Investigating Behavioral and Neuronal Changes in Adolescent Mice Following Prenatal Exposure to Electronic Cigarette (E-Cigarette) Vapor Containing Nicotine
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. The Exposure Paradigm
2.3. Body Weight Tracking
2.4. Sucrose Preference Test (SPT)
2.5. Marble-Burying Test (MBT)
2.6. Novel Object Recognition
2.7. Oral Nicotine Preference Test
2.8. Molecular Studies: Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
3. Results
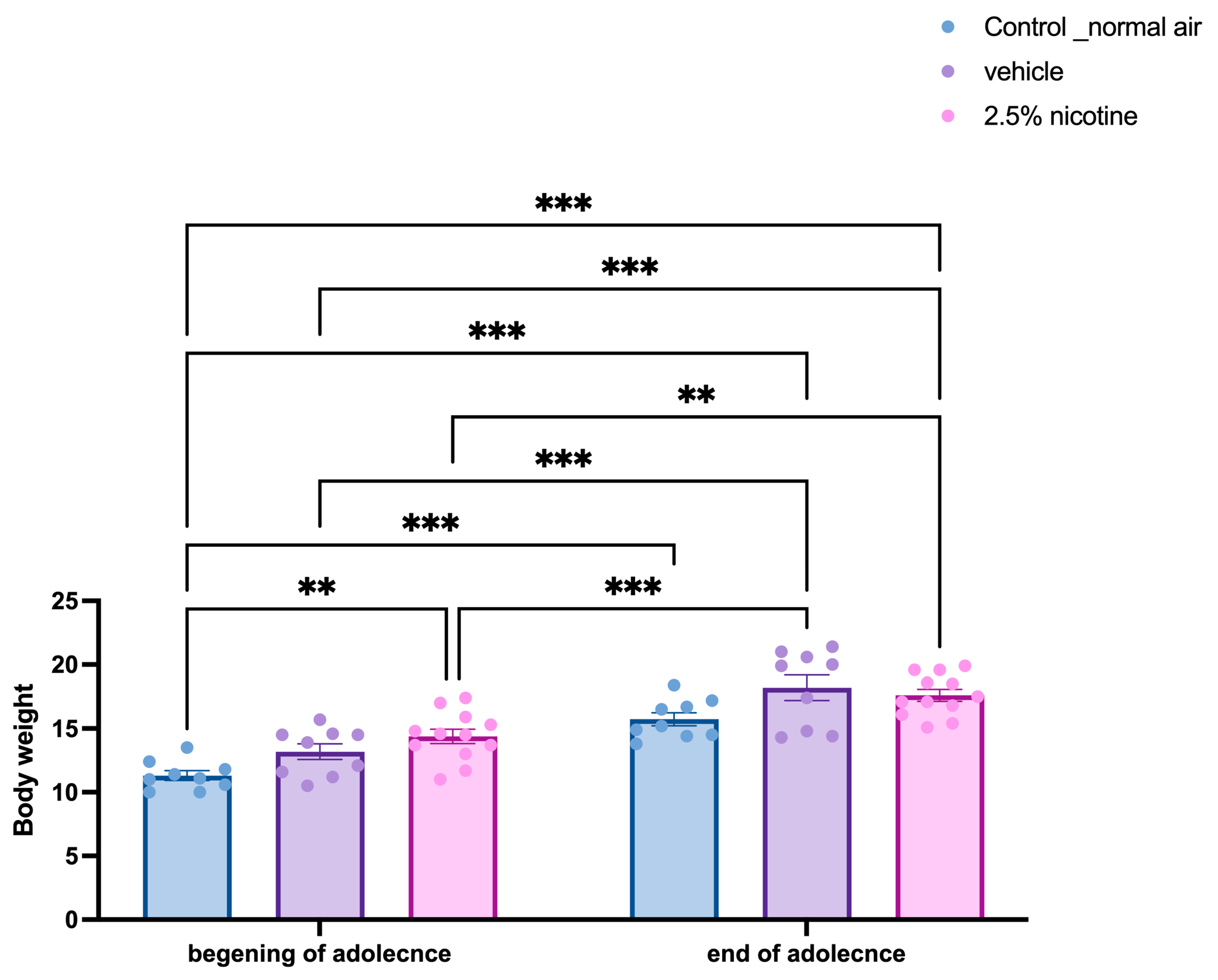
3.1. Body Weight Analyses of Adolescent Mice Following First-Trimester Prenatal Exposure to E-Cigarettes
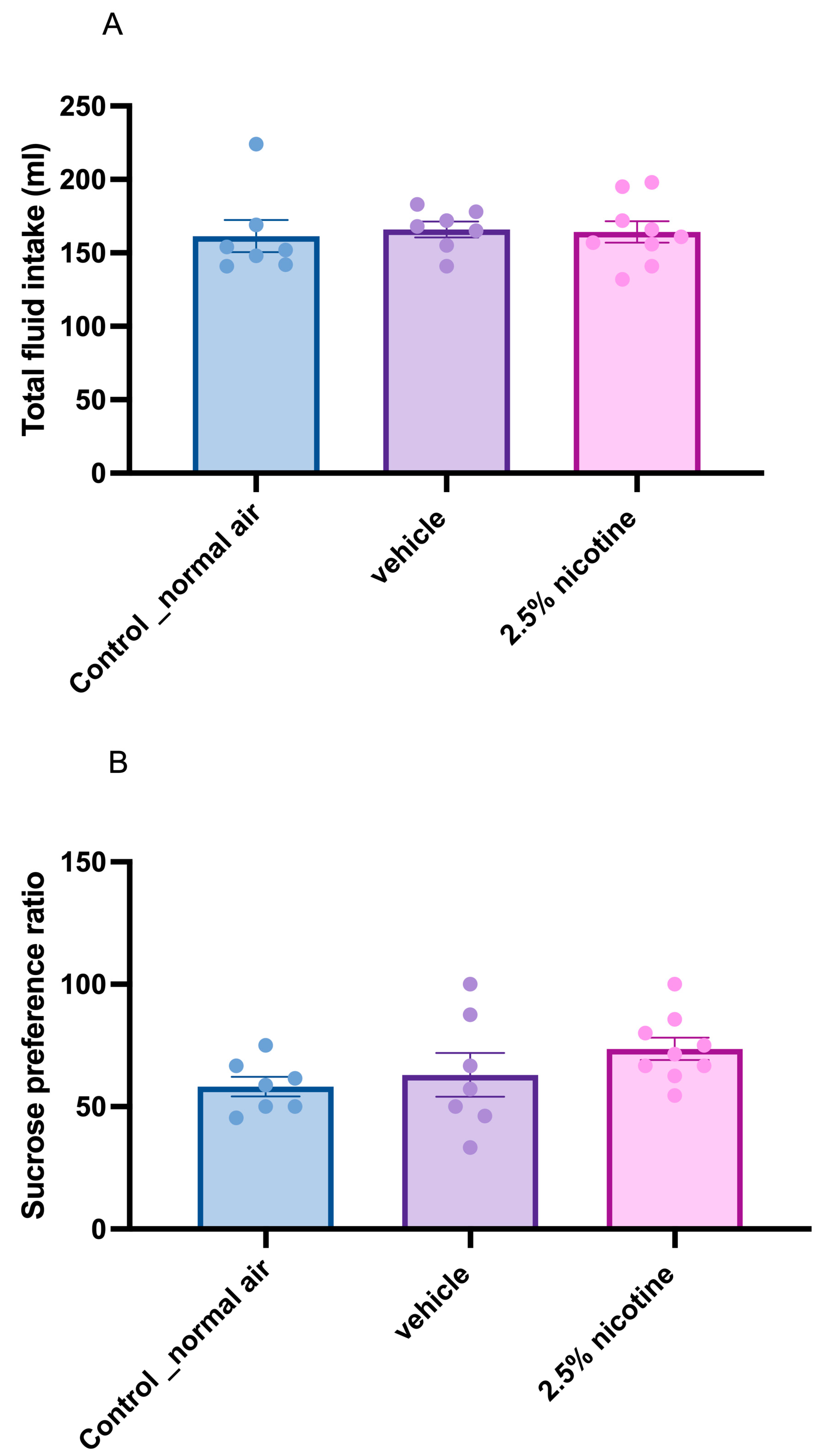
3.2. Sucrose Neophobia among Tested Groups
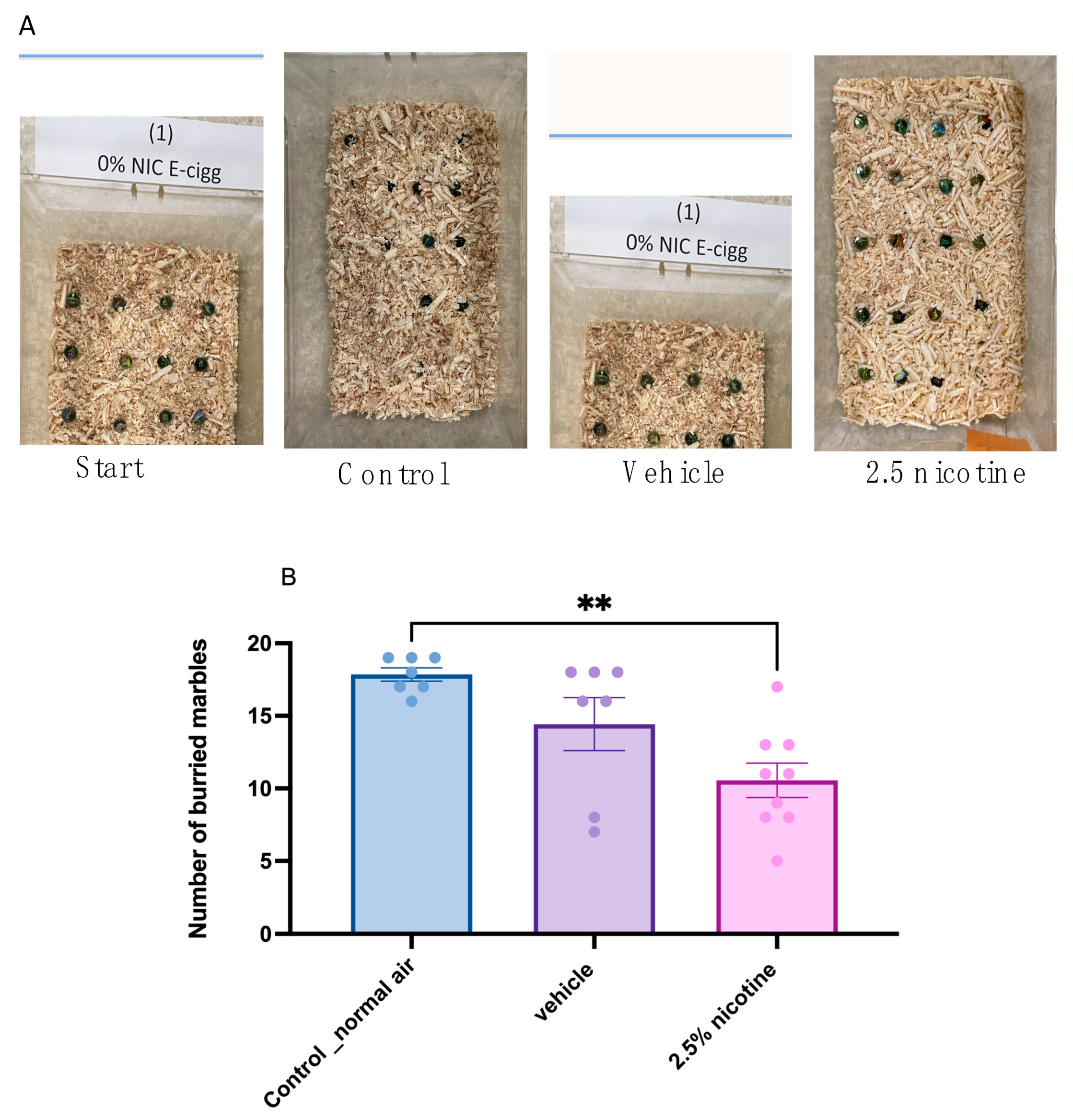
3.3. Marble-Burying Behavior
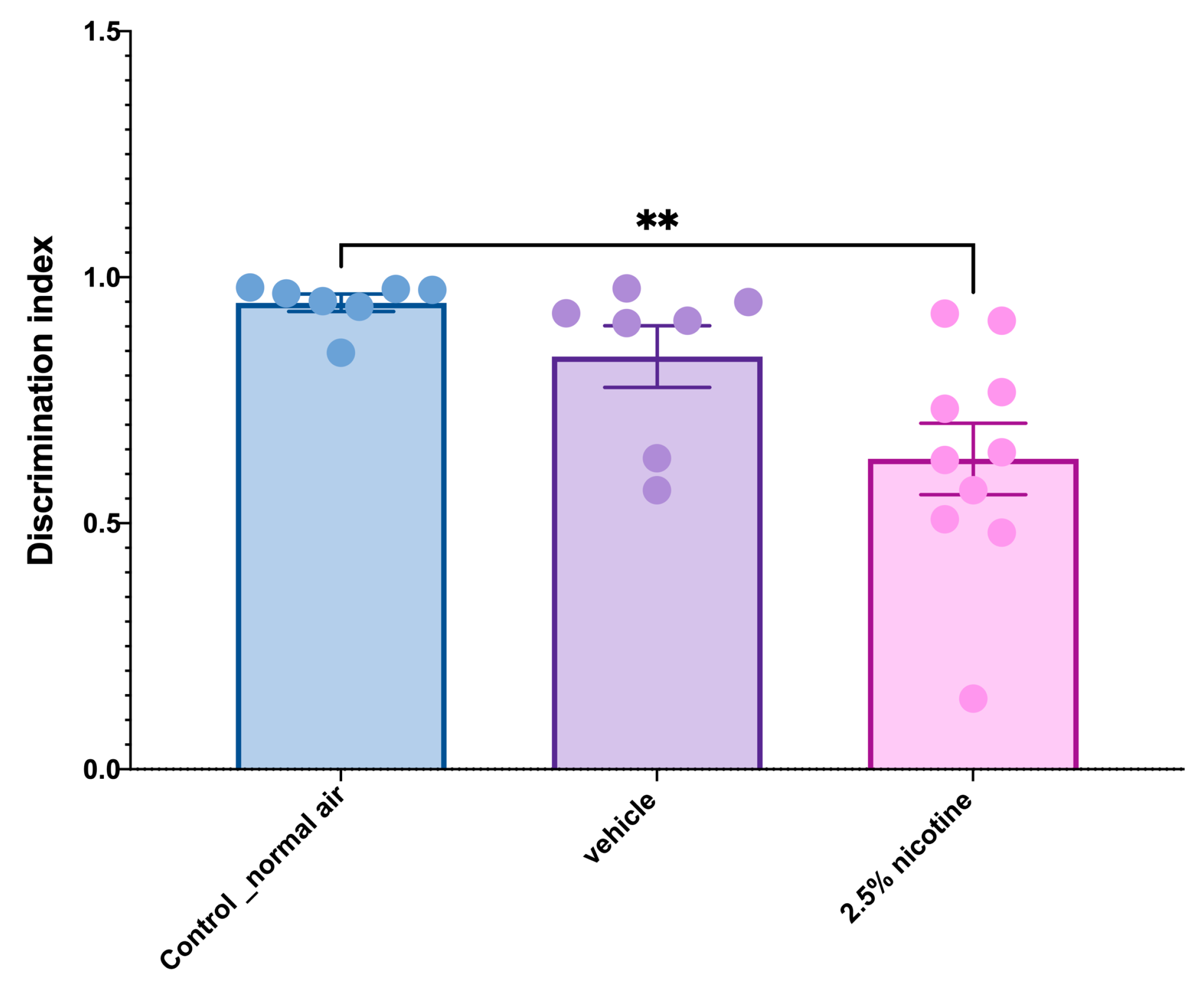
3.4. Examining Recognition Memory Using a Novel Object Task
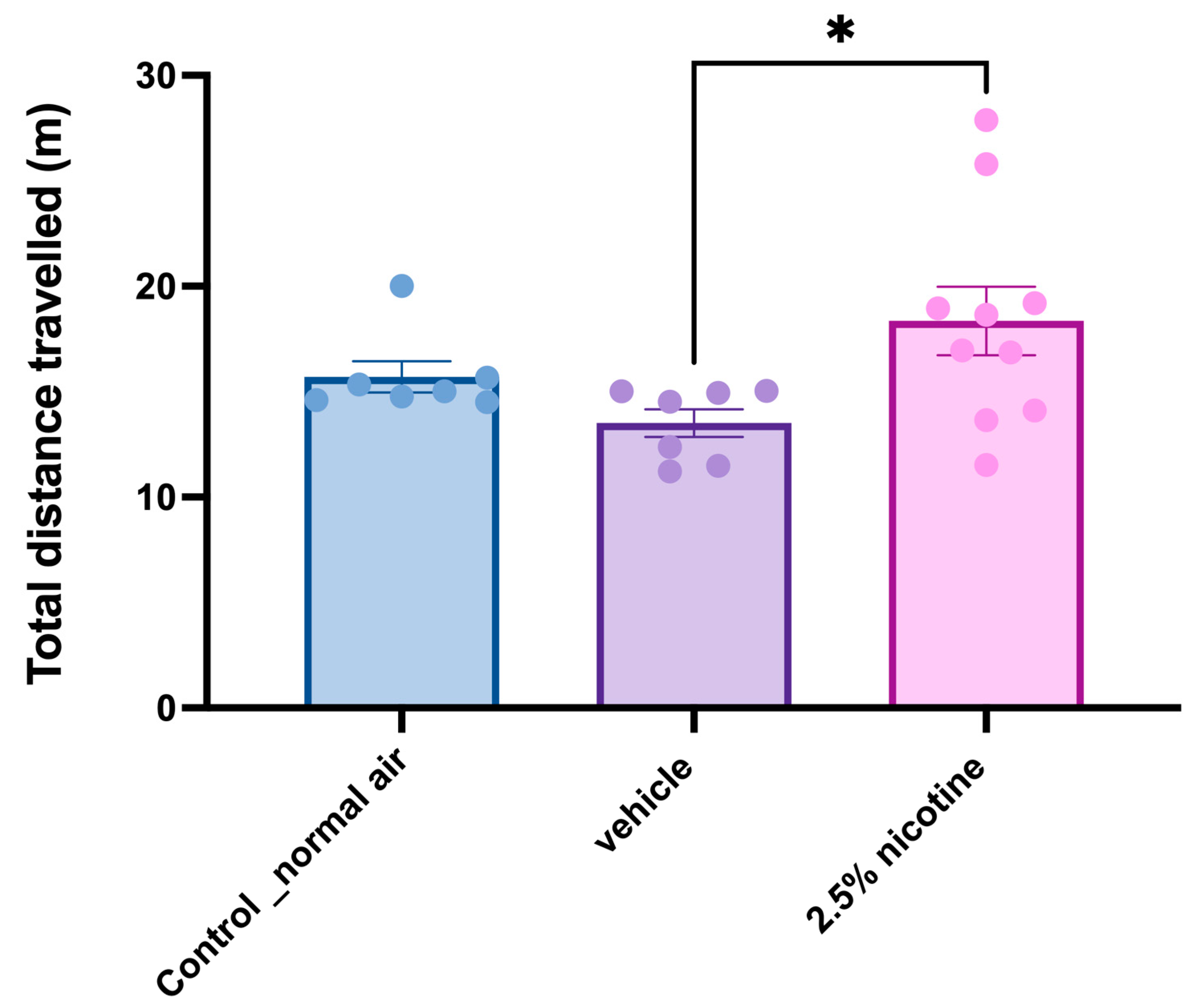
3.5. Investigating Locomotor Activity while Performing Novel Object Tasks
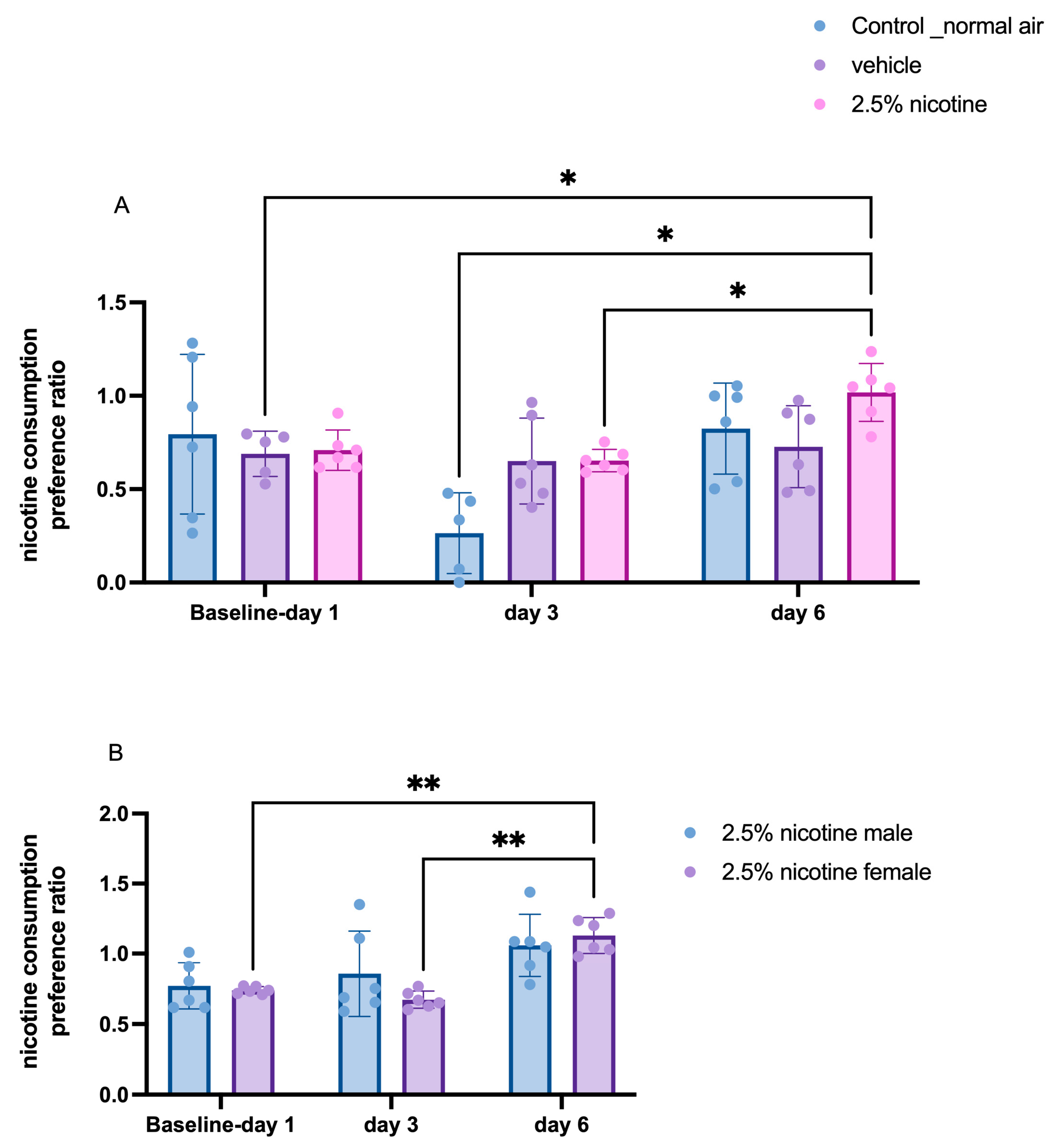
3.6. Tracking Daily Nicotine Consumption Ratio among Tested Groups
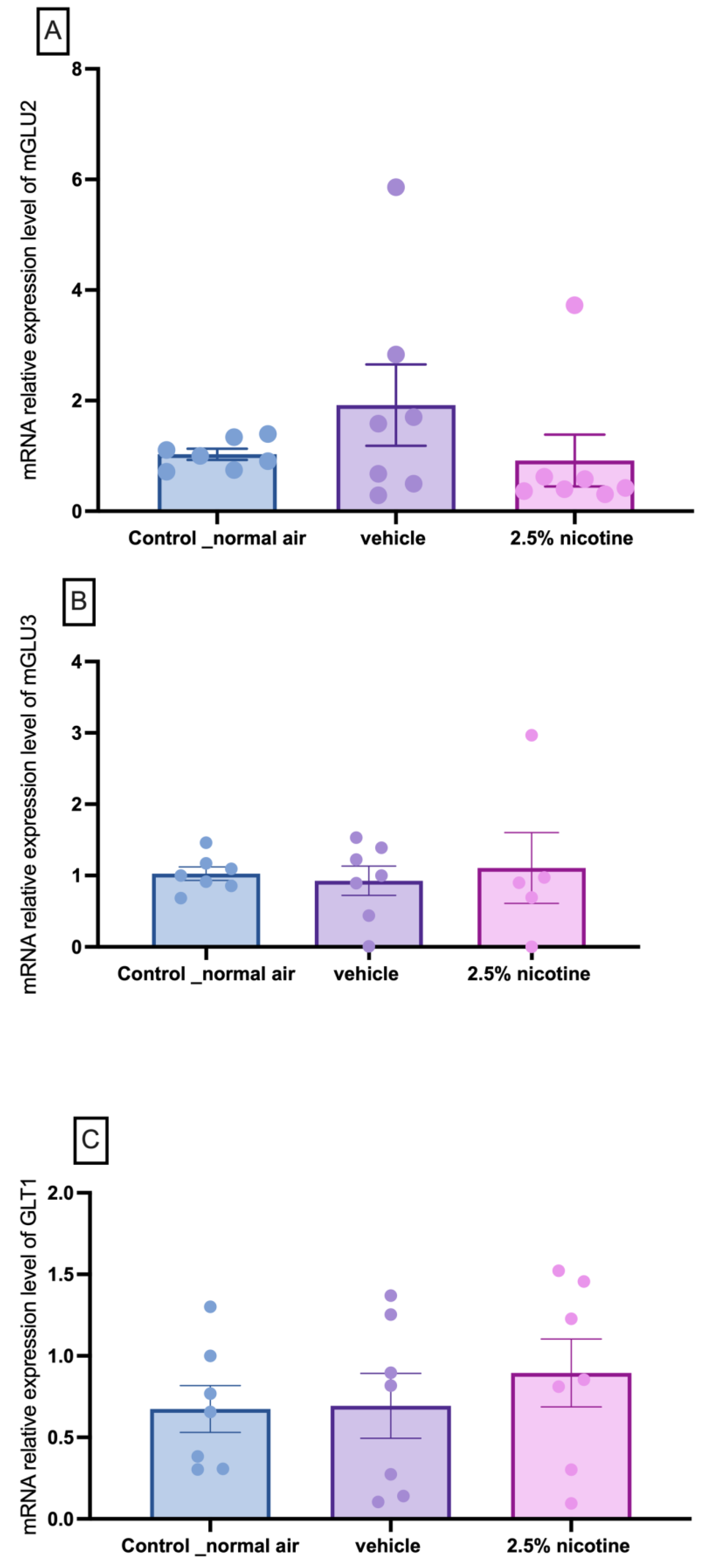
3.7. Molecular Assessment of Metabotropic Glutamate Receptors and Trannsporter mRNA Expression in the Nucleus Accumbens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schoenborn, C.A.; Gindi, R.M. Electronic Cigarette Use Among Adults: United States, 2014. NCHS Data Brief 2015, 217, 1–8. [Google Scholar]
- Rooke, C.; Amos, A. News media representations of electronic cigarettes: An analysis of newspaper coverage in the UK and Scotland. Tob. Control 2014, 23, 507. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, A.; Fallin-Bennett, A.; Barnett, J.; Ashford, K. Perceptions and use of electronic cigarettes in pregnancy. Health Educ. Res. 2017, 32, 22–32. [Google Scholar] [CrossRef]
- Dani, J.A.; Bertrand, D. Nicotinic Acetylcholine Receptors and Nicotinic Cholinergic Mechanisms of the Central Nervous System. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.B.; McQuown, S.C.; Leslie, F.M. The dynamic effects of nicotine on the developing brain. Pharmacol. Ther. 2009, 122, 125–139. [Google Scholar] [CrossRef]
- Albillos, A.; McIntosh, J.M. Human nicotinic receptors in chromaffin cells: Characterization and pharmacology. Pflügers Arch. Eur. J. Physiol. 2018, 470, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.K.; Sharma, V.; Pandey, R.K.; Shukla, S.S. Nicotine Addiction: Neurobiology and Mechanism. J. Pharmacopunct. 2020, 23, 1–7. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Moussawi, K.; Kalivas, P.W. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur. J. Pharmacol. 2010, 639, 115–122. [Google Scholar] [CrossRef]
- Moro, F.; Orrù, A.; Marzo, C.M.; Di Clemente, A.; Cervo, L. mGluR2/3 mediates short-term control of nicotine-seeking by acute systemic N-acetylcysteine. Addict. Biol. 2018, 23, 28–40. [Google Scholar] [CrossRef]
- Kruyer, A.; Kalivas, P.W. Astrocytes as cellular mediators of cue reactivity in addiction. Curr. Opin. Pharmacol. 2021, 56, 1–6. [Google Scholar] [CrossRef]
- Alasmari, F.; Crotty Alexander, L.E.; Nelson, J.A.; Schiefer, I.T.; Breen, E.; Drummond, C.A.; Sari, Y. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α-7 nicotinic acetylcholine receptor in female CD-1 mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 77, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Crotty Alexander, L.E.; Hammad, A.M.; Bojanowski, C.M.; Moshensky, A.; Sari, Y. Effects of Chronic Inhalation of Electronic Cigarette Vapor Containing Nicotine on Neurotransmitters in the Frontal Cortex and Striatum of C57BL/6 Mice. Front. Pharmacol. 2019, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Knackstedt, L.A.; LaRowe, S.; Mardikian, P.; Malcolm, R.; Upadhyaya, H.; Hedden, S.; Markou, A.; Kalivas, P.W. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol. Psychiatry 2009, 65, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Aherrera, A.; Lopez, A.; Neptune, E.; Winickoff, J.P.; Klein, J.D.; Chen, G.; Lazarus, P.; Collaco, J.M.; McGrath-Morrow, S.A. Adult Behavior in Male Mice Exposed to E-Cigarette Nicotine Vapors during Late Prenatal and Early Postnatal Life. PLoS ONE 2015, 10, e0137953. [Google Scholar] [CrossRef]
- Al-Sawalha, N.; Alzoubi, K.; Khabour, O.; Karaoghlanian, N.; Ismail, Z.; Shihadeh, A.; Eissenberg, T. Effect of electronic cigarette aerosol exposure during gestation and lactation on learning and memory of adult male offspring rats. Physiol. Behav. 2020, 221, 112911. [Google Scholar] [CrossRef]
- Green, T.A.; Alibhai, I.N.; Roybal, C.N.; Winstanley, C.A.; Theobald, D.E.H.; Birnbaum, S.G.; Graham, A.R.; Unterberg, S.; Graham, D.L.; Vialou, V.; et al. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biol. Psychiatry 2010, 67, 28–35. [Google Scholar] [CrossRef]
- Bunney, P.E.; Burroughs, D.; Hernandez, C.; LeSage, M.G. The effects of nicotine self-administration and withdrawal on concurrently available chow and sucrose intake in adult male rats. Physiol. Behav. 2016, 154, 49–59. [Google Scholar] [CrossRef][Green Version]
- Alkhlaif, Y.; Bagdas, D.; Jackson, A.; Park, A.J.; Damaj, I.M. Assessment of nicotine withdrawal-induced changes in sucrose preference in mice. Pharmacol. Biochem. Behav. 2017, 161, 47–52. [Google Scholar] [CrossRef]
- Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J. Neurosci. Methods 2014, 234, 139–146. [Google Scholar] [CrossRef]
- Alkam, T.; Kim, H.-C.; Hiramatsu, M.; Mamiya, T.; Aoyama, Y.; Nitta, A.; Yamada, K.; Nabeshima, T. Evaluation of emotional behaviors in young offspring of C57BL/6J mice after gestational and/or perinatal exposure to nicotine in six different time-windows. Behav. Brain Res. 2013, 239, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Carboni, L.; Ponzoni, L.; Braida, D.; Sala, M.; Gotti, C.; Zoli, M. Altered mRNA Levels of Stress-Related Peptides in Mouse Hippocampus and Caudate-Putamen in Withdrawal after Long-Term Intermittent Exposure to Tobacco Smoke or Electronic Cigarette Vapour. Int. J. Mol. Sci. 2021, 22, 599. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, C.J.; Fontaine-Palmer, N.S.; Vincent, M.; Endean, E.P.; Aggleton, J.P.; Brown, M.W.; Warburton, E.C. Differing time dependencies of object recognition memory impairments produced by nicotinic and muscarinic cholinergic antagonism in perirhinal cortex. Learn. Mem. 2011, 18, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.C.; Pogun, S.; Nesil, T.; Kanit, L. Oral Nicotine Self-Administration in Rodents. J. Addict. Res. Ther. 2012, S2, 224. [Google Scholar] [CrossRef]
- Alshammari, T.K.; Alghamdi, H.; Alkhader, L.F.; Alqahtani, Q.; Alrasheed, N.M.; Yacoub, H.; Alnaem, N.; AlNakiyah, M.; Alshammari, M.A. Analysis of the molecular and behavioral effects of acute social isolation on rats. Behav. Brain Res. 2020, 377, 112191. [Google Scholar] [CrossRef]
- Ponzoni, L.; Moretti, M.; Sala, M.; Fasoli, F.; Mucchietto, V.; Lucini, V.; Cannazza, G.; Gallesi, G.; Castellana, C.N.; Clementi, F.; et al. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur. Neuropsychopharmacol. 2015, 25, 1775–1786. [Google Scholar] [CrossRef]
- Guimarães, A.T.B.; Ferreira, R.d.O.; Rabelo, L.M.; e Silva, B.C.; de Souza, J.M.; da Silva, W.A.M.; de Menezes, I.P.P.; Rodrigues, A.S.d.L.; Vaz, B.G.; de Oliveira Costa, D.R.; et al. The C57BL/6J mice offspring originated from a parental generation exposed to tannery effluents shows object recognition deficits. Chemosphere 2016, 164, 593–602. [Google Scholar] [CrossRef]
- Robinson, S.F.; Marks, M.J.; Collins, A.C. Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology 1996, 124, 332–339. [Google Scholar] [CrossRef]
- Siu, E.C.K.; Wildenauer, D.B.; Tyndale, R.F. Nicotine self-administration in mice is associated with rates of nicotine inactivation by CYP2A5. Psychopharmacology 2006, 184, 401–408. [Google Scholar] [CrossRef]
- Butt, C.M.; King, N.M.; Hutton, S.R.; Collins, A.C.; Stitzel, J.A. Modulation of nicotine but not ethanol preference by the mouse Chrna4 A529T polymorphism. Behav. Neurosci. 2005, 119, 26–37. [Google Scholar] [CrossRef]
- Alshammari, T.K.; Alghamdi, H.M.; Alduhailan, H.E.; Saja, M.F.; Alrasheed, N.M.; Alshammari, M.A. Examining the central effects of chronic stressful social isolation on rats. Biomed. Rep. 2020, 13, 56. [Google Scholar] [CrossRef]
- Gravel, J.; Potter, B.; Dubois, L. Prenatal Exposure to Maternal Cigarette Smoke and Offspring Risk of Excess Weight Is Independent of Both Birth Weight and Catch-Up Growth. ISRN Epidemiol. 2013, 2013, 206120. [Google Scholar] [CrossRef][Green Version]
- Gao, Y.J.; Holloway, A.C.; Zeng, Z.H.; Lim, G.E.; Petrik, J.J.; Foster, W.G.; Lee, R.M. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes. Res. 2005, 13, 687–692. [Google Scholar] [CrossRef]
- Barra, N.G.; VanDuzer, T.A.; Holloway, A.C.; Hardy, D.B. Maternal Nicotine Exposure Leads to Augmented Expression of the Antioxidant Adipose Tissue Triglyceride Lipase Long-Term in the White Adipose of Female Rat Offspring. Toxicol. Sci. 2018, 164, 72–84. [Google Scholar] [CrossRef]
- Syme, C.; Abrahamowicz, M.; Mahboubi, A.; Leonard, G.T.; Perron, M.; Richer, L.; Veillette, S.; Gaudet, D.; Paus, T.; Pausova, Z. Prenatal exposure to maternal cigarette smoking and accumulation of intra-abdominal fat during adolescence. Obesity 2010, 18, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, L.; Perono, G.A.; Jamshed, S.; Holloway, A.C. Early Life Exposure to Nicotine: Postnatal Metabolic, Neurobehavioral and Respiratory Outcomes and the Development of Childhood Cancers. Toxicol. Sci. 2020, 178, 3–15. [Google Scholar] [CrossRef]
- Gimenez-Llort, L.; Alveal-Mellado, D. Digging Signatures in 13-Month-Old 3xTg-AD Mice for Alzheimer’s Disease and Its Disruption by Isolation Despite Social Life Since They Were Born. Front. Behav. Neurosci. 2021, 14, 611384. [Google Scholar] [CrossRef]
- Deacon, R.M.J.; Raley, J.M.; Perry, V.H.; Rawlins, J.N.P. Burrowing into prion disease. NeuroReport 2001, 12, 2053–2057. [Google Scholar] [CrossRef]
- Deacon, R.M.J.; Croucher, A.; Rawlins, J.N.P. Hippocampal cytotoxic lesion effects on species-typical behaviours in mice. Behav. Brain Res. 2002, 132, 203–213. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.R.; Rose, G.M.; Jensik, P.J. Impaired memory and marble burying activity in deformed epidermal autoregulatory factor 1 (Deaf1) conditional knockout mice. Behav. Brain Res. 2020, 380, 112383. [Google Scholar] [CrossRef]
- Santana-Santana, M.; Bayascas, J.-R.; Giménez-Llort, L. Sex-Dependent Signatures, Time Frames and Longitudinal Fine-Tuning of the Marble Burying Test in Normal and AD-Pathological Aging Mice. Biomedicines 2021, 9, 994. [Google Scholar] [CrossRef]
- Jedynak, P.; Jaholkowski, P.; Wozniak, G.; Sandi, C.; Kaczmarek, L.; Filipkowski, R.K. Lack of cyclin D2 impairing adult brain neurogenesis alters hippocampal-dependent behavioral tasks without reducing learning ability. Behav. Brain Res. 2012, 227, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Njung’e, K.; Handley, S.L. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav. 1991, 38, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.J. Digging and marble burying in mice: Simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006, 1, 122–124. [Google Scholar] [CrossRef]
- Zhang, L.; Spencer, T.J.; Biederman, J.; Bhide, P.G. Attention and working memory deficits in a perinatal nicotine exposure mouse model. PLoS ONE 2018, 13, e0198064. [Google Scholar] [CrossRef]
- Gavini, K.; Yang, E.; Parameshwaran, K. Developmental nicotine exposure impairs memory and reduces acetylcholine levels in the hippocampus of mice. Brain Res. Bull. 2021, 176, 1–7. [Google Scholar] [CrossRef]
- Polli, F.S.; Scharff, M.B.; Ipsen, T.H.; Aznar, S.; Kohlmeier, K.A.; Andreasen, J.T. Prenatal nicotine exposure in mice induces sex-dependent anxiety-like behavior, cognitive deficits, hyperactivity, and changes in the expression of glutamate receptor associated-genes in the prefrontal cortex. Pharmacol. Biochem. Behav. 2020, 195, 172951. [Google Scholar] [CrossRef]
- Pauly, J.R.; Sparks, J.A.; Hauser, K.F.; Pauly, T.H. In utero nicotine exposure causes persistent, gender-dependant changes in locomotor activity and sensitivity to nicotine in C57Bl/6 mice. Int. J. Dev. Neurosci. 2004, 22, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, V.; Patkina, N.; Tammimäki, A.; Talka, R.; Salminen, O.; Belozertseva, I.; Galankin, T.; Tuominen, R.; Zvartau, E. Nicotine exposure throughout early development promotes nicotine self-administration in adolescent mice and induces long-lasting behavioural changes. Eur. J. Pharmacol. 2010, 640, 87–93. [Google Scholar] [CrossRef]
- Cornelius, M.D.; Leech, S.L.; Goldschmidt, L.; Day, N.L. Prenatal tobacco exposure: Is it a risk factor for early tobacco experimentation? Nicotine Tob. Res. 2000, 2, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.D.; Rezvani, A.H.; Montoya, D.; Rose, J.E.; Swartzwelder, H.S. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology 2003, 169, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Pogun, S.; Yararbas, G.; Nesil, T.; Kanit, L. Sex differences in nicotine preference. J. Neurosci. Res. 2017, 95, 148–162. [Google Scholar] [CrossRef]
- Cornelius, M.E.; Wang, T.W.; Jamal, A.; Loretan, C.G.; Neff, L.J. Tobacco Product Use Among Adults—United States, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Al-Rejaie, S.S.; AlSharari, S.D.; Sari, Y. Targeting glutamate homeostasis for potential treatment of nicotine dependence. Brain Res. Bull. 2016, 121, 1–8. [Google Scholar] [CrossRef]
- Reid, M.S.; Fox, L.; Ho, L.B.; Berger, S.P. Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: Neuropharmacological characterization. Synapse 2000, 35, 129–136. [Google Scholar] [CrossRef]
- Saellstroem Baum, S.; Huebner, A.; Krimphove, M.; Morgenstern, R.; Badawy, A.A.; Spies, C.D. Nicotine stimulation on extracellular glutamate levels in the nucleus accumbens of ethanol-withdrawn rats in vivo. Alcohol. Clin. Exp. Res. 2006, 30, 1414–1421. [Google Scholar] [CrossRef]
- Science, A.I.f.B. Allen Developing Mouse Brain Atlas 2008. Available online: https://alleninstitute.org/division/brain-science/ (accessed on 1 March 2022).
- Smith, L.C.; Kallupi, M.; Tieu, L.; Shankar, K.; Jaquish, A.; Barr, J.; Su, Y.; Velarde, N.; Sedighim, S.; Carrette, L.L.G.; et al. Validation of a nicotine vapor self-administration model in rats with relevance to electronic cigarette use. Neuropsychopharmacology 2020, 45, 1909–1919. [Google Scholar] [CrossRef]
- Scofield, M.D.; Heinsbroek, J.A.; Gipson, C.D.; Kupchik, Y.M.; Spencer, S.; Smith, A.C.; Roberts-Wolfe, D.; Kalivas, P.W. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol. Rev. 2016, 68, 816–871. [Google Scholar] [CrossRef]
- Alhaddad, H.; Wong, W.; Sari, A.T.; Crotty Alexander, L.E.; Sari, Y. Effects of 3-Month Exposure to E-Cigarette Aerosols on Glutamatergic Receptors and Transporters in Mesolimbic Brain Regions of Female C57BL/6 Mice. Toxics 2020, 8, 95. [Google Scholar] [CrossRef]
- Willoughby, M.T.; Kollins, S.H.; McClernon, F.J. Association between smoking and retrospectively reported attention-deficit/hyperactivity disorder symptoms in a large sample of new mothers. Nicotine Tob. Res. 2009, 11, 313–322. [Google Scholar] [CrossRef]
- Kollins, S.H.; McClernon, F.J.; Fuemmeler, B.F. Association Between Smoking and Attention-Deficit/Hyperactivity Disorder Symptoms in a Population-Based Sample of Young Adults. Arch. Gen. Psychiatry 2005, 62, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Xie, M.; Li, Y.; Gong, X.; Li, J. Topiramate Reverses Physiological and Behavioral Alterations by Postoperative Cognitive Dysfunction in Rat Model Through Inhibiting TNF Signaling Pathway. Neuromol. Med. 2020, 22, 227–238. [Google Scholar] [CrossRef]
- Khurana, K.; Kumar, M.; Bansal, N. Lacidipine Attenuates Symptoms of Nicotine Withdrawal in Mice. Neurotox. Res. 2021, 39, 1920–1936. [Google Scholar] [CrossRef] [PubMed]
- Roni, M.A.; Rahman, S. The effects of lobeline on nicotine withdrawal-induced depression-like behavior in mice. Psychopharmacology 2014, 231, 2989–2998. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Batool, Z.; Ahmed, S.; Tabassum, S.; Khaliq, S.; Mehdi, B.J.; Sajid, I.; Ahmad, S.; Saleem, S.; Naqvi, F.; et al. Enriched environment palliates nicotine-induced addiction and associated neurobehavioral deficits in rats. Pak. J. Pharm. Sci. 2017, 30, 2375–2381. [Google Scholar]
- Ng, S.P.; Conklin, D.J.; Bhatnagar, A.; Bolanowski, D.D.; Lyon, J.; Zelikoff, J.T. Prenatal Exposure to Cigarette Smoke Induces Diet- and Sex-Dependent Dyslipidemia and Weight Gain in Adult Murine Offspring. Environ. Health Perspect. 2009, 117, 1042–1048. [Google Scholar] [CrossRef]

| Gene | Name | NCBI ID# | Forward | Reverse |
|---|---|---|---|---|
| Slc1a2 (GLT1) | Glial high affinity glutamate transporter) | NM_001077514.4 | 5′GCA CGA GAG CTA TGG TGT ATT3 A3′ | 5′CTG CTT GAG TTT GGG ATT G3′ |
| mGLU2 | Metabotropic glutamate receptor 2 | NM_001160353.1 | 5′CGC TAC AAC ATC TTC ACC TAT CT3′ | 5′AGT ATC CAG AGT CAG ACC TTC T 3′ |
| mGLU3 | Metabotropic glutamate receptor 3 | NM_181850.2 | 5′CCA TGT GAG CCC TAT GAA TAC C3′ | 5′GGA AGG TTG TAG CAT CCA GAT AG3′ |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase isoform 1 | NM_001289726.1 | 5′GTG GCA AAG TGG AGA TTG TTG3′ | 5′CGT TGA ATT TGC CGT GAG TG3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlHarthi, A.; Alasmari, F.; AlSharari, S.D.; Alrasheed, N.M.; Alshammari, M.A.; Alshammari, T.K. Investigating Behavioral and Neuronal Changes in Adolescent Mice Following Prenatal Exposure to Electronic Cigarette (E-Cigarette) Vapor Containing Nicotine. Brain Sci. 2023, 13, 1417. https://doi.org/10.3390/brainsci13101417
AlHarthi A, Alasmari F, AlSharari SD, Alrasheed NM, Alshammari MA, Alshammari TK. Investigating Behavioral and Neuronal Changes in Adolescent Mice Following Prenatal Exposure to Electronic Cigarette (E-Cigarette) Vapor Containing Nicotine. Brain Sciences. 2023; 13(10):1417. https://doi.org/10.3390/brainsci13101417
Chicago/Turabian StyleAlHarthi, Alaa, Fawaz Alasmari, Shakir D. AlSharari, Nouf M. Alrasheed, Musaad A. Alshammari, and Tahani K. Alshammari. 2023. "Investigating Behavioral and Neuronal Changes in Adolescent Mice Following Prenatal Exposure to Electronic Cigarette (E-Cigarette) Vapor Containing Nicotine" Brain Sciences 13, no. 10: 1417. https://doi.org/10.3390/brainsci13101417
APA StyleAlHarthi, A., Alasmari, F., AlSharari, S. D., Alrasheed, N. M., Alshammari, M. A., & Alshammari, T. K. (2023). Investigating Behavioral and Neuronal Changes in Adolescent Mice Following Prenatal Exposure to Electronic Cigarette (E-Cigarette) Vapor Containing Nicotine. Brain Sciences, 13(10), 1417. https://doi.org/10.3390/brainsci13101417







