Body Processing in Children and Adolescents with Traumatic Brain Injury: An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Inclusion Criteria
2.2. Procedure
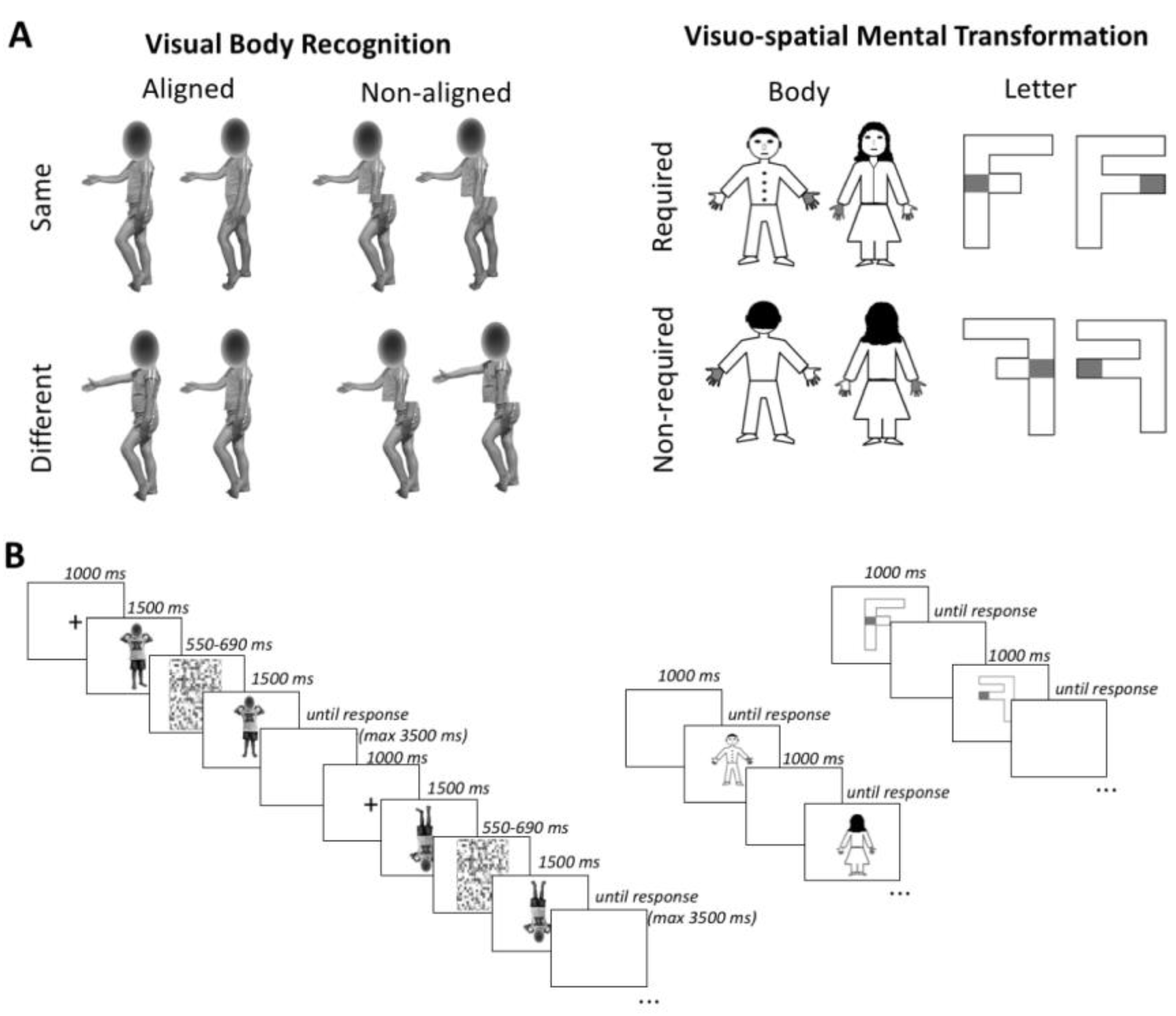
2.2.1. Visual Body Recognition Paradigm (Configural and Holistic Processing)
2.2.2. Visuospatial Imagery Paradigm (Motor and Visual Imagery)
2.3. Data Handling and Statistical Analyses
3. Results
3.1. Demographic and Clinical Variables
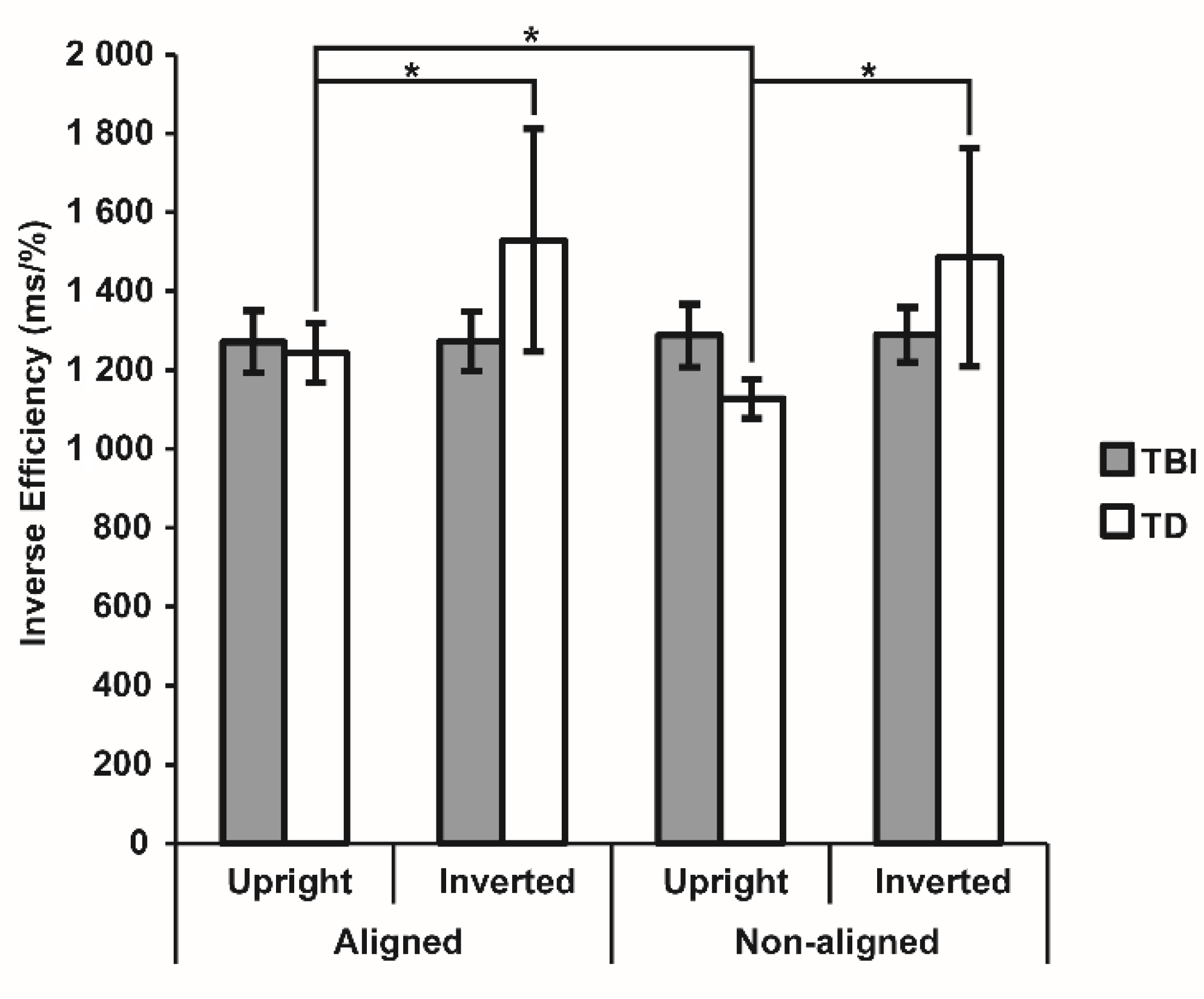
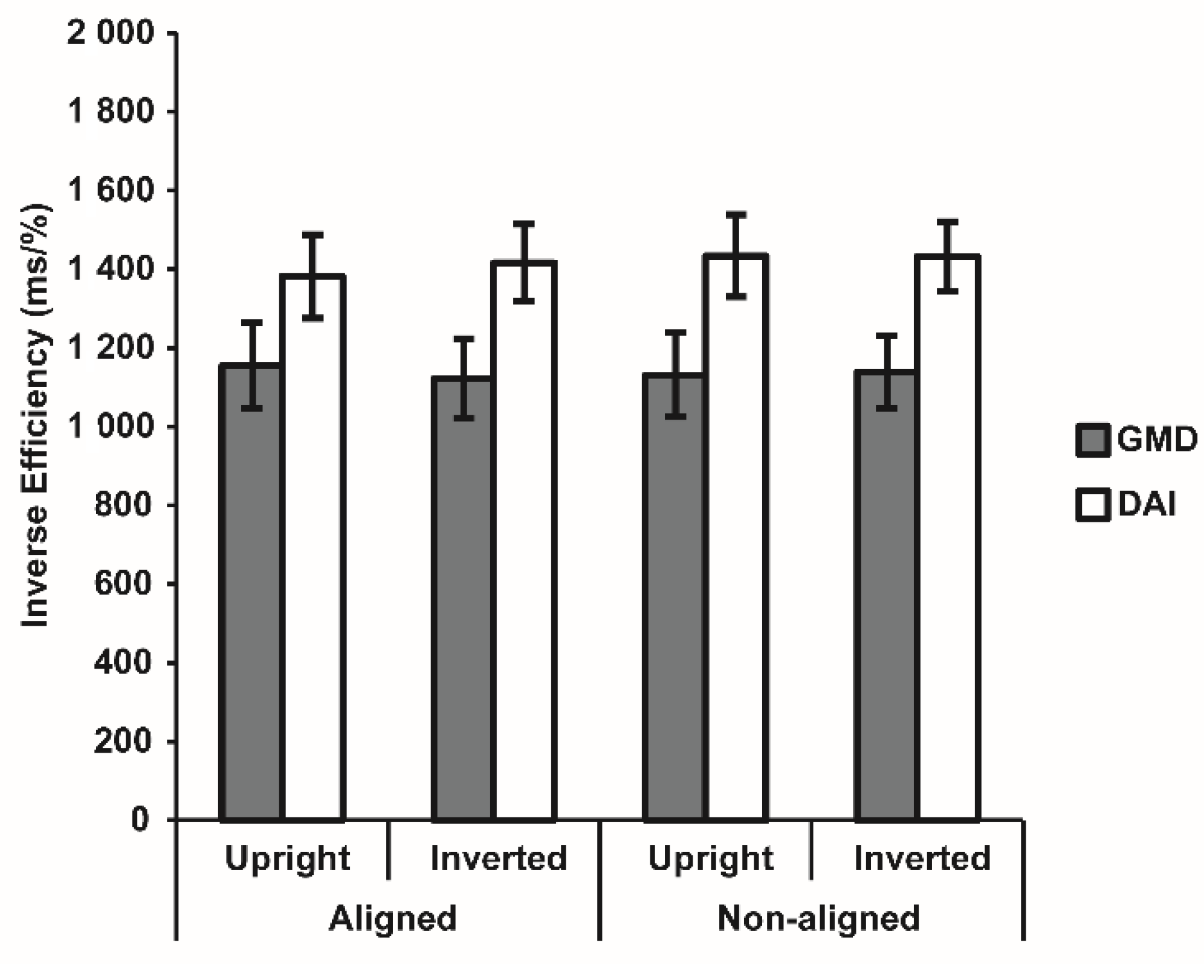
3.2. Visual Body Recognition Task
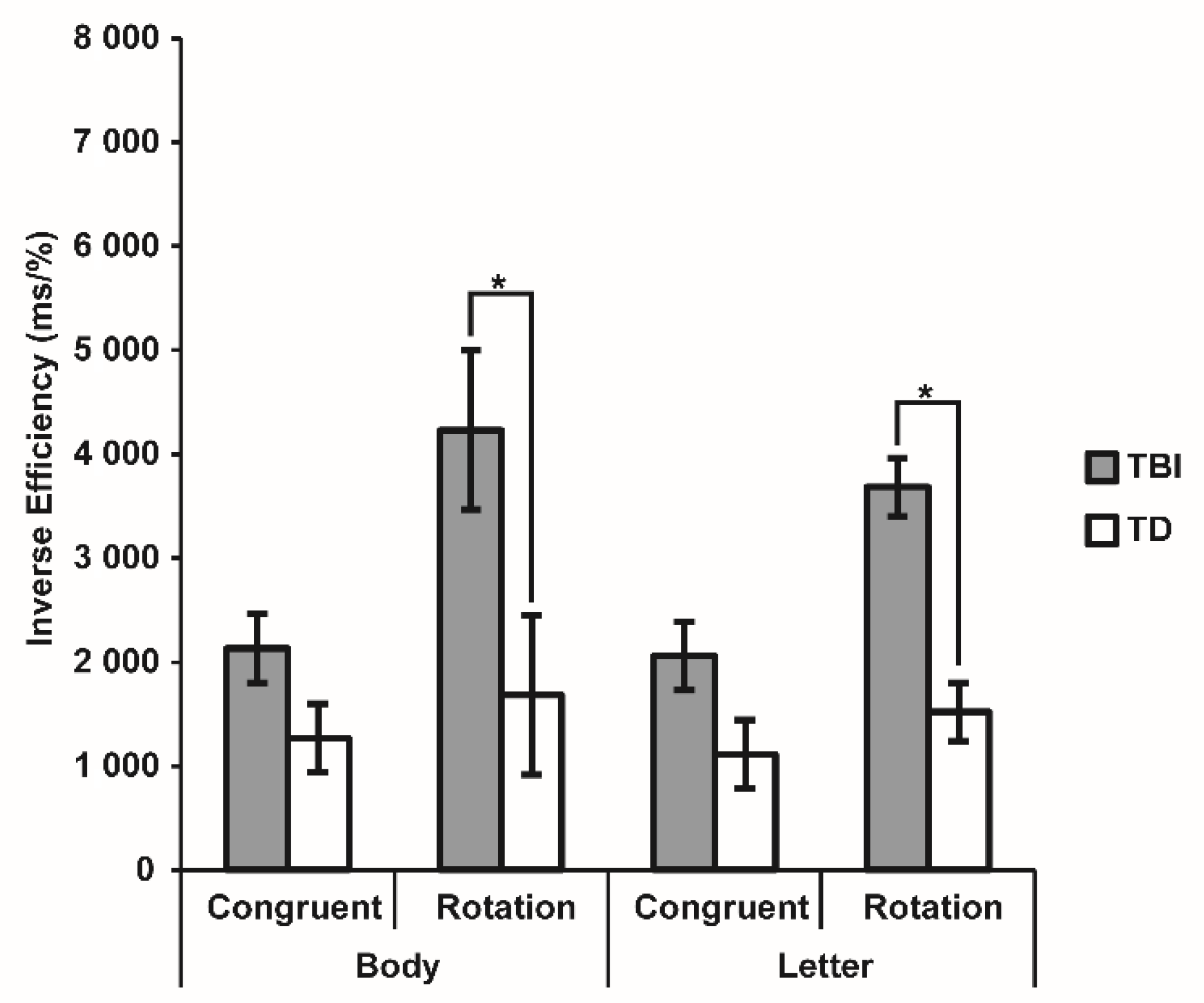
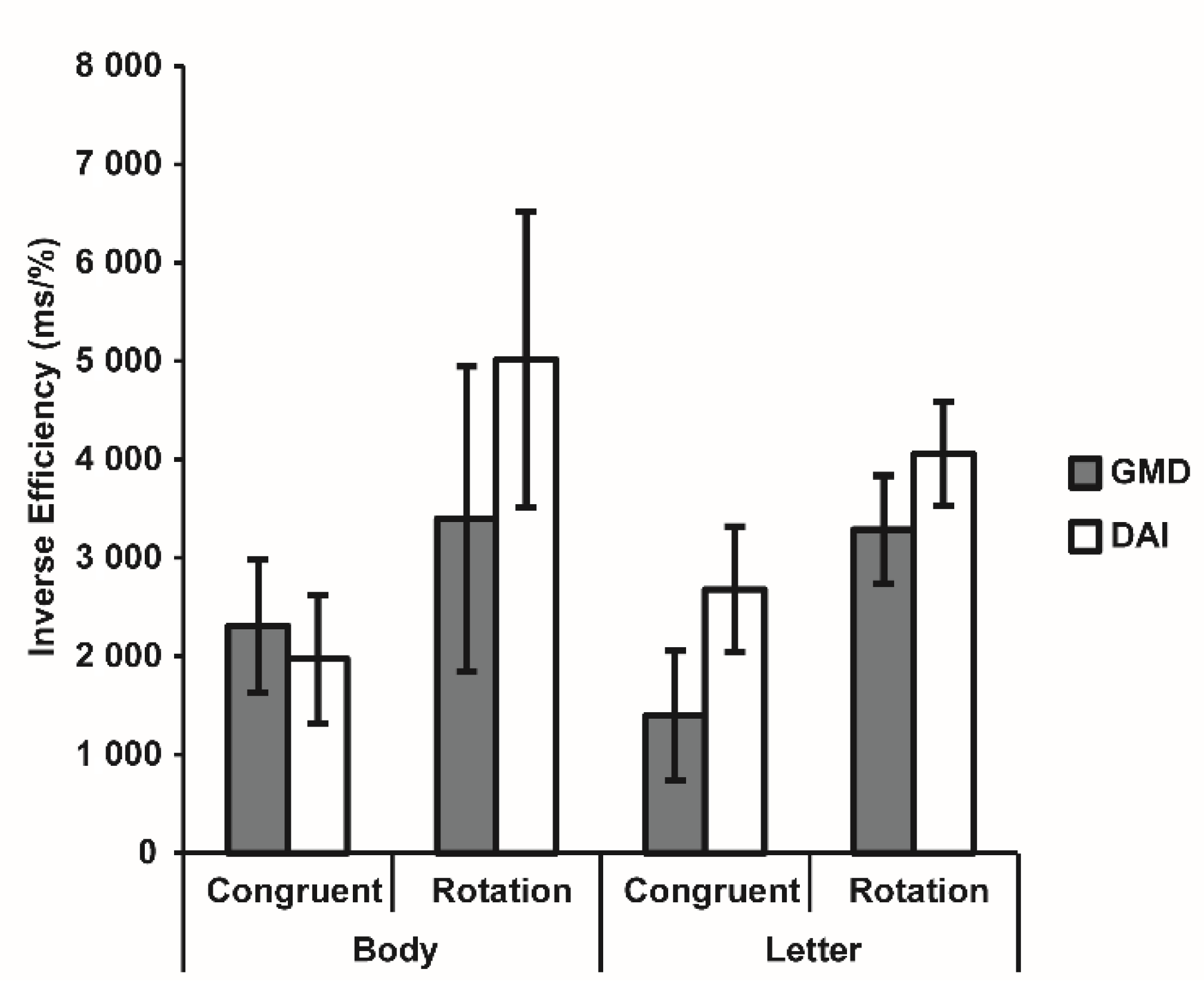
3.3. Visuospatial Imagery Paradigm
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Gelder, B. Why bodies? Twelve reasons for including bodily expressions in affective neuroscience. Philos. T. Roy. Soc. B 2009, 364, 3475–3484. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, B.; de Borst, A.W.; Watson, R. The perception of emotion in body expressions. Wires. Cogn. Sci. 2015, 6, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Papeo, L.; Stein, T.; Soto-Faraco, S. The Two-Body Inversion Effect. Psychol. Sci. 2017, 28, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Cignetti, F.; Vaugoyeau, M.; Nazarian, B.; Roth, M.; Anton, J.L.; Assaiante, C. Boosted activation of right inferior frontoparietal network: A basis for illusory movement awareness. Hum. Brain Mapp. 2014, 35, 5166–5178. [Google Scholar] [CrossRef] [PubMed]
- Naito, E.; Morita, T.; Amemiya, K. Body representations in the human brain revealed by kinesthetic illusions and their essential contributions to motor control and corporeal awareness. Neurosci. Res. 2016, 104, 16–30. [Google Scholar] [CrossRef]
- Nesbitt, A.; Sabiston, C.M.; deJonge, M.; Solomon-Krakus, S.; Welsh, T.N. Barbie’s new look: Exploring cognitive body representation among female children and adolescents. PLoS ONE 2019, 25, e0218315. [Google Scholar] [CrossRef]
- Corradi-Dell’Acqua, C.; Tessari, A. Is the body in the eye of the beholder? Visual processing of bodies in individuals with anomalous anatomical sensory and motor features. Neuropsychologia 2010, 48, 689–702. [Google Scholar] [CrossRef]
- Kaltner, S.; Riecke, B.E.; Jansen, P. Embodied mental rotation: A special link between egocentric transformation and the bodily self. Front. Psychol. 2014, 3, 5–505. [Google Scholar] [CrossRef]
- Medina, J.; Coslett, H.B. From maps to form to space: Touch and the body schema. Neuropsychologia 2010, 48, 645–654. [Google Scholar] [CrossRef]
- Steggemann, Y.; Engbert, K.; Weigelt, M. Selective effects of motor expertise in mental body rotation tasks: Comparing object-based and perspective transformations. Brain Cogn. 2011, 76, 97–105. [Google Scholar] [CrossRef]
- Corradi-Dell’Acqua, C.; Tomasino, B.; Fink, G.R. What is the position of an arm relative to the body? Neural correlates of body schema and body structural description. J. Neurosci. 2009, 29, 4162–4171. [Google Scholar] [CrossRef]
- Fontes, P.L.B.; Moura, R.; Haase, V.G. Body representation in children with hemiplegic cerebral palsy. Child Neuropsychol. 2017, 23, 838–863. [Google Scholar] [CrossRef]
- Raimo, S.; Boccia, M.; Di Vita, A.; Iona, T.; Cropano, M.; Ammendolia, A.; Colar, R.; Angelillo, V.; Maiorino, A.; Guariglia, C.; et al. Body Representation Alterations in Patients with Unilateral Brain Damage. J. Int. Neuropsychl. Soc. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Urgesi, C.; Berlucchi, G.; Aglioti, S.M. Magnetic stimulation of extrastriate body area impairs visual processing of nonfacial body parts. Curr. Biol. 2004, 14, 2130–2134. [Google Scholar] [CrossRef]
- Tanaka, J.W.; Gordon, I. Features, configuration, and holistic face processing. In Oxford Handbook of Face Perception; Calder, A., Rhodes, G., Johnson, M., Haxby, J., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 177–194. [Google Scholar]
- Minnebusch, D.A.; Daum, I. Neuropsychological mechanisms of visual face and body perception. Neurosci. Biobehav. R 2009, 33, 1133–1144. [Google Scholar] [CrossRef]
- Butti, N.; Montirosso, R.; Borgatti, R.; Urgesi, C. Maternal sensitivity is associated with configural processing of infant’s cues in preterm and full-term mothers. Early Hum. Dev. 2018, 125, 35–45. [Google Scholar] [CrossRef]
- Harris, A.; Vyas, D.B.; Reed, C.L. Holistic processing for bodies and body parts: New evidence from stereoscopic depth manipulations. Psychon. B Rev. 2016, 23, 1513–1519. [Google Scholar] [CrossRef]
- Reed, C.L.; Stone, V.E.; Grubb, J.D.; McGoldrick, J.E. Turning configural processing upside down: Part and whole body postures. J. Exp. Psychol. Hum. 2006, 32, 73–87. [Google Scholar] [CrossRef]
- Reed, C.; Vyas, D.; Harris, A. Holistic Processing of Body Postures. J. Vision 2015, 15, 248. [Google Scholar] [CrossRef]
- Murphy, J.; Gray, K.L.H.; Cook, R. The composite face illusion. Psychon. B Rev. 2017, 24, 245–261. [Google Scholar] [CrossRef]
- Downing, P.E.; Peelen, M.V. The role of occipitotemporal body-selective regions in person perception. Cogn. Neurosci. 2011, 2, 186–203. [Google Scholar] [CrossRef]
- Downing, P.E.; Peelen, M.V. Body selectivity in occipitotemporal cortex: Causal evidence. Neuropsychologia 2016, 83, 138–148. [Google Scholar] [CrossRef]
- Hodzic, A.; Kaas, A.; Muckli, L.; Stirn, A.; Singer, W. Distinct cortical networks for the detection and identification of human body. Neuroimage 2009, 45, 1264–1271. [Google Scholar] [CrossRef]
- Peelen, M.V.; Glaser, B.; Vuilleumier, P.; Eliez, S. Differential development of selectivity for faces and bodies in the fusiform gyrus. Dev. Sci. 2009, 12, 16–25. [Google Scholar] [CrossRef]
- Brandman, T.; Yovel, G. Bodies are represented as wholes rather than their sum of parts in the occipital-temporal cortex. Cereb. Cortex. 2016, 26, 530–543. [Google Scholar] [CrossRef]
- Barsalou, L.W. Grounded cognition. Ann. Rev. Psychol. 2008, 59, 617–645. [Google Scholar] [CrossRef]
- Gallese, V.; Sinigaglia, C. What is so special about embodied simulation? Trends Cogn. Sci. 2011, 15, 512–519. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Sinigaglia, C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat. Rev. Neurosci. 2010, 11, 264–274. [Google Scholar] [CrossRef]
- McAvinue, L.P.; Robertson, I.H. Relationship between visual and motor imagery. Percept. Motor. Skills 2007, 104, 823–843. [Google Scholar] [CrossRef]
- Mutsaarts, M.; Steenbergen, B.; Bekkering, H. Impaired motor imagery in right hemiparetic cerebral palsy. Neuropsychologia 2007, 45, 853–859. [Google Scholar] [CrossRef]
- ter Horst, A.C.; Cole, J.; van Lier, R.; Steenbergen, B. The Effect of Chronic Deafferentation on Mental Imagery: A Case Study. PLoS ONE 2012, 7, e42742. [Google Scholar] [CrossRef] [PubMed]
- Conson, M.; Mazzarella, E.; Trojano, L. Self-touch affects motor imagery: A study on posture interference effect. Exp. Brain Res. 2011, 215, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Sirigu, A.; Duhamel, J.R. Motor and visual imagery as two complementary but neurally dissociable mental processes. J. Cogn. Neurosci. 2001, 13, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Crescentini, C.; Fabbro, F.; Urgesi, C. Mental spatial transformations of objects and bodies: Different developmental trajectories in children from 7 to 11 years of age. Dev. Psychol. 2014, 5, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Pelgrims, B.; Andres, M.; Olivier, E. Double dissociation between motor and visual imagery in posterior parietal cortex. Cereb. Cortex. 2009, 19, 2298–2307. [Google Scholar] [CrossRef]
- Zacks, J.; Rypma, B.; Gabrieli, J.D.E.; Tversky, B.; Glover, G.H. Imagined transformations of bodies: An fMRI investigation. Neuropsychologia 1999, 37, 1029–1040. [Google Scholar] [CrossRef]
- Zacks, J.M.; Tversky, B. Multiple systems for spatial imagery: Transformations of objects and bodies. Spat. Cogn. Comput. 2005, 5, 271–306. [Google Scholar] [CrossRef]
- Hétu, S.; Grégoire, M.; Saimpont, A.; Coll, M.P.; Eugène, F.; Michon, P.E.; Jackson, P.L. The neural network of motor imagery: An ALE meta-analysis. Neurosci. Biobehav. R 2013, 37, 930–949. [Google Scholar] [CrossRef]
- Butti, N.; Finisguerra, A.; Urgesi, C. Holistic processing of body stimuli: Evidence of body composite illusion in adults and children. Dev. Psychol. 2022, 58, 1286–1297. [Google Scholar] [CrossRef]
- Heck, A.; Chroust, A.; White, H.; Jubran, R.; Bhatt, R.S. Development of body emotion perception in infancy: From discrimination to recognition. Infant. Behav. Dev. 2018, 50, 42–51. [Google Scholar] [CrossRef]
- Hock, A.; White, H.; Jubran, R.; Bhatt, R.S. The whole picture: Holistic body posture recognition in infancy. Pharm. Biomed. Res. 2016, 23, 426–431. [Google Scholar] [CrossRef]
- Frassinetti, F.; Fiori, S.; D’Angelo, V.; Magnani, B.; Guzzetta, A.; Brizzolara, D.; Cioni, G. Body knowledge in brain-damaged children: A double-dissociation in self and other’s body processing. Neuropsychologia 2012, 50, 181–188. [Google Scholar] [CrossRef]
- Berlucchi, G.; Aglioti, S.M. The body in the brain revisited. Exp. Brain Res. 2010, 200, 25–35. [Google Scholar] [CrossRef]
- Soria Bauser, D.A.; Schriewer, E.; Suchan, B. Dissociation between the behavioural and electrophysiological effects of the face and body composite illusions. Brit. J. Psychol. 2014, 106, 1–19. [Google Scholar] [CrossRef]
- Frassinetti, F.; Maini, M.; Benassi, M.; Avanzi, S.; Cantagallo, A.; Farne, A. Selective impairment of self body-parts processing in right brain-damaged patients. Cortex 2009, 46, 322–328. [Google Scholar] [CrossRef]
- Guariglia, C.; Piccardi, L.; Puglisi, A.M.C.; Traballesi, M. Is autotopoagnosia real? EC says yes. A case study. Neuropsychologia 2002, 40, 1744–1749. [Google Scholar] [CrossRef]
- Moro, V.; Urgesi, C.; Pernigo, S.; Lanteri, P.; Pazzaglia, M.; Aglioti, S.M. The neural basis of body form and body action agnosia. Neuron 2008, 60, 235–246. [Google Scholar] [CrossRef]
- Moro, V.; Pernigo, S.; Avesani, R.; Bulgarelli, C.; Urgesi, C.; Candidi, M.; Aglioti, S.M. Visual body recognition in a prosopagnosic patient. Neuropsychologia 2012, 50, 104–117. [Google Scholar] [CrossRef]
- Aglioti, S.M.; Smania, N.; Manfredi, M.; Berlucchi, G. Disownership of left hand and objects related to it in a patient with right brain damage. Neuroreport 1996, 8, 293–296. [Google Scholar] [CrossRef][Green Version]
- Schwoebel, J.; Coslett, H.B. Evidence for multiple, distinct representations of the human body. J. Cogn. Neurosci. 2005, 17, 543–553. [Google Scholar] [CrossRef]
- Passarotti, A.M.; Paul, B.M.; Bussiere, J.R.; Buxton, R.B.; Wong, E.C.; Stiles, J. The development of face and location processing: An fMRI study. Dev. Sci. 2003, 6, 100–117. [Google Scholar] [CrossRef]
- Martel, M.; Finos, L.; Koun, E.; Farnè, A.; Roy, C.A. The long developmental trajectory of body representation plasticity following tool use. Sci. Rep. 2021, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Butti, N.; Montirosso, R.; Giusti, L.; Piccinini, L.; Borgatti, R.; Urgesi, C. Early brain damage affects body schema and person perception abilities in children and adolescents with spastic diplegia. Neural Plast. 2019, 2019, 1678984. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, M.L.; Baas, C.M.; Sangen, A.F.; Aarts, P.B.; van der Lubbe, R.H.; Meulenbroek, R.G.; Steenbergen, B. Children with unilateral cerebral palsy show diminished implicit motor imagery with the affected hand. Dev. Med. Child Neurol. 2016, 58, 277–284. [Google Scholar] [CrossRef]
- Nuara, A.; Papangelo, P.; Avanzini, P.; Fabbri-Destro, M. Body Representation in Children with Unilateral Cerebral Palsy. Front. Psychol. 2019, 10, 354. [Google Scholar] [CrossRef]
- Steenbergen, P.; Buitenweg, J.R.; Trojan, J.; Veltink, P.H. Reproducibility of somatosensory spatial perceptual maps. Exp. Brain Res. 2013, 224, 417–427. [Google Scholar] [CrossRef]
- Corti, C.; Poggi, G.; Massimino, M.; Bardoni, A.; Borgatti, R.; Urgesi, C. Visual perception and spatial transformation of the body in children and adolescents with brain tumor. Neuropsychologia 2018, 120, 124–136. [Google Scholar] [CrossRef]
- Rodgers, S.P.; Trevino, M.; Zawaski, J.A.; Gaber, M.W.; Leasure, J.L. Neurogenesis, exercise, and cognitive late effects of pediatric radiotherapy. Neural Plast. 2013, 2013, 698528. [Google Scholar] [CrossRef]
- Butti, N.; Montirosso, R.; Giusti, L.; Borgatti, R.; Urgesi, C. Premature birth affects visual body representation and body schema in preterm children. Brain Cogn. 2020, 145, 105612. [Google Scholar] [CrossRef]
- Maurer, D.; Le Grand, R.; Mondloch, C.J. The many faces of configural processing. Trends Cogn. Sci. 2002, 6, 255–260. [Google Scholar] [CrossRef]
- Soria Bauser, D.A.; Suchan, B.; Daum, I. Differences between perception of human faces and body shapes: Evidence from the composite illusion. Vis. Res. 2011, 51, 195–202. [Google Scholar] [CrossRef]
- Gomez, J.; Pestilli, F.; Witthoft, N.; Golarai, G.; Liberman, A.; Poltoratski, S.; Joon, J.; Grill-Spector, K. Functionally defined white matter reveals segregated pathways in human ventral temporal cortex associated with category-specific processing. Neuron 2015, 85, 216–227. [Google Scholar] [CrossRef]
- Pyles, J.A.; Verstynen, T.D.; Schneider, W.; Tarr, M.J. Explicating the Face Perception Network with White Matter Connectivity. PLoS ONE 2013, 8, e61611. [Google Scholar] [CrossRef]
- Thomas, C.; Avidan, G.; Humphreys, K.; Jung, K.J.; Gao, F.; Behrmann, M. Reduced structural connectivity in ventral visual cortex in congential prosopagnosia. Nat. Neurosci. 2009, 12, 29–31. [Google Scholar] [CrossRef]
- Sokolov, A.A.; Miall, R.C.; Ivry, R.B. The Cerebellum: Adaptive Prediction for Movement and Cognition. Trends Cogn. Sci. 2017, 21, 313–332. [Google Scholar] [CrossRef]
- Tanaka, H.; Ishikawa, T.; Lee, J.; Kakei, S. The Cerebro-Cerebellum as a Locus of Forward Model: A Review. Front. System Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef]
- Tomasino, B.; Guarracino, I.; Ius, T.; Budai, R.; Skrap, M. Real-Time Neuropsychological Testing of Sensorimotor Cognition During Awake Surgery in Precentral and Postsomatosensory Areas. World Neurosurg. 2022; in press. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papagiannis, G.I.; Triantafyllou, A.I.; Koulouvaris, P.; Anastasiou, A.I.; Kontoangelos, K.; Anastasiou, I.P. Neurological and neurourological complications of electrical injuries. Neurol. Neurochir. Pol. 2021, 55, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kush, J.C.; Canivez, G.L. The higher order structure of the WISC–IV Italian adaptation using hierarchical exploratory factor analytic procedures. Int. J. Educ. Psychol. 2019, 7, 15–28. [Google Scholar] [CrossRef]
- Orsini, A.; Pezzuti, L.; Picone, L. WISC-IV. In Contributo Alla Taratura Italiana; Giunti OS: Firenze, Italy, 2012. [Google Scholar]
- Pezzuti, L.; Lang, M.; Rossetti, S.; Michelotti, C. CHC model according to Weiss: Evidence from the WAIS-IV administration to Italian adults and elders. J. Individ. Differ. 2018, 39, 53–59. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Pezzuti, L.; Michelotti, C.; Lauriola, M.; Lang, M. Advanced interpretation of WAIS-IV. The application of the CHC model to a WAIS-IV protocol. BPA Appl. Psychol. Bull. (Boll. Psicol. Appl.) 2020, 68, 289. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Li, X.Y.; Feng, D.F. Diffuse axonal injury: Novel insights into detection and treatment. J. Clin. Neurosci. 2009, 16, 614–619. [Google Scholar] [CrossRef]
- Robbins, A.; Coltheart, M. Left-right holistic integration of human bodies. Q. J. Exp. Psychol. 2012, 65, 1962–1974. [Google Scholar] [CrossRef]
- Urgesi, C.; Fornasari, L.; Canalaz, F.; Perini, L.; Cremaschi, S.; Faleschini, L.; Zappoli Thyrion, E.; Zuliani, M.; Balestrieri, M.; Fabbro, F.; et al. Impaired configural body processing in anorexia nervosa: Evidence from the body inversion effect. Brit. J. Psychol. 2014, 105, 486–508. [Google Scholar] [CrossRef]
- Rossion, B. The composite face illusion: A whole window into our understanding of holistic face perception. Vis. Cogn. 2013, 21, 139–253. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A fexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioural Science, 2nd ed.; Lawrence Erlbaum Associates: New York, NY, USA, 1988. [Google Scholar]
- Aviezer, H.; Trope, Y.; Todorov, A. Holistic person processing: Faces with bodies tell the whole story. J. Pers. Soc. Psychol. 2012, 103, 20–37. [Google Scholar] [CrossRef]
- Palermo, R.; Willis, M.L.; Rivolta, D.; McKone, E.; Wilson, C.E.; Calder, A.J. Impaired holistic coding of facial expression and facial identity in congenital prosopagnosia. Neuropsychologia 2011, 49, 1226–1235. [Google Scholar] [CrossRef]
- Soria Bauser, D.A.; Thoma, P.; Aizenberg, V.; Brüne, M.; Juckel, G.; Daum, I. Face and body perception in schizophrenia: A configural processing deficit? Psychiat. Res. 2012, 195, 9–17. [Google Scholar] [CrossRef]
- Corallo, F.; Tarda, D.; Coppola, V.; Bonanno, L.; Lo Buono, V.; Palmeri, R.; De Cola, M.C.; Di Cara, M.; Romeo, L.; Raciti, L.; et al. The relationship between body image and emotional and cognitive impairment after brain damage: A preliminary study. Brain Behav. 2021, 11, e02181. [Google Scholar] [CrossRef]
- van den Stock, J.; de Jong, S.J.; Hodiamont, P.P.G.; de Gelder, B. Perceiving emotions from bodily expressions and multisensory integration of emotion cues in schizophrenia. Soc. Neurosci. 2011, 6, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Reed, C.L.; Beall, P.M.; Stone, V.E.; Kopelioff, L.; Pulham, D.J.; Hepburn, S.L. Brief report: Perception of body posture-what individuals with autism spectrum disorder might be missing. J. Autism. Dev. Disord. 2007, 37, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Ropar, D.; Greenfield, K.; Smith, A.D.; Carey, M.; Newport, R. Body representation difficulties in children and adolescents with autism may be due to delayed development of visuo-tactile temporal binding. Cogn. Neurosci. 2018, 29, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Suchan, B.; Bauser, D.S.; Busch, M.; Schulte, D.; Grönemeyer, D.; Herpertz, S.; Vocks, S. Reduced connectivity between the left fusiform body area and the extrastriate body area in anorexia nervosa is associated with body image distortion. Behav. Brain Res. 2013, 241, 80–85. [Google Scholar] [CrossRef]
- Suchan, B.; Vocks, S.; Waldorf, M. Alterations in activity, volume, and connectivity of body-processing brain areas in anorexia nervosa: A review. Eur. Psychol. 2015, 20, 27–33. [Google Scholar] [CrossRef]
- Pavlova, M.; Sokolov, A.N.; Birbaumer, N.; KrägelohMann, I. Perception and understanding of others’ actions and brain connectivity. J. Cogn. Neurosci. 2008, 20, 494–504. [Google Scholar] [CrossRef]
- Biotti, F.; Gray, K.L.H.; Cook, R. Impaired body perception in developmental prosopagnosia. Cortex 2017, 93, 41–49. [Google Scholar] [CrossRef]
- Caeyenberghs, K.; van Roon, D.; Swinnen, S.P.; Smits-Engelsman, B.C. Deficits in executed and imagined aiming performance in brain-injured children. Brain Cogn. 2009, 69, 154–161. [Google Scholar] [CrossRef]
- Vocks, S.; Busch, M.; Grönemeyer, D.; Schulte, D.; Herpertz, S.; Suchan, B. Neural correlates of viewing photographs of one’s own and another female’s body in anorexia and bulimia nervosa: An fMRI study. J. Psychiatr. Neurosci. 2010, 35, 163–176. [Google Scholar] [CrossRef]
- Vocks, S.; Schulte, D.; Busch, M.; Grönemeyer, D.; Herpertz, S.; Suchan, B. Changes in neuronal correlates of body image processing by means of cognitive-behavioral body image therapy for eating disorders: A randomized-controlled fMRI study. Psychol. Med. 2011, 41, 1651–1664. [Google Scholar] [CrossRef]
- Di Nuovo, S.; De La Cruz, V.; Conti, D.; Buono, S.; Di Nuovo, A. Mental Imagery: Rehabilitation through simulation. Life Span. Disabil. 2014, 17, 89–118. [Google Scholar]
- Mulder, T. Motor imagery and action observation: Cognitive tools for rehabilitation. J. Neural Transm. 2007, 114, 1265–1278. [Google Scholar] [CrossRef]
- Fusco, A.; Giovannini, S.; Castelli, L.; Coraci, D.; Gatto, D.M.; Reale, G.; Pastorino, R.; Padua, L. Virtual Reality and Lower Limb Rehabilitation: Effects on Motor and Cognitive Outcome-A Crossover Pilot Study. J. Clin. Med. 2022, 11, 2300. [Google Scholar] [CrossRef]
- Georgiev, D.; Georgieva, I.; Gong, Z.; Nanjappan, V.; Georgiev, G. Virtual Reality for Neurorehabilitation and Cognitive Enhancement. Brain Sci. 2021, 11, 221. [Google Scholar] [CrossRef]
- Morganti, F.; Gaggioli, A.; Castelnuovo, G.; Bulla, D.; Vettorello, M.; Riva, G. The use of technology-supported mental imagery in neurological rehabilitation: A research protocol. Cyberpsychol. Behav. 2003, 6, 421–427. [Google Scholar] [CrossRef]
- Shen, J.; Xiang, H.; Luna, J.; Grishchenko, A.; Patterson, J.; Strouse, R.V.; Roland, M.; Lundine, J.P.; Koterba, C.H.; Lever, K.; et al. Virtual Reality-Based Executive Function Rehabilitation System for Children with Traumatic Brain Injury: Design and Usability Study. JMIR Serious Games 2020, 8, e16947. [Google Scholar] [CrossRef]
- Garcia-Hernandez, N.; Guzman-Alvarado, M.; Parra-Vega, V. Virtual body representation for rehabilitation influences on motor performance of cerebral palsy children. Virtual Real. 2021, 25, 669–680. [Google Scholar] [CrossRef]
- Richler, J.J.; Palmeri, T.J.; Gauthier, I. Meanings, mechanisms, and measures of holistic processing. Front. Psychol. 2012, 3, 553. [Google Scholar] [CrossRef]
- Richler, J.J.; Gauthier, I. When intuition fails to align with data: A reply to Rossion. Vis. Cogn. 2013, 21, 254–276. [Google Scholar] [CrossRef]
- Richler, J.J.; Gauthier, I.A. Meta-analysis and review of holistic face processing. Psychol. Bull. 2014, 140, 1281–1302. [Google Scholar] [CrossRef]
- Mondloch, C.J.; Pathman, T.; Maurer, D.; Le Grand, R.; de Schonen, S. The composite face effect in six-year-old children: Evidence of adult-like holistic face processing. Vis. Cogn. 2007, 15, 564–577. [Google Scholar] [CrossRef]
- Vander Heyden, K.M.; Huizinga, M.; Raijmakers, M.E.J.; Jolles, J. Children’s representations of another person’s spatial perspective: Different strategies for different viewpoints? J. Exp. Child Psychol. 2017, 153, 57–73. [Google Scholar] [CrossRef]
- Vander Heyden, K.M.; Huizinga, M.; Kan, K.J.; Jolles, J. A developmental perspective on spatial reasoning: Dissociating object transformation from viewer transformation ability. Cogn. Dev. 2016, 38, 63–74. [Google Scholar] [CrossRef]
| GMD (N = 16) | DAI (N = 17) | t/Chi2 | p Value | |||
|---|---|---|---|---|---|---|
| Mean (SD) | N (%) | Mean (SD) | N (%) | |||
| Demographic variables | ||||||
| Sex (males) | 9 (56%) | 12 (71%) | 0.73 | 0.392 | ||
| Age at evaluation (years) | 13.38 (2.58) | 13.59 (2.18) | 0.26 | 0.799 | ||
| Clinical variables | ||||||
| Time since TBI (years) | 5.93 (4.66) | 3.19 (3.89) | 1.84 | 0.076 | ||
| GCS | 7.69 (3.02) | 6.88 (3.67) | 0.68 | 0.499 | ||
| Motor impairments | 9 (56%) | 11 (65%) | 0.25 | 0.619 | ||
| Visual impairments | 10 (63%) | 6 (35%) | 2.44 | 0.118 | ||
| Cognitive functioning | ||||||
| FSIQ | 98.25 (16.21) | 86.17 (21.47) | 1.81 | 0.079 | ||
| VCI | 101.38 (165.60) | 94.82 (16.52) | 1.17 | 0.251 | ||
| PRI | 103.50 (18.68) | 93.00 (20.64) | 1.53 | 0.136 | ||
| Accuracy (%) | RT (ms) | ||||
|---|---|---|---|---|---|
| Alignment | Orientation | TBI Group | TD Group | TBI Group | TD Group |
| Aligned | Upright | 86.73 ± 1.63 | 87.85 ± 1.36 | 1076.59 ± 54.40 | 1083.12 ± 62.44 |
| Inverted | 87.53 ± 1.62 | 85.79 ± 2.77 | 1093.73 ± 54.35 | 1114.89 ± 73.58 | |
| Non-aligned | Upright | 86.30 ± 1.80 | 90.48 ± 1.19 | 1082.82 ± 55.48 | 1022.27 ± 50.47 |
| Inverted | 86.45 ± 1.59 | 86.15 ± 2.64 | 1095.07 ± 50.54 | 1086.08 ± 67.86 | |
| Accuracy (%) | RT (ms) | ||||
|---|---|---|---|---|---|
| Stimulus | Transformation | TBI Group | TD Group | TBI Group | TD Group |
| Body | Congruent | 84.39 ± 4.21 | 94.84 ± 0.80 | 1256.20 ± 89.58 | 1195.26 ± 76.83 |
| Rotation | 68.71 ± 6.07 | 91.26 ± 1.29 | 1459.24 ± 1117.63 | 1490.25 ± 108.96 | |
| Letter | Congruent | 84.94 ± 3.56 | 96.00 ± 0.83 | 1380.92 ± 108.96 | 1066.58 ± 48.86 |
| Rotation | 47.87 ± 1.62 | 90.52 ± 2.18 | 1644.77 ± 148.40 | 1328.25 ± 73.66 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corti, C.; Butti, N.; Bardoni, A.; Strazzer, S.; Urgesi, C. Body Processing in Children and Adolescents with Traumatic Brain Injury: An Exploratory Study. Brain Sci. 2022, 12, 962. https://doi.org/10.3390/brainsci12080962
Corti C, Butti N, Bardoni A, Strazzer S, Urgesi C. Body Processing in Children and Adolescents with Traumatic Brain Injury: An Exploratory Study. Brain Sciences. 2022; 12(8):962. https://doi.org/10.3390/brainsci12080962
Chicago/Turabian StyleCorti, Claudia, Niccolò Butti, Alessandra Bardoni, Sandra Strazzer, and Cosimo Urgesi. 2022. "Body Processing in Children and Adolescents with Traumatic Brain Injury: An Exploratory Study" Brain Sciences 12, no. 8: 962. https://doi.org/10.3390/brainsci12080962
APA StyleCorti, C., Butti, N., Bardoni, A., Strazzer, S., & Urgesi, C. (2022). Body Processing in Children and Adolescents with Traumatic Brain Injury: An Exploratory Study. Brain Sciences, 12(8), 962. https://doi.org/10.3390/brainsci12080962







