al. Hypothermia for Neuroprotection in Convulsive Status Epilepticus. N. Engl. J. Med. 2016, 375, 2457–2467. [Google Scholar] [CrossRef]
- Rossetti, A.O.; Lowenstein, D.H. Management of refractory status epilepticus in adults: Still more questions than answers. Lancet Neurol. 2011, 10, 922–930. [Google Scholar] [CrossRef] [PubMed]
- San-Juan, D.; Davila-Rodriguez, D.O.; Jimenez, C.R.; Gonzalez, M.S.; Carranza, S.M.; Hernandez Mendoza, J.R.; Anschel, D.J. Neuromodulation techniques for status epilepticus: A review. Brain Stimul. 2019, 12, 835–844. [Google Scholar] [CrossRef]
- Weiner, R.D.; Reti, I.M. Key updates in the clinical application of electroconvulsive therapy. Int. Rev. Psychiatry 2017, 29, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, F.A.; Matuszczak, M.; Teitelbaum, J.; Gillman, L.M.; Kazina, C.J. Electroconvulsive therapy for refractory status epilepticus: A systematic review. Seizure 2016, 35, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Semkovska, M.; McLoughlin, D.M. Objective cognitive performance associated with electroconvulsive therapy for depression: A systematic review and meta-analysis. Biol. Psychiatry 2010, 68, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Kellner, C.H.; Tobias, K.G.; Wiegand, J. Electrode placement in electroconvulsive therapy (ECT): A review of the literature. J. ECT 2010, 26, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lisanby, S.H.; Medalia, A.; Prudic, J. A conceptual introduction to cognitive remediation for memory deficits associated with right unilateral electroconvulsive therapy. J. ECT 2011, 27, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.R.; Junqueira, Y.N.; Correa, N.B.; Fonseca, E.O.; Brito, N.B.M.; Menezes, T.A.; Magini, M.; Fidalgo, T.K.S.; Ferreira, D.; de Lima, R.L.; et al. The efficacy of transcranial direct current stimulation and transcranial magnetic stimulation for chronic orofacial pain: A systematic review. PLoS ONE 2019, 14, e0221110. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, C.; Wang, J. Updated Review on the Clinical Use of Repetitive Transcranial Magnetic Stimulation in Psychiatric Disorders. Neurosci. Bull. 2017, 33, 747–756. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Matuszczak, M.; Teitelbaum, J.; Gillman, L.M.; Kazina, C.J. Transcranial Magnetic Stimulation for Status Epilepticus. Epilepsy Res. Treat. 2015, 2015, 678074. [Google Scholar] [CrossRef]
- Kobayashi, M.; Pascual-Leone, A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003, 2, 145–156. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- San-Juan, D.; Espinoza Lopez, D.A.; Vazquez Gregorio, R.; Trenado, C.; Fernandez-Gonzalez Aragon, M.; Morales-Quezada, L.; Hernandez Ruiz, A.; Hernandez-Gonzalez, F.; Alcaraz-Guzman, A.; Anschel, D.J.; et al. Transcranial Direct Current Stimulation in Mesial Temporal Lobe Epilepsy and Hippocampal Sclerosis. Brain Stimul. 2017, 10, 28–35. [Google Scholar] [CrossRef] [PubMed]
- LivaNova. VNS Therapy® System Physician’s Manual. Available online: https://www.livanova.com/epilepsy-vnstherapy/en-us/hcp/product-manuals (accessed on 1 September 2023).
- Giordano, F.; Zicca, A.; Barba, C. Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia 2017, 58 (Suppl. S1), 85–90. [Google Scholar] [CrossRef] [PubMed]
- Dibue-Adjei, M.; Brigo, F.; Yamamoto, T.; Vonck, K.; Trinka, E. Vagus nerve stimulation in refractory and super-refractory status epilepticus—A systematic review. Brain Stimul. 2019, 12, 1101–1110. [Google Scholar] [CrossRef]
- Trinka, E.; Brigo, F. Neurostimulation in the treatment of refractory and super-refractory status epilepticus. Epilepsy Behav. 2019, 101, 106551. [Google Scholar] [CrossRef]
- Aum, D.J.; Tierney, T.S. Deep brain stimulation: Foundations and future trends. Front. Biosci. 2018, 23, 162–182. [Google Scholar] [CrossRef]
- Laxpati, N.G.; Kasoff, W.S.; Gross, R.E. Deep brain stimulation for the treatment of epilepsy: Circuits, targets, and trials. Neurotherapeutics 2014, 11, 508–526. [Google Scholar] [CrossRef] [PubMed]
- Zangiabadi, N.; Ladino, L.D.; Sina, F.; Orozco-Hernández, J.P.; Carter, A.; Téllez-Zenteno, J.F. Deep Brain Stimulation and Drug-Resistant Epilepsy: A Review of the Literature. Front. Neurol. 2019, 10, 601. [Google Scholar] [CrossRef]
- Salanova, V. Deep brain stimulation for epilepsy. Epilepsy Behav. 2018, 88, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Salanova, V.; Witt, T.; Worth, R.; Henry, T.; Gross, R.; Oommen, K.; Osorio, I.; Nazzaro, J.; Labar, D.; et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010, 51, 899–908. [Google Scholar] [CrossRef]
- Fisher, R.S.; Uematsu, S.; Krauss, G.L.; Cysyk, B.J.; McPherson, R.; Lesser, R.P.; Gordon, B.; Schwerdt, P.; Rise, M. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia 1992, 33, 841–851. [Google Scholar] [CrossRef]
- Jitkritsadakul, O.; Bhidayasiri, R.; Kalia, S.K.; Hodaie, M.; Lozano, A.M.; Fasano, A. Systematic review of hardware-related complications of Deep Brain Stimulation: Do new indications pose an increased risk? Brain Stimul. 2017, 10, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Picillo, M.; Lozano, A.M.; Kou, N.; Munhoz, R.P.; Fasano, A. Programming Deep Brain Stimulation for Tremor and Dystonia: The Toronto Western Hospital Algorithms. Brain Stimul. 2016, 9, 438–452. [Google Scholar] [CrossRef]
- Picillo, M.; Lozano, A.M.; Kou, N.; Puppi Munhoz, R.; Fasano, A. Programming Deep Brain Stimulation for Parkinson’s Disease: The Toronto Western Hospital Algorithms. Brain Stimul. 2016, 9, 425–437. [Google Scholar] [CrossRef]
- Morrell, M.J. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011, 77, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.D.; Raslan, A.M.; Wabulya, A.; Shin, H.W.; Cash, S.S.; Yang, J.C.; Sagi, V.; King-Stephens, D.; Damisah, E.C.; Ramos, A.; et al. Responsive neurostimulation as a treatment for super-refractory focal status epilepticus: A systematic review and case series. J. Neurosurg. 2023, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.D.; Krause, K.L.; Kellogg, M.A.; Raslan, A.M.; Spencer, D.C. Novel Use of Responsive Neurostimulation (RNS System) in the Treatment of Super Refractory Status Epilepticus. J. Clin. Neurophysiol. 2019, 36, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Harid, N.M.; Nascimento, F.A.; Kokkinos, V.; Shaughnessy, A.; Lam, A.D.; Westover, M.B.; Leslie-Mazwi, T.M.; Hochberg, L.R.; Rosenthal, E.S.; et al. Responsive neurostimulation for focal motor status epilepticus. Ann. Clin. Transl. Neurol. 2021, 8, 1353–1361. [Google Scholar] [CrossRef]
- Ryvlin, P.; Jehi, L.E. Neuromodulation for Refractory Epilepsy. Epilepsy Curr. 2022, 22, 11–17. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hect, J.L.; Fernandez, L.D.; Welch, W.P.; Abel, T.J. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of FIRES. Epilepsia Open 2022, 7, 187–193. [Google Scholar] [CrossRef]
- García-López, B.; Gómez-Menéndez, A.I.; Vázquez-Sánchez, F.; Pérez-Cabo, E.; Isidro-Mesas, F.; Zabalegui-Pérez, A.; Muñoz-Siscart, I.; Lloria-Gil, M.C.; Soto-Cámara, R.; González-Bernal, J.J.; et al. Electroconvulsive Therapy in Super Refractory Status Epilepticus: Case Series with a Defined Protocol. Int. J. Environ. Res. Public Health 2020, 17, 4023. [Google Scholar] [CrossRef] [PubMed]
- Winston, K.R.; Levisohn, P.; Miller, B.R.; Freeman, J. Vagal nerve stimulation for status epilepticus. Pediatr. Neurosurg. 2001, 34, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, N.; Rychlicki, F.; Corpaci, L.; Cesaroni, E.; Trignani, R. Vagus nerve stimulation (VNS) is effective in treating catastrophic 1 epilepsy in very young children. Neurosurg. Rev. 2008, 31, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Grioni, D.; Landi, A.; Fiori, L.; Sganzerla, E.P. Does emergent implantation of a vagal nerve stimulator stop refractory status epilepticus in children? Seizure 2018, 61, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Mostacci, B.; Bisulli, F.; Muccioli, L.; Minardi, I.; Bandini, M.; Licchetta, L.; Zucchelli, M.; Leta, C.; Michelucci, R.; Zanello, M.; et al. Super refractory status epilepticus in Lafora disease interrupted by vagus nerve stimulation: A case report. Brain Stimul. 2019, 12, 1605–1607. [Google Scholar] [CrossRef] [PubMed]
- Specchio, N.; Ferretti, A.; Pietrafusa, N.; Trivisano, M.; Calabrese, C.; Carfì Pavia, G.; De Benedictis, A.; Marras, C.E.; de Palma, L.; Vigevano, F. Refractory Status Epilepticus in Genetic Epilepsy-Is Vagus Nerve Stimulation an Option? Front. Neurol. 2020, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Incecik, F.; Horoz, O.O.; Herguner, O.M.; Yildizdas, D.; Altunbasak, S. Electroconvulsive therapy for refractory status epilepticus in a child: A case report. Ann. Indian Acad. Neurol. 2015, 18, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Miras Veiga, A.; Moreno, D.C.; Menendez, A.I.; Siscart, I.M.; Fernandez, M.D.; Sanchez, E.G.; Gonzalez, M.G.; Saez, F.G. Effectiveness of Electroconvulsive Therapy for Refractory Status Epilepticus in Febrile Infection-Related Epilepsy Syndrome. Neuropediatrics 2017, 48, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.W.; O’Donovan, C.A.; Boggs, J.G.; Grefe, A.; Harper, A.; Bell, W.L.; McCall, W.V.; Rosenquist, P. Successful ECT treatment for medically refractory nonconvulsive status epilepticus in pediatric patient. Seizure 2011, 20, 433–436. [Google Scholar] [CrossRef]
- Nath, M.; Shah, Y.D.; Theroux, L.M.; Petrides, G.; Karkare, S.; Sanghani, S.N.; Kothare, S.V. A Role for Electroconvulsive Therapy in the Management of New Onset Refractory Status Epilepticus (NORSE) in a Young Child. Neurol. India 2021, 69, 1374–1379. [Google Scholar] [CrossRef]
- De Herdt, V.; Waterschoot, L.; Vonck, K.; Dermaut, B.; Verhelst, H.; Van Coster, R.; De Jaeger, A.; Van Roost, D.; Boon, P. Vagus nerve stimulation for refractory status epilepticus. Eur. J. Paediatr. Neurol. 2009, 13, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.B.; Katanyuwong, K.; Mackay, M.T.; Bailey, C.A.; Scheffer, I.E.; Freeman, J.L.; Berkovic, S.F.; Harvey, A.S. Long-term follow-up of febrile infection-related epilepsy syndrome. Epilepsia 2012, 53, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Wang, Y.; Lu, G.; Zhou, Y.; Wang, Y. Vagus nerve stimulation for super-refractory status epilepticus in febrile infection-related epilepsy syndrome: A pediatric case report and literature review. Child’S Nerv. Syst. 2022, 38, 1401–1404. [Google Scholar] [CrossRef] [PubMed]
- Sa, M.; Singh, R.; Pujar, S.; D’Arco, F.; Desai, N.; Eltze, C.; Hughes, E.; Al Obaidi, M.; Eleftheriou, D.; Tisdall, M.; et al. Centromedian thalamic nuclei deep brain stimulation and Anakinra treatment for FIRES—Two different outcomes. Eur. J. Paediatr. Neurol. 2019, 23, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, I.; Selway, R.; Hasegawa, H.; Hughes, E.; Rittey, C.; Jiménez-Jiménez, D.; Valentin, A. Low frequency centromedian thalamic nuclei deep brain stimulation for the treatment of super refractory status epilepticus: A case report and a review of the literature. Brain Stimul. 2021, 14, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lim, S.N.; Wu, T.; Lee, S.T. Successful Treatment of Refractory Status Epilepticus Using Anterior Thalamic Nuclei Deep Brain Stimulation. World Neurosurg. 2017, 99, 14–18. [Google Scholar] [CrossRef]
- Lehtimaki, K.; Langsjo, J.W.; Ollikainen, J.; Heinonen, H.; Mottonen, T.; Tahtinen, T.; Haapasalo, J.; Tenhunen, J.; Katisko, J.; Ohman, J.; et al. Successful management of super-refractory status epilepticus with thalamic deep brain stimulation. Ann. Neurol. 2017, 81, 142–146. [Google Scholar] [CrossRef]
- Hill, C.E.; Parikh, A.O.; Ellis, C.; Myers, J.S.; Litt, B. Timing is everything: Where status epilepticus treatment fails. Ann. Neurol. 2017, 82, 155–165. [Google Scholar] [CrossRef]
- Betjemann, J.P.; Lowenstein, D.H. Status epilepticus in adults. Lancet Neurol. 2015, 14, 615–624. [Google Scholar] [CrossRef]
- Claassen, J.; Lokin, J.K.; Fitzsimmons, B.F.; Mendelsohn, F.A.; Mayer, S.A. Predictors of functional disability and mortality after status epilepticus. Neurology 2002, 58, 139–142. [Google Scholar] [CrossRef]
- Farias-Moeller, R.; Bartolini, L.; Staso, K.; Schreiber, J.M.; Carpenter, J.L. Early ictal and interictal patterns in FIRES: The sparks before the blaze. Epilepsia 2017, 58, 1340–1348. [Google Scholar] [CrossRef]
- van Baalen, A.; Vezzani, A.; Häusler, M.; Kluger, G. Febrile infection–related epilepsy syndrome: Clinical review and hypotheses of epileptogenesis. Neuropediatrics 2017, 48, 005–018. [Google Scholar]
- Wickström, R.; Taraschenko, O.; Dilena, R.; Payne, E.T.; Specchio, N.; Nabbout, R.; Koh, S.; Gaspard, N.; Hirsch, L.J.; The International NORSE Consensus Group. International consensus recommendations for management of new onset refractory status epilepticus (NORSE) including febrile infection-related epilepsy syndrome (FIRES): Summary and clinical tools. Epilepsia 2022, 63, 2827–2839. [Google Scholar] [CrossRef]
- Singh, A.; Kar, S.K. How Electroconvulsive Therapy Works? Understanding the Neurobiological Mechanisms. Clin. Psychopharmacol. Neurosci. 2017, 15, 210–221. [Google Scholar] [CrossRef]
- Jang, S.S.; Royston, S.E.; Lee, G.; Wang, S.; Chung, H.J. Seizure-Induced Regulations of Amyloid-beta, STEP61, and STEP61 Substrates Involved in Hippocampal Synaptic Plasticity. Neural Plast. 2016, 2016, 2123748. [Google Scholar] [CrossRef]
- Krahl, S.E.; Clark, K.B. Vagus nerve stimulation for epilepsy: A review of central mechanisms. Surg. Neurol. Int. 2012, 3, S255–S259. [Google Scholar] [CrossRef]
- George, M.S.; Nahas, Z.; Bohning, D.E.; Mu, Q.; Andrew Kozel, F.; Borckhardt, J.; Denslow, S. Mechanisms of action of vagus nerve stimulation (VNS). Clin. Neurosci. Res. 2004, 4, 71–79. [Google Scholar] [CrossRef]
- Ashkan, K.; Rogers, P.; Bergman, H.; Ughratdar, I. Insights into the mechanisms of deep brain stimulation. Nat. Reviews. Neurol. 2017, 13, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Chiken, S.; Nambu, A. Disrupting neuronal transmission: Mechanism of DBS? Front. Syst. Neurosci. 2014, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Valentin, A.; Nguyen, H.Q.; Skupenova, A.M.; Agirre-Arrizubieta, Z.; Jewell, S.; Mullatti, N.; Moran, N.F.; Richardson, M.P.; Selway, R.P.; Alarcon, G. Centromedian thalamic nuclei deep brain stimulation in refractory status epilepticus. Brain Stimul. 2012, 5, 594–598. [Google Scholar] [CrossRef]
- Martín-López, D.; Jiménez-Jiménez, D.; Cabañés-Martínez, L.; Selway, R.P.; Valentín, A.; Alarcón, G. The role of thalamus versus cortex in epilepsy: Evidence from human ictal centromedian recordings in patients assessed for deep brain stimulation. Int. J. Neural Syst. 2017, 27, 1750010. [Google Scholar] [CrossRef]
- Cooper, Y.A.; Pianka, S.T.; Alotaibi, N.M.; Babayan, D.; Salavati, B.; Weil, A.G.; Ibrahim, G.M.; Wang, A.C.; Fallah, A. Repetitive transcranial magnetic stimulation for the treatment of drug-resistant epilepsy: A systematic review and individual participant data meta-analysis of real-world evidence. Epilepsia Open 2018, 3, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Perez-Carbonell, L.; Faulkner, H.; Higgins, S.; Koutroumanidis, M.; Leschziner, G. Vagus nerve stimulation for drug-resistant epilepsy. Pract. Neurol. 2019, 20, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, S.; Liang, S.; Liu, N.; Yu, X.; Liang, S. Deep brain stimulation of the anterior nucleus of the thalamus in a patient with super-refractory convulsive status epilepticus. Epileptic Disord. Int. Epilepsy J. Videotape 2019, 21, 379–384. [Google Scholar] [CrossRef]
- Nissen, T.; Wynn, R. The clinical case report: A review of its merits and limitations. BMC Res. Notes 2014, 7, 264. [Google Scholar] [CrossRef]
- Liu, A.; Pang, T.; Herman, S.; Pascual-Leone, A.; Rotenberg, A. Transcranial magnetic stimulation for refractory focal status epilepticus in the intensive care unit. Seizure 2013, 22, 893–896. [Google Scholar] [CrossRef]
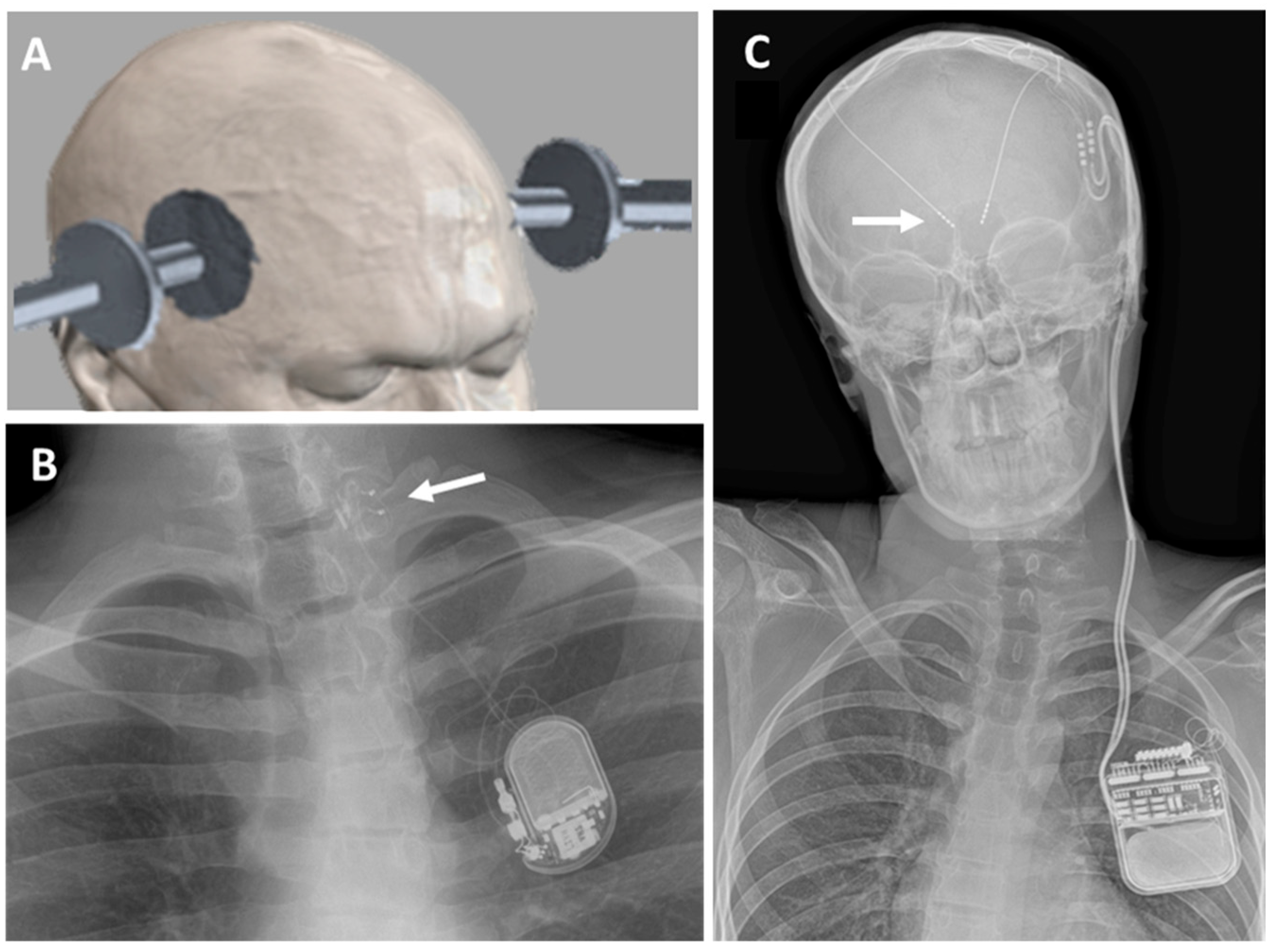
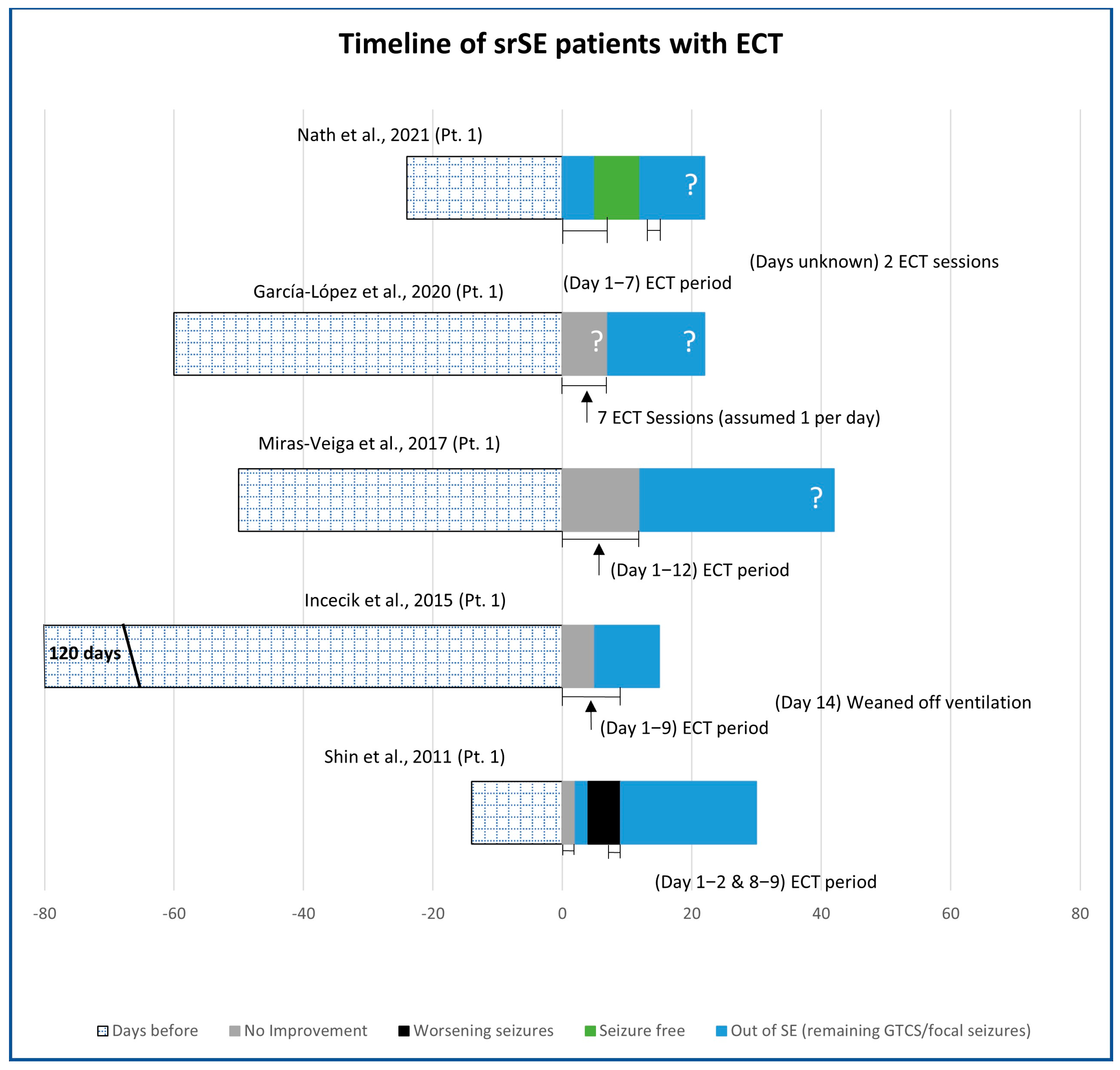
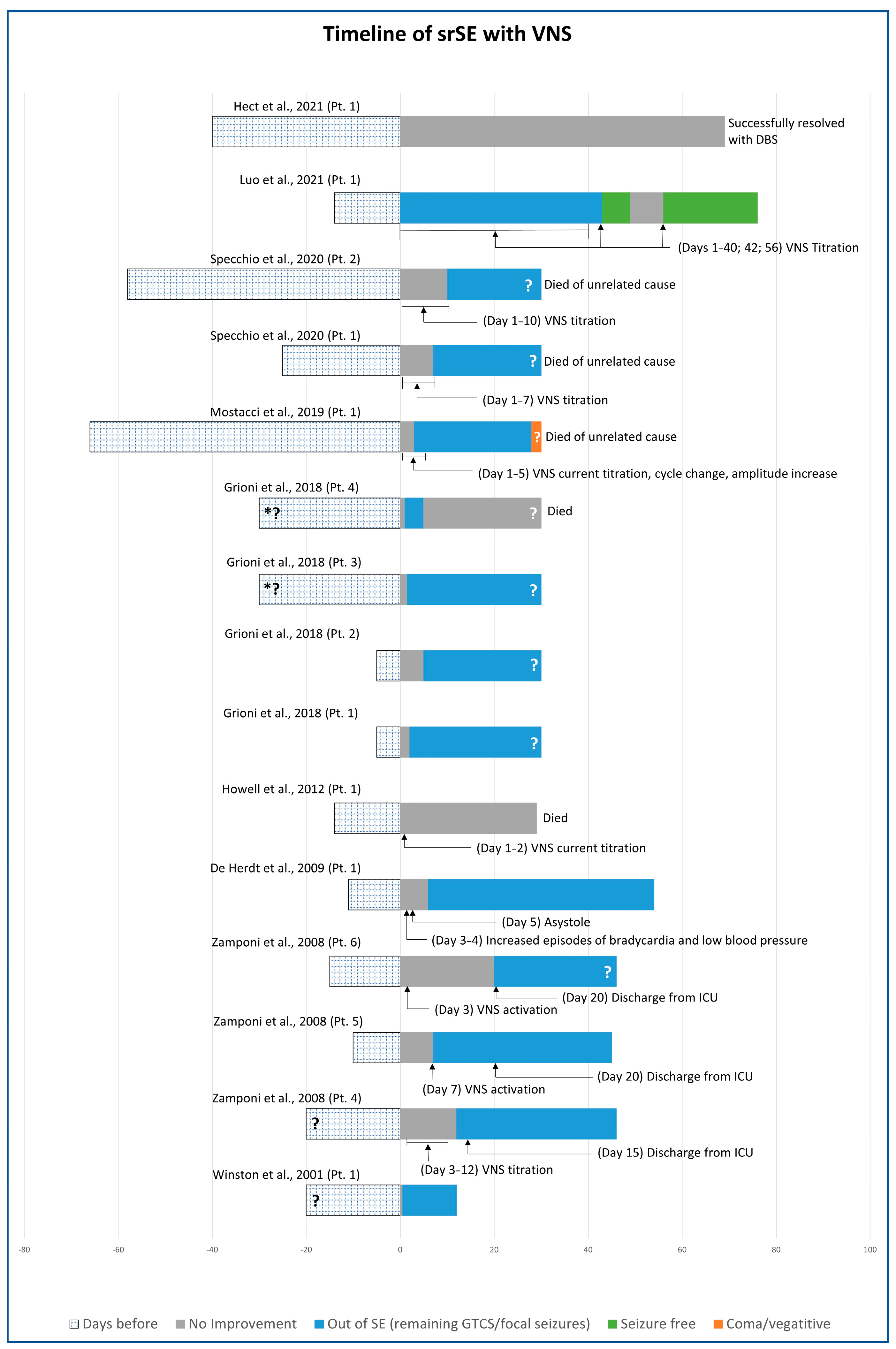
| Number of Patients | Male | Female | Stimulation Age (Years) | De Novo | Post Febrile | |
|---|---|---|---|---|---|---|
| ECT | 5 | 2 | 3 | 6.8 (3−–−16) | 3 | 2 |
| VNS | 15 * | 8 | 7 | 5.73 (0.5–16) | 3 | 3 |
| DBS | 6 * | 3 | 3 | 12.5 (5–17) | 4 | 3 |
| All | 25 | 13 | 12 | 7.4 (0.5–17) | 9 | 7 |
| SE Duration before Stim (Days) | Stim Duration before SE Resolution (Days) | Recovered from SE | Severe Sequelae Post-SE | Died during SE | |
|---|---|---|---|---|---|
| ECT | 53.63 (14–120) N = 5 | 4.75 (0–12) * N = 4 | 5 | 3 | 0 |
| VNS | 23.9 (5–66) N = 11 ** | 6.2 (0–20) N = 12 | 12 | 0 | 2 *** |
| DBS | 49 (27–86) N = 6 | 7.7 (0–29) N = 6 | 6 | 1 | 0 |
| All | 37.5 (5–120) N = 21 | 6.3 (0–29) * N = 22 | 23 | 4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavropoulos, I.; Pak, H.L.; Alarcon, G.; Valentin, A. Neuromodulation Techniques in Children with Super-Refractory Status Epilepticus. Brain Sci. 2023, 13, 1527. https://doi.org/10.3390/brainsci13111527
Stavropoulos I, Pak HL, Alarcon G, Valentin A. Neuromodulation Techniques in Children with Super-Refractory Status Epilepticus. Brain Sciences. 2023; 13(11):1527. https://doi.org/10.3390/brainsci13111527
Chicago/Turabian StyleStavropoulos, Ioannis, Ho Lim Pak, Gonzalo Alarcon, and Antonio Valentin. 2023. "Neuromodulation Techniques in Children with Super-Refractory Status Epilepticus" Brain Sciences 13, no. 11: 1527. https://doi.org/10.3390/brainsci13111527
APA StyleStavropoulos, I., Pak, H. L., Alarcon, G., & Valentin, A. (2023). Neuromodulation Techniques in Children with Super-Refractory Status Epilepticus. Brain Sciences, 13(11), 1527. https://doi.org/10.3390/brainsci13111527









