Disrupted Gray Matter Networks Associated with Cognitive Dysfunction in Cerebral Small Vessel Disease
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Subjects
2.2. Image Acquisition
2.3. Preprocessing for Voxel-Based Morphometry
2.4. Network Construction
2.5. Network Topological Analysis
2.6. Between-Group Statistical Comparison and Correlation Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Subjects
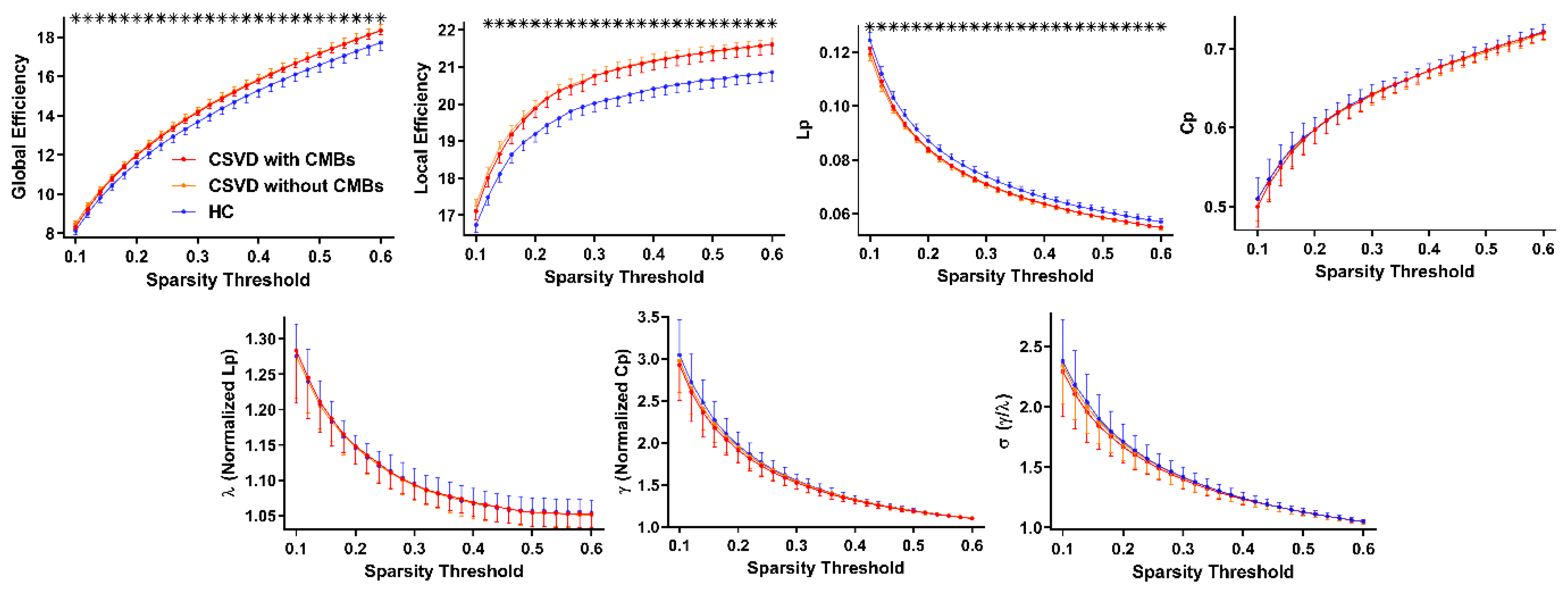
3.2. Alterations in the Global Properties of GM Networks in Patients with CSVD
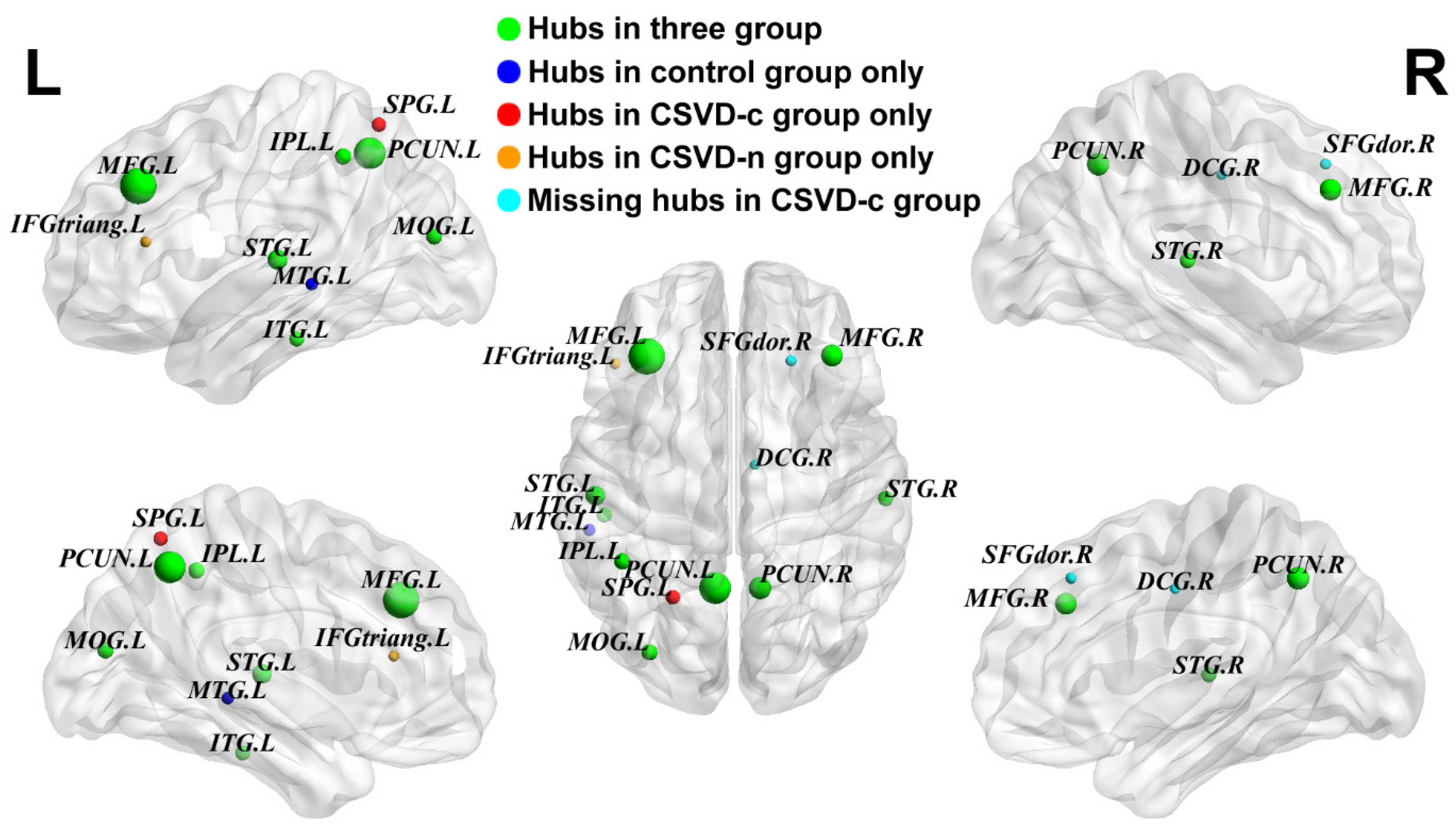
3.3. Partially Reorganized Hub Distributions of GM Networks among Groups
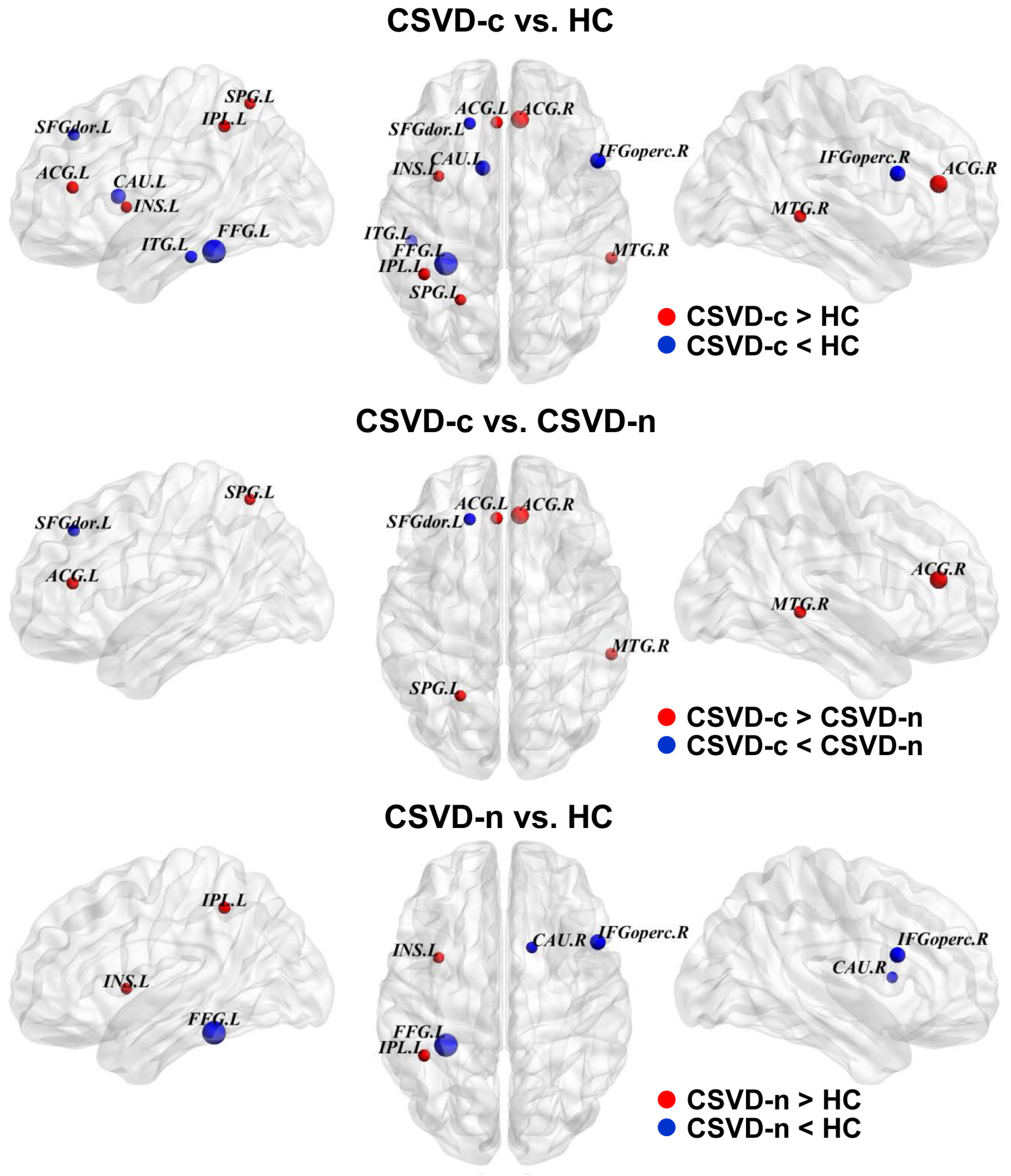
3.4. Alterations in the Regional Properties of GM Networks in Patients with CSVD
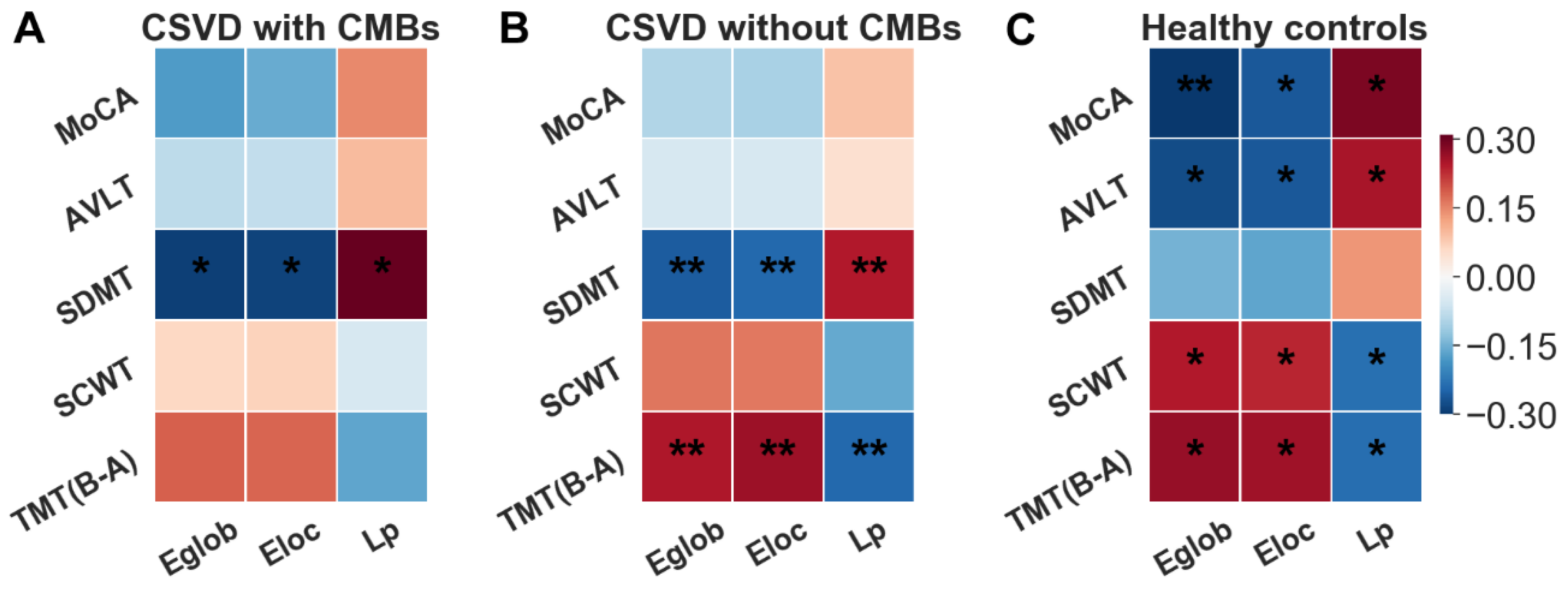
3.5. Correlations between Network Topological Alterations and Cognitive Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Dichgans, M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef] [PubMed]
- Cannistraro, R.J.; Badi, M.; Eidelman, B.H.; Dickson, D.W.; Middlebrooks, E.H.; Meschia, J.F. CNS small vessel disease: A clinical review. Neurology 2019, 92, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Hong, J.; Jeon, J.C. Cerebral Small Vessel Disease and Alzheimer’s Disease: A Review. Front Neurol. 2020, 11, 927. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Reis, C.; Tao, T.; Li, W.; Li, X.; Zhang, J.H. Cerebral Small Vessel Disease. Int. J. Mol. Sci. 2020, 21, 9729. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sohn, E.H.; Oh, E.; Lee, A.Y. Characteristics of Cerebral Microbleeds. Dement. Neurocognitive Disord. 2018, 17, 73–82. [Google Scholar] [CrossRef]
- Nannoni, S.; Ohlmeier, L.; Brown, R.B.; Morris, R.G.; MacKinnon, A.D.; Markus, H.S. Cognitive impact of cerebral microbleeds in patients with symptomatic small vessel disease. Int. J. Stroke 2022, 17, 415–424. [Google Scholar] [CrossRef]
- Godin, O.; Tzourio, C.; Rouaud, O.; Zhu, Y.; Maillard, P.; Pasquier, F.; Crivello, F.; Alpérovitch, A.; Mazoyer, B.; Dufouil, C. Joint effect of white matter lesions and hippocampal volumes on severity of cognitive decline: The 3C-Dijon MRI study. J. Alzheimers Dis. 2010, 20, 453–463. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef]
- Chételat, G.; Desgranges, B.; De La Sayette, V.; Viader, F.; Eustache, F.; Baron, J.-C. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport 2002, 13, 1939–1943. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Yang, S.; Luo, Y.; Zhu, W.; Zhu, W.; Shi, L. Structural changes in the lobar regions of brain in cerebral small-vessel disease patients with and without cognitive impairment: An MRI-based study with automated brain volumetry. Eur. J. Radiol. 2020, 126, 108967. [Google Scholar] [CrossRef]
- Su, N.; Liang, X.; Zhai, F.; Zhou, L.; Ni, J.; Yao, M.; Tian, F.; Zhang, S.; Jin, Z.; Cui, L.; et al. The consequence of cerebral small vessel disease: Linking brain atrophy to motor impairment in the elderly. Hum. Brain Mapp. 2018, 39, 4452–4461. [Google Scholar] [CrossRef] [PubMed]
- Raji, C.A.; Lopez, O.L.; Kuller, L.H.; Carmichael, O.T.; Longstreth, W.T.; Gach, H.M.; Boardman, J.; Bernick, C.B.; Thompson, P.M.; Becker, J.T. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol. Aging 2012, 33, 834.e7–834.e16. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shi, L.; Zhao, Q.; Zhang, M.; Ding, D.; Yu, B.; Fu, J. Coexisting cortical atrophy plays a crucial role in cognitive impairment in moderate to severe cerebral small vessel disease patients. Discov. Med. 2017, 23, 175–182. [Google Scholar]
- Babulal, G.M.; Chen, L.; Carr, D.B.; Johnson, A.M.; Shimony, J.S.; Doherty, J.; Murphy, S.; Walker, A.; Domash, H.; Hornbeck, R.; et al. Cortical atrophy and leukoaraiosis, imaging markers of cerebrovascular small vessel disease, are associated with driving behavior changes among cognitively normal older adults. J. Neurol. Sci. 2023, 448, 120616. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Fleischman, D.A.; Arfanakis, K.; Leurgans, S.E.; Barnes, L.L.; Bennett, D.A. Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct. Funct. 2016, 221, 2135–2146. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Wang, T.; Nie, S.; Yin, H.; Liu, J. Correlation between White Matter Hyperintensities Related Gray Matter Volume and Cognition in Cerebral Small Vessel Disease. J. Stroke Cerebrovasc. Dis. 2020, 29, 105275. [Google Scholar] [CrossRef]
- He, Y.; Chen, Z.; Evans, A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s disease. J. Neurosci. 2008, 28, 4756–4766. [Google Scholar] [CrossRef]
- Kuang, C.; Zha, Y.; Liu, C.; Chen, J. Altered Topological Properties of Brain Structural Covariance Networks in Patients With Cervical Spondylotic Myelopathy. Front. Hum. Neurosci. 2020, 14, 364. [Google Scholar] [CrossRef]
- Li, X.; Lei, D.; Niu, R.; Li, L.; Suo, X.; Li, W.; Yang, C.; Yang, T.; Ren, J.; Pinaya, W.H.L.; et al. Disruption of gray matter morphological networks in patients with paroxysmal kinesigenic dyskinesia. Hum. Brain Mapp. 2021, 42, 398–411. [Google Scholar] [CrossRef]
- Lim, H.K.; Jung, W.S.; Aizenstein, H.J. Aberrant topographical organization in gray matter structural network in late life depression: A graph theoretical analysis. Int. Psychogeriatr. 2013, 25, 1929–1940. [Google Scholar] [CrossRef]
- Singh, M.K.; Kesler, S.R.; Hosseini, S.H.; Kelley, R.G.; Amatya, D.; Hamilton, J.P.; Chen, M.C.; Gotlib, I.H. Anomalous gray matter structural networks in major depressive disorder. Biol. Psychiatry 2013, 74, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Orta, C.; Mendiola-Santibañez, J.D.; Ibrahimi, D.; Rodríguez-Reséndiz, J.; Díaz-Florez, G.; Olvera-Olvera, C.A. Hyperconnected Openings Codified in a Max Tree Structure: An Application for Skull-Stripping in Brain MRI T1. Sensors 2022, 22, 1378. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, B.C.; Worsley, K.J.; Besson, P.; Concha, L.; Lerch, J.P.; Evans, A.C.; Bernasconi, N. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: Insights on the relation between mesiotemporal connectivity and cortical atrophy. Neuroimage 2008, 42, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Mitelman, S.A.; Buchsbaum, M.S.; Brickman, A.M.; Shihabuddin, L. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage 2005, 27, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Lerch, J.P.; Worsley, K.; Shaw, W.P.; Greenstein, D.K.; Lenroot, R.K.; Giedd, J.; Evans, A.C. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage 2006, 31, 993–1003. [Google Scholar] [CrossRef]
- Raamana, P.R.; Strother, S.C. Does size matter? The relationship between predictive power of single-subject morphometric networks to spatial scale and edge weight. Brain Struct. Funct. 2020, 225, 2475–2493. [Google Scholar] [CrossRef]
- Wen, H.; Liu, Y.; Rekik, I.; Wang, S.; Zhang, J.; Zhang, Y.; Peng, Y.; He, H. Disrupted topological organization of structural networks revealed by probabilistic diffusion tractography in Tourette syndrome children. Hum. Brain Mapp. 2017, 38, 3988–4008. [Google Scholar] [CrossRef]
- Tuladhar, A.M.; Tay, J.; Van Leijsen, E.; Lawrence, A.J.; Van Uden IW, M.; Bergkamp, M.; De Leeuw, F.E. Structural network changes in cerebral small vessel disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 196–203. [Google Scholar] [CrossRef]
- Nestor, S.M.; Mišić, B.; Ramirez, J.; Zhao, J.; Graham, S.J.; Verhoeff, N.P.; Black, S.E. Small vessel disease is linked to disrupted structural network covariance in Alzheimer’s disease. Alzheimers Dement. 2017, 13, 749–760. [Google Scholar] [CrossRef]
- Sheng, X.; Chen, H.; Shao, P.; Qin, R.; Zhao, H.; Xu, Y.; Bai, F.; Initiative, T.A.D.N. Brain Structural Network Compensation Is Associated With Cognitive Impairment and Alzheimer’s Disease Pathology. Front. Neurosci. 2021, 15, 630278. [Google Scholar] [CrossRef]
- Chesebro, A.G.; Amarante, E.; Lao, P.J.; Meier, I.B.; Mayeux, R.; Brickman, A.M. Automated detection of cerebral microbleeds on T2*-weighted MRI. Sci. Rep. 2021, 11, 4004. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Al Olama, A.A.; Wason, J.; Tuladhar, A.M.; MC Van Leijsen, E.; Koini, M.; Hofer, E.; Morris, R.G.; Schmidt, R.; De Leeuw, F.-E.; Markus, H. Simple MRI score aids prediction of dementia in cerebral small vessel disease. Neurology 2020, 94, e1294–e1302. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Schoenberg, M.R.; Dawson, K.A.; Duff, K.; Patton, D.; Scott, J.G.; Adams, R.L. Test performance and classification statistics for the Rey Auditory Verbal Learning Test in selected clinical samples. Arch. Clin. Neuropsychol. 2006, 21, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Llinàs-Reglà, J.; Vilalta-Franch, J.; López-Pousa, S.; Calvó-Perxas, L.; Torrents Rodas, D.; Garre-Olmo, J. The Trail Making Test. Assessment 2017, 24, 183–196. [Google Scholar] [CrossRef]
- Periáñez, J.A.; Lubrini, G.; García-Gutiérrez, A.; Ríos-Lago, M. Construct Validity of the Stroop Color-Word Test: Influence of Speed of Visual Search, Verbal Fluency, Working Memory, Cognitive Flexibility, and Conflict Monitoring. Arch Clin. Neuropsychol. 2021, 36, 99–111. [Google Scholar] [CrossRef]
- Silva, P.; Spedo, C.; Barreira, A.; Leoni, R. Symbol Digit Modalities Test adaptation for Magnetic Resonance Imaging environment: A systematic review and meta-analysis. Mult. Scler. Relat. Disord 2018, 20, 136–143. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Ruffle, J.K.; Hyare, H.; Howard, M.A.; Farmer, A.D.; Apkarian, A.V.; Williams, S.C.; Aziz, Q.; Nachev, P. The autonomic brain: Multi-dimensional generative hierarchical modelling of the autonomic connectome. Cortex 2021, 143, 164–179. [Google Scholar] [CrossRef]
- Wen, H.; Liu, Y.; Rekik, I.; Wang, S.; Chen, Z.; Zhang, J.; Zhang, Y.; Peng, Y.; He, H. Combining Disrupted and Discriminative Topological Properties of Functional Connectivity Networks as Neuroimaging Biomarkers for Accurate Diagnosis of Early Tourette Syndrome Children. Mol. Neurobiol. 2018, 55, 3251–3269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Chen, J.; Liu, B.; Wang, J. Widespread increase of functional connectivity in Parkinson’s disease with tremor: A resting-state FMRI study. Front. Aging Neurosci. 2015, 7, 6. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xia, M.; Liao, X.; Evans, A.; He, Y. GRETNA: A graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci. 2015, 9, 386. [Google Scholar] [PubMed]
- He, Y.; Wang, J.; Wang, L.; Chen, Z.J.; Yan, C.; Yang, H.; Tang, H.; Zhu, C.; Gong, Q.; Zang, Y.; et al. Uncovering intrinsic modular organization of spontaneous brain activity in humans. PLoS ONE 2009, 4, e5226. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef]
- ter Telgte, A.; van Leijsen, E.M.C.; Wiegertjes, K.; Klijn, C.J.M.; Tuladhar, A.M.; de Leeuw, F.-E. Cerebral small vessel disease: From a focal to a global perspective. Nat. Rev. Neurol. 2018, 14, 387–398. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, X.; Deng, B.; Chang, Z.; Jin, D.; Huang, Y.; Zhang, J.H.; Yenari, M.A.; Jin, K.; Wang, Q. Cerebral small vessel disease alters neurovascular unit regulation of microcirculation integrity involved in vascular cognitive impairment. Neurobiol. Dis. 2022, 170, 105750. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosc. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Zhang, B.; Qi, S.; Liu, S.; Liu, X.; Wei, X.; Ming, D. Altered spontaneous neural activity in the precuneus, middle and superior frontal gyri, and hippocampus in college students with subclinical depression. BMC Psychiatry 2021, 21, 280. [Google Scholar] [CrossRef]
- Hornak, J.; Bramham, J.; Rolls, E.T.; Morris, R.G.; O’doherty, J.; Bullock, P.R.; Polkey, C.E. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain 2003, 126, 1691–1712. [Google Scholar] [CrossRef]
- Zhang, R.; Geng, X.; Lee, T.M.C. Large-scale functional neural network correlates of response inhibition: An fMRI meta-analysis. Brain Struct. Funct. 2017, 222, 3973–3990. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.S.; Mok, E.H.; Beharelle, A.R.; Goldin-Meadow, S.; Small, S.L. Frontal and temporal contributions to understanding the iconic co-speech gestures that accompany speech. Hum. Brain Mapp. 2014, 35, 900–917. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.; Thompson, H.E.; Hallam, G.; Karapanagiotidis, T.; Murphy, C.; De Caso, I.; Krieger-Redwood, K.; Bernhardt, B.C.; Smallwood, J.; Jefferies, E. Exploring the role of the posterior middle temporal gyrus in semantic cognition: Integration of anterior temporal lobe with executive processes. Neuroimage 2016, 137, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, S.H.; Alexander, M.P. Pure agraphia and unilateral optic ataxia associated with a left superior parietal lobule lesion. J. Neurol. Neurosurg. Psychiatry 1981, 44, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Schrooten, M.; Ghumare, E.G.; Seynaeve, L.; Theys, T.; Dupont, P.; Van Paesschen, W.; Vandenberghe, R. Electrocorticography of Spatial Shifting and Attentional Selection in Human Superior Parietal Cortex. Front. Hum. Neurosci. 2017, 11, 240. [Google Scholar] [CrossRef]
- Segal, E.; Petrides, M. The anterior superior parietal lobule and its interactions with language and motor areas during writing. Eur. J. Neurosci. 2012, 35, 309–322. [Google Scholar] [CrossRef]
- Koenigs, M.; Barbey, A.K.; Postle, B.R.; Grafman, J. Superior parietal cortex is critical for the manipulation of information in working memory. J. Neurosci. 2009, 29, 14980–14986. [Google Scholar] [CrossRef]
- Hänggi, J.; Streffer, J.; Jäncke, L.; Hock, C. Volumes of lateral temporal and parietal structures distinguish between healthy aging, mild cognitive impairment, and Alzheimer’s disease. J. Alzheimers Dis. 2011, 26, 719–734. [Google Scholar] [CrossRef]
- Dick, A.S.; Goldin-Meadow, S.; Hasson, U.; Skipper, J.I.; Small, S.L. Co-speech gestures influence neural activity in brain regions associated with processing semantic information. Hum. Brain Mapp. 2009, 30, 3509–3526. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci USA 2003, 100, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Chen, Q.F.; Yang, Z.T.; Shi, H.B. Aberrant topological organization of the functional brain network associated with prior overt hepatic encephalopathy in cirrhotic patients. Brain Imaging Behav. 2019, 13, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Yakushiji, Y.; Yokota, C.; Yamada, N.; Kuroda, Y.; Minematsu, K. Clinical characteristics by topographical distribution of brain microbleeds, with a particular emphasis on diffuse microbleeds. J. Stroke Cerebrovasc. Dis. 2011, 20, 214–221. [Google Scholar] [CrossRef]
- Van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef]
- Akoudad, S.; Wolters, F.J.; Viswanathan, A.; de Bruijn, R.F.; van der Lugt, A.; Hofman, A.; Vernooij, M.W. Association of Cerebral Microbleeds With Cognitive Decline and Dementia. JAMA Neurol. 2016, 73, 934–943. [Google Scholar] [CrossRef]
- Li, M.; Meng, Y.; Wang, M.; Yang, S.; Wu, H.; Zhao, B.; Wang, G. Cerebral gray matter volume reduction in subcortical vascular mild cognitive impairment patients and subcortical vascular dementia patients, and its relation with cognitive deficits. Brain Behav. 2017, 7, e00745. [Google Scholar] [CrossRef]
- Cao, W.W.; Wang, Y.; Dong, Q.; Chen, X.; Li, Y.S.; Zhou, Y.; Xu, Q. Deep microbleeds and periventricular white matter disintegrity are independent predictors of attention/executive dysfunction in non-dementia patients with small vessel disease. Int. Psychogeriatr. 2017, 29, 793–803. [Google Scholar] [CrossRef]
- Briggs, R.G.; Tanglay, O.; Dadario, N.B.; Young, I.M.; Fonseka, R.D.; Hormovas, J.; Dhanaraj, V.; Lin, Y.-H.; Kim, S.J.; Bouvette, A.; et al. The Unique Fiber Anatomy of Middle Temporal Gyrus Default Mode Connectivity. Oper. Neurosurg. 2021, 21, E8–E14. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Hu, X.; Xiao, C.; Rao, J.; Li, Z.; Liu, D.; Zou, Y.; Chen, J.; Liu, H. Altered Rich-Club Organization and Regional Topology Are Associated With Cognitive Decline in Patients With Frontal and Temporal Gliomas. Front. Hum. Neurosci. 2020, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.S.; Rombouts, S.A.R.B.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.H.; Karapanagiotidis, T.; Coggan, D.D.; Wailes-Newson, K.; Smallwood, J. Brain networks underlying bistable perception. Neuroimage 2015, 119, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhang, Y.; Liu, Y.; Wu, Z.; Song, Z.; Ma, M.; Wang, Y.; Dai, H. White Matter Structural Network Analysis to Differentiate Alzheimer’s Disease and Subcortical Ischemic Vascular Dementia. Front. Aging Neurosci. 2021, 13, 650377. [Google Scholar] [CrossRef]
- Hartwigsen, G. Flexible Redistribution in Cognitive Networks. Trends Cogn. Sci. 2018, 22, 687–698. [Google Scholar] [CrossRef]
- Cuadrado-Godia, E.; Dwivedi, P.; Sharma, S.; Santiago, A.O.; Gonzalez, J.R.; Balcells, M.; Laird, J.; Turk, M.; Suri, H.S.; Nicolaides, A.; et al. Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies. J. Stroke 2018, 20, 302–320. [Google Scholar] [CrossRef]
- Yates, P.A.; Villemagne, V.L.; Ellis, K.A.; Desmond, P.M.; Masters, C.L.; Rowe, C.C. Cerebral microbleeds: A review of clinical, genetic, and neuroimaging associations. Front. Neurol. 2014, 4, 205. [Google Scholar] [CrossRef]
- Miwa, K.; Tanaka, M.; Okazaki, S.; Furukado, S.; Sakaguchi, M.; Kitagawa, K. Relations of blood inflammatory marker levels with cerebral microbleeds. Stroke 2011, 42, 3202–3206. [Google Scholar] [CrossRef]
- Pétrault, M.; Casolla, B.; Ouk, T.; Cordonnier, C.; Bérézowski, V. Cerebral microbleeds: Beyond the macroscope. Int. J. Stroke 2019, 14, 468–475. [Google Scholar] [CrossRef]
- Ikeda, M.; Kodaira, S.; Kasahara, H.; Takai, E.; Nagashima, K.; Fujita, Y.; Ikeda, Y. Cerebral Microbleeds, Cerebrospinal Fluid, and Neuroimaging Markers in Clinical Subtypes of Alzheimer’s Disease. Front. Neurol. 2021, 12, 543866. [Google Scholar] [CrossRef]
- Mustapha, M.; Nassir, C.M.N.C.M.; Aminuddin, N.; Safri, A.A.; Ghazali, M.M. Cerebral Small Vessel Disease (CSVD)-Lessons From the Animal Models. Front. Physiol. 2019, 10, 1317. [Google Scholar] [CrossRef]
- Ifthikharuddin, S.F.; Shrier, D.A.; Numaguchi, Y.; Tang, X.; Ning, R.; Shibata, D.K.; Kurlan, R. MR volumetric analysis of the human basal ganglia: Normative data. Acad. Radiol. 2000, 7, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Torres, I.; Acker, J. Age, gender, and hemispheric differences in human striatum: A quantitative review and new data from in vivo MRI morphometry. Neurobiol. Learn Mem. 1995, 63, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Looi, J.C.L.; Maller, J.J.; Pagani, M.; Högberg, G.; Lindberg, O.; Liberg, B.; Botes, L.; Engman, E.-L.; Zhang, Y.; Svensson, L.; et al. Caudate volumes in public transportation workers exposed to trauma in the Stockholm train system. Psychiatry Res. 2009, 171, 138–143. [Google Scholar] [CrossRef]
- Cvejic, R.C.; Hocking, D.R.; Wen, W.; Georgiou-Karistianis, N.; Cornish, K.M.; Godler, D.E.; Rogers, C.; Trollor, J.N. Reduced caudate volume and cognitive slowing in men at risk of fragile X-associated tremor ataxia syndrome. Brain Imaging Behav. 2019, 13, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S. Molecular and Cellular Basis of Neurodegeneration in Alzheimer’s Disease. Mol. Cells 2017, 40, 613–620. [Google Scholar]
- Lei, Y.; Su, J.; Guo, Q.; Yang, H.; Gu, Y.; Mao, Y. Regional Gray Matter Atrophy in Vascular Mild Cognitive Impairment. J. Stroke Cerebrovasc. Dis. 2016, 25, 95–101. [Google Scholar] [CrossRef]
- Hutton, C.; Draganski, B.; Ashburner, J.; Weiskopf, N. A comparison between voxel-based cortical thickness and voxel-based morphometry in normal aging. Neuroimage 2009, 48, 371–380. [Google Scholar] [CrossRef]
- Liang, X.; Zou, Q.; He, Y.; Yang, Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc. Natl. Acad. Sci. USA 2013, 110, 1929–1934. [Google Scholar] [CrossRef]


| Characteristic | CSVD-c (n = 49) | CSVD-n (n = 121) | HC (n = 74) | p Value (ANOVA/χ2) | p Value (Post Hoc) | ||
|---|---|---|---|---|---|---|---|
| CSVD-c vs. HC | CSVD-c vs. CSVD-n | CSVD-n vs. HC | |||||
| Sex, female (%) | 19 (38.8%) | 59 (48.8%) | 41 (55.4%) | 0.196 χ2 | - | - | - |
| Age (y) | 63.69 ± 8.37 | 63.08 ± 7.73 | 60.85 ± 9.05 | 0.096 a | - | - | - |
| Education (y) | 11.51 ± 2.94 | 11.77 ± 3.24 | 12.68 ± 3.59 | 0.094 a | - | - | - |
| Smoke | 19 (38.8%) | 26 (21.5%) | 19 (25.7%) | 0.067 χ2 | - | - | - |
| Alcohol | 24 (49.0%) | 30 (24.8%) | 20 (27.0%) | 0.006 χ2 | 0.002 | 0.013 | - |
| Hypertension | 26 (53.1%) | 63 (52.1%) | 33 (44.6%) | 0.534 χ2 | - | - | - |
| Hyperlipidemia | 25 (51.0%) | 48 (39.7%) | 28 (37.8%) | 0.300 χ2 | - | - | - |
| Lacune | 16 (32.7%) | 23 (19.0%) | 0 | 0.055 χ2 | - | - | - |
| WMH | 47 (95.9%) | 108 (89.3%) | 0 | 0.165 χ2 | - | - | - |
| PVS | 32 (65.3%) | 48 (39.7%) | 0 | 0.002 χ2 | - | - | - |
| CMBs | 49 (100.0%) | 0 (0.0%) | 0 | <0.001 χ2 | - | - | - |
| CMBs-lobar | 23 (46.9%) | - | - | - | - | - | - |
| CMBs-deep | 18 (36.7%) | - | - | - | - | - | - |
| CMBs-mixed | 8 (16.3%) | - | - | - | - | - | - |
| MoCA | 24.34 ± 3.05 | 25.23 ± 3.65 | 26.51 ± 3.58 | 0.003 a | 0.001 | 0.143 | 0.015 |
| AVLT | 55.81 ± 14.87 | 60.37 ± 11.31 | 64.51 ± 11.89 | 0.001 a | <0.001 | 0.032 | 0.024 |
| SDMT | 27.43 ± 12.31 | 31.32 ± 11.94 | 40.01 ± 13.37 | <0.001 a | <0.001 | 0.071 | <0.001 |
| SCWT | 169.15 ± 58.97 | 151.10 ± 45.11 | 133.32 ± 30.52 | <0.001 a | <0.001 | 0.019 | 0.008 |
| TMT(B-A) | 152.51 ± 97.74 | 130.64 ± 101.03 | 106.00 ± 80.72 | 0.030 a | 0.009 | 0.182 | 0.083 |
| TIV | 1.60 ± 0.15 | 1.57 ± 0.14 | 1.61 ± 0.16 | 0.143 a | - | - | - |
| Global Property (AUC Value) | CSVD-c | CSVD-n | HC | p Value (ANCONA) | p Value (Post Hoc) | ||
|---|---|---|---|---|---|---|---|
| CSVD-c vs. HC | CSVD-c vs. CSVD-n | CSVD-n vs. HC | |||||
| Eglob | 14.50 ± 1.20 | 15.56 ± 1.21 | 14.03 ± 1.36 | 0.014 | 0.041 | 0.792 | 0.005 |
| Eloc | 20.54 ± 1.67 | 20.58 ± 1.72 | 19.84 ± 1.91 | 0.015 | 0.034 | 0.906 | 0.006 |
| Lp (×e−2) | 7.27 ± 0.62 | 7.23 ± 0.58 | 7.52 ± 0.72 | 0.006 | 0.031 | 0.690 | 0.002 |
| Cp (×e−1) | 6.45 ± 0.08 | 6.45 ± 0.08 | 6.46 ± 0.08 | 0.545 | - | - | - |
| γ | 1.59 ± 0.09 | 1.61 ± 0.10 | 1.61 ± 0.10 | 0.521 | - | - | - |
| λ | 1.11 ± 0.02 | 1.10 ± 0.02 | 1.11 ± 0.02 | 0.836 | - | - | - |
| σ | 1.42 ± 0.08 | 1.43 ± 0.09 | 1.44 ± 0.09 | 0.462 | - | - | - |
| CSVD with CMBs | CSVD without CMBs | HC | |||
|---|---|---|---|---|---|
| Regions | Bnodal | Regions | Bnodal | Regions | Bnodal |
| MFG.L | 77.27 ± 37.42 | MFG.L | 75.24 ± 44.94 | MFG.L | 69.23 ± 37.91 |
| MFG.R | 53.95 ± 34.08 | MFG.R | 53.92 ± 39.83 | MFG.R | 50.63 ± 37.49 |
| MOG.L | 48.07 ± 32.85 | MOG.L | 47.13 ± 34.00 | MOG.L | 39.60 ± 26.70 |
| IPL.L | 51.30 ± 32.29 | IPL.L | 47.46 ± 39.94 | IPL.L | 36.51 ± 25.35 |
| PCUN.L | 66.59 ± 34.33 | PCUN.L | 68.01 ± 33.36 | PCUN.L | 66.99 ± 37.05 |
| PCUN.R | 56.46 ± 38.80 | PCUN.R | 52.27 ± 34.29 | PCUN.R | 53.21 ± 31.44 |
| STG.L | 48.73 ± 31.73 | STG.L | 47.90 ± 28.32 | STG.L | 53.63 ± 29.89 |
| STG.R | 44.87 ± 23.02 | STG.R | 46.12 ± 30.12 | STG.R | 39.84 ± 31.54 |
| ITG.L | 36.99 ± 24.88 | ITG.L | 43.25 ± 28.14 | ITG.L | 50.17 ± 29.77 |
| SPG.L | 42.54 ± 40.94 | SFGdor.R | 38.09 ± 29.21 | SFGdor.R | 37.34 ± 22.37 |
| IFGtriang.L | 37.33 ± 31.29 | DCG.R | 37.56 ± 25.24 | ||
| DCG.R | 35.46 ± 24.02 | MTG.L | 39.52 ± 24.86 |
| Bnodal | p-Value (ANCONA) | p-Value (Post Hoc) | ||||||
|---|---|---|---|---|---|---|---|---|
| Module | Region | CSVD-c | CSVD-n | Control | CSVD-c vs. HC | CSVD-c vs. CSVD-n | CSVD-n vs. HC | |
| DMN | SFGdor.L | 17.99 ± 15.30 | 27.23 ± 25.47 | 28.00 ± 24.18 | 0.039 | 0.021 | 0.020 | N.S. |
| DMN | ACG.L | 21.1 ± 24.39 | 13.72 ± 13.54 | 14.45 ± 17.33 | 0.038 | 0.038 | 0.013 | N.S. |
| DMN | ACG.R | 20.3 ± 18.15 | 13.60 ± 14.12 | 12.36 ± 12.91 | 0.009 | 0.004 | 0.008 | N.S. |
| DMN | MTG.R | 26.24 ± 23.96 | 18.92 ± 18.12 | 17.74 ± 16.29 | 0.035 | 0.015 | 0.023 | N.S. |
| attention | IFGoperc.R | 16.64 ± 15.54 | 16.50 ± 15.18 | 23.62 ± 21.85 | 0.016 | 0.032 | N.S. | 0.006 |
| attention | IPL.L | 51.30 ± 32.63 | 47.46 ± 40.11 | 36.51 ± 25.52 | 0.038 | 0.022 | N.S. | 0.034 |
| attention | ITG.L | 36.99 ± 25.14 | 43.25 ± 28.26 | 50.17 ± 29.97 | 0.038 | 0.012 | N.S. | N.S. |
| sensory/motor | INS.L | 17.52 ± 16.61 | 16.35 ± 17.03 | 11.45 ± 11.18 | 0.048 | 0.033 | N.S. | 0.032 |
| sensory/motor | SPG.L | 42.54 ± 41.36 | 31.24 ± 32.10 | 27.53 ± 30.30 | 0.048 | 0.016 | 0.048 | N.S. |
| subcortical | CAU.L | 6.96 ± 8.75 | 11.18 ± 12.34 | 14.63 ± 20.13 | 0.018 | 0.005 | N.S. | N.S. |
| subcortical | CAU.R | 15.92 ± 17.28 | 15.02 ± 16.06 | 20.57 ± 17.75 | 0.047 | N.S | N.S. | 0.015 |
| vision | FFG.L | 7.40 ± 7.14 | 8.86 ± 10.69 | 14.55 ± 15.13 | 0.001 | 0.001 | N.S. | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, S.; Xin, H.; Feng, M.; Zhang, Q.; Sui, C.; Guo, L.; Liang, C.; Wen, H. Disrupted Gray Matter Networks Associated with Cognitive Dysfunction in Cerebral Small Vessel Disease. Brain Sci. 2023, 13, 1359. https://doi.org/10.3390/brainsci13101359
Gao Y, Wang S, Xin H, Feng M, Zhang Q, Sui C, Guo L, Liang C, Wen H. Disrupted Gray Matter Networks Associated with Cognitive Dysfunction in Cerebral Small Vessel Disease. Brain Sciences. 2023; 13(10):1359. https://doi.org/10.3390/brainsci13101359
Chicago/Turabian StyleGao, Yian, Shengpei Wang, Haotian Xin, Mengmeng Feng, Qihao Zhang, Chaofan Sui, Lingfei Guo, Changhu Liang, and Hongwei Wen. 2023. "Disrupted Gray Matter Networks Associated with Cognitive Dysfunction in Cerebral Small Vessel Disease" Brain Sciences 13, no. 10: 1359. https://doi.org/10.3390/brainsci13101359
APA StyleGao, Y., Wang, S., Xin, H., Feng, M., Zhang, Q., Sui, C., Guo, L., Liang, C., & Wen, H. (2023). Disrupted Gray Matter Networks Associated with Cognitive Dysfunction in Cerebral Small Vessel Disease. Brain Sciences, 13(10), 1359. https://doi.org/10.3390/brainsci13101359






