Abstract
Current findings on brain structural alterations in complex regional pain syndrome (CRPS) are heterogenous and controversial. This study aimed to perform a systematic review and meta-analysis to explore the significant gray matter volume (GMV) abnormalities between patients with CRPS and healthy controls (HCs). A systematic search of the PubMed, Web of Science, and MEDLINE databases was performed, updated through 27 January 2022. A total of five studies (93 CRPS patients and 106 HCs) were included. Peak coordinates and effect sizes were extracted and meta-analyzed by anisotropic effect size–signed differential mapping (AES-SDM). Heterogeneity, sensitivity, and publication bias of the main results were checked by the Q test, jackknife analysis, and the Egger test, respectively. Meta-regression analysis was performed to explore the potential impact of risk factors on GMV alterations in patients with CRPS. The main analysis exhibited that patients with CRPS had increased GMV in the left medial superior frontal gyrus (SFGmedial.L), left striatum, and an undefined area (2, 0, −8) that may be in hypothalamus, as well as decreased GMV in the corpus callosum (CC) (extending to right supplementary motor area (SMA.R), right median cingulate/paracingulate gyri (MCC.R)), and an undefined area (extending to the right caudate nucleus (CAU.R), and right thalamus (THA.R)). Meta-regression analysis showed a negative relationship between increased GMV in the SFGmedial.L and disease duration, and the percentage of female patients with CRPS. Brain structure abnormalities in the sensorimotor regions (e.g., SFGmedial.L, SMA.R, CAU.R, MCC.R, and THA.R) may be susceptible in patients with CRPS. Additionally, sex differences and disease duration may have a negative effect on the increased GMV in SFGmedial.L.
1. Introduction
Complex regional pain syndrome (CRPS), commonly caused by limb trauma, is a chronic pain that is characterized by spontaneous and evoked regional pain in the extremities [1,2]. The prevalence of CRPS is about 5.4~26.2 per 100,000 person-years with a female predominance [3,4]. One of the significant impairments in CRPS is pain-induced neuroplasticity in the central nervous system (CNS). For instance, it was reported that cognitive function impairments and abnormal body representation were showed in CRPS patients without brain injury [5]. Resting-state functional magnetic resonance imaging (Rs-fMRI) studies have shown that CRPS involves significant default-mode network (DMN) alterations by comparing CRPS and non-CRPS patients [6,7]. Although multiple factors are involved in the process of CRPS, insufficient understanding of the underlying mechanism leads to poor clinical intervention and management [8]. Thus, further study of CNS changes is of great significance for targeted treatment and improving intervention efficiency.
Neuroimaging studies have focused on the brain structure alterations involved in CRPS. Voxel-based morphometry (VBM) is one of the most important methods for measuring gray matter volume (GMV) abnormalities [9,10]. According to the accumulating VBM evidence, patients with CRPS show GMV abnormalities in specific brain regions (e.g., cingulate cortex and amygdala) that play a key role in somatosensory and emotional functions. [7]. In addition, a neuroimaging study reported that GM atrophy might be limited in the emotion-related brain regions including the insula, ventromedial prefrontal cortex (VMPFC), and nucleus accumbens (NAc) [11]. In the early stage of CRPS, patients showed decreased GMV in cerebral areas that are involved in spatial body perception, and somatosensory and limbic systems [12]. Although brain structure abnormalities have been found to be closely related to the dysfunctions within CRPS, the findings on GMV alterations in specific brain regions can be heterogenous and controversial. For instance, it was reported that GMV in the dorsomedial prefrontal cortex of patients with CRPS was higher than that of HCs [1], whereas Barad et al. found that patients with CRPS had higher GMV in the right hypothalamus, left dorsal putamen, and left inferior temporal lobe, as well as lower GMV in the left orbitofrontal cortex, left middle cingulate cortex, right middle cingulate cortex, left posterior middle cingulate cortex, left dorsal insula, and left anterior middle cingulate cortex at the same time [13]. Due to the limited research samples, heterogeneous and unreliable findings may exist in CRPS studies [14]. Therefore, it is of great necessity to perform a systematic review and meta-analysis exploring the reliable GMV alterations in CRPS.
In this study, we aimed to conduct a voxel-wise meta-analysis including only VBM studies to reduce potential bias and explore reliable GMV abnormalities between CRPS patients and HCs, providing a valuable reference for future research and clinical management.
2. Materials and Methods
2.1. Search Strategy and Study Selection
This systematic review and meta-analysis followed the PRISMA checklist (Supplementary Table S1) [15], and the protocol was registered in PROSPERO (registration number CRD42022307238) (https://www.crd.york.ac.uk/prospero/). A systematic search was performed using the PubMed, Web of Science, and MEDLINE databases, updated through 27 January 2022, with the items as follows: (“complex regional pain syndromes” OR “CRPS” OR “complex regional pain syndrome, type i” OR “complex regional pain syndrome type ii”) AND (“gray matter” OR “gray matter volume” OR “VBM” OR “voxel-based morphometry” OR “GMV” OR “GM”). The detailed search strategy is shown in Supplementary Table S2. Additionally, references from review articles were searched manually.
Studies meeting all the following criteria were included: (1) the exploration of GMV alterations between CRPS patients and HCs; (2) adult participants; (3) available coordinate information (x, y, z) and effect size (t value or z score) reported in the standard coordinate space, including Montreal Neurological Institute (MNI), or Talairach (TAL) coordinates; (4) VBM method used to measure the GMV alteration. Studies meeting one of the following criteria were excluded: (1) animal study; (2) VBM method not used; (3) not CRPS; (4) not original study; (5) children participants; (6) not related to GMV.
2.2. Quality Assessment and Data Extraction
A quality assessment of the studies included was performed using a 12-point checklist (Supplementary Table S3), which has commonly been used in neuroimaging meta-analysis studies [16,17]. Both demographic and technical information were collected and presented in this study. Available coordinate information (x, y, z) and effect size (t value or z score) of the GMV alteration between CRPS patients and HCs were extracted for meta-analysis, and the transformation between z score and t value was performed using the SDM website (https://www.sdmproject.com/utilities/?show=Statistics). All work was independently accomplished by two reviewers (TM and ZYL), and any controversial assessments were solved by the third reviewer (LFY).
2.3. Voxel-Wise Meta-Analysis
Significant GMV abnormality between CRPS patients and HCs was analyzed by using AES-SDM version 5.15 (www.sdmproject.com), which has been widely used in brain structure meta-analysis [18,19]. This process was reported in previous studies [20,21], and is briefly described as follows: The significant coordinates and effect sizes of GMV abnormality between CRPS patients and HCs for each dataset were extracted and recreated as a new statistical map in the MNI space by using an anisotropic Gaussian kernel. Then, the voxel-wise calculation of the random-effects mean of the dataset maps, weighted by the sample size, intra-dataset variability, and between-dataset heterogeneity, was used to generate a mean map. The SDM default thresholds (FWHM = 20 mm, p = 0.005, peak height Z = 1, and cluster extent = 10 voxels) were used in this study. The 50 randomization test was used to generate stable results [22]. No subgroup analysis was performed because of limited datasets.
2.4. Heterogeneity, Sensitivity, and Publication Bias Assessment
The default Q maps of SDM were used to check the specific brain regions that present between-study heterogeneity when compared with a global set of voxels. The p threshold, peak height Z, and cluster extent in the random-effects model were set to 0.005, 1, and 10 voxels, respectively [21]. The robustness of the meta-analysis results was assessed by a whole-brain, voxel-based jackknife sensitivity analysis, which was performed by repeating the same steps as the main analysis after removing each study, one by one. Additionally, the total repeated times of the main results in all or most analysis results were regarded as reliable. Potential publication bias was checked by the Egger test with an SDM default. A p-value less than 0.05 was regarded as having publication bias.
2.5. Meta-Regression Analysis
The linear model in SDM was selected to perform meta-regression. Conservative thresholds (probability = 0.0005; peak height threshold = 1.000; extent threshold = 10) were used to explore the relationship between risk factors (age, sexual difference, pain score (VAS), and disease duration) and GMV alterations in CRPS.
3. Results
3.1. Characteristics of Studies Included
A total of 411 studies were found from three databases; 129 results were removed because of duplication, 270 of the remaining 282 studies were removed after screening the title and abstract, and 7 of the remaining 12 studies were removed after browsing the full text, meaning 5 studies were included in this meta-analysis [1,11,12,13,14]. A detailed search flow diagram is shown in Figure 1. No study was found from searching the references. In the end, a total of 199 participants (93 CRPS patients and 106 HCs, 50 male and 149 female) aged between 40.5 and 54.42 years old were included in this study. Demographic information, including author, publication year, sample size, age, disease duration, tools for pain severity assessment, and quality assessment of the study is shown in Table 1. Technical information, including the main GMV abnormality findings in CRPS patients, magnetic field, neuroimaging method, standard space, voxel size, and p-value, is shown in Table 2.
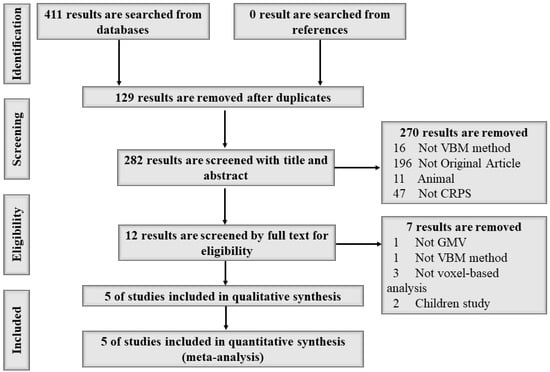
Figure 1.
The search flow diagram following PRISMA guidelines.

Table 1.
Demographic information of five studies included.

Table 2.
Technical information of five studies included.
3.2. Main Analysis of GMV Abnormality between CRPS Patients and HCs
Compared with HCs, patients with CRPS showed significantly and consistently increased GMV in the medial left superior frontal gyrus (SFGmedial) (MNI: 2, 52, 20; SDM-Z = 1.005; p < 0.001; voxels = 324; jackknife analysis: 3/5), left striatum (MNI: −22, −4, −6; SDM-Z = 1.029; p < 0.001; voxels = 79; jackknife analysis: 4/5), and an undefined area (MNI: 2, 0, −8; SDM-Z = 1.034; p < 0.001; voxels = 56; jackknife analysis: 3/5) (Table 3; Figure 2), whereas decreased GMV in the CC (MNI: −2, −18, 26; SDM-Z = −1.769; p < 0.001; voxels = 1601; jackknife analysis: 4/5) and an undefined area (MNI: −8, −18, 20; SDM-Z = −1.693; p < 0.001; voxels = 312; jackknife analysis: 3/5) (Table 3; Figure 2) was observed. Detailed jackknife analysis information is shown in Table 4.

Table 3.
Significant and consistent GMV abnormality in the main analysis.
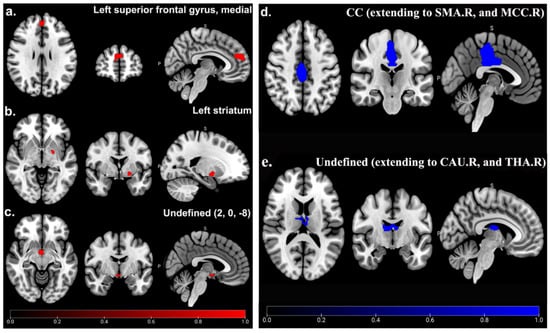
Figure 2.
GMV abnormality between CRPS patients and HCs in main analysis. Significant increased (red) GMV of SFGmedial.L (a), left striatum (b), and undefined area (2, 0, −8) (c), as well as decreased (blue) GMV of CC (extending to SMA.R and MCC.R) (d), and undefined area (extending to CAU.R and THA.R) (e) in CRPS patients compared to HCs.

Table 4.
Jackknife analysis of main analysis results.
3.3. Heterogeneity and Publication Bias Assessment
No heterogeneity was found between specific brain regions and the global set of voxels (Q positive = 0.299, p > 0.05; Q negative = 0.951, p > 0.05) (Supplementary Table S4, Figure 3). The Egger test showed no publication bias in the SFGmedial (bias: −0.30, t: −0.13, df: 3, p > 0.05), left striatum (bias: 1.50, t: 1.17, df: 3, p > 0.05), and CC (bias: −0.39, t: −1.45, df: 3, p > 0.05), as well as in two undefined areas ((2, 0, −8) (bias: 7.41, t: 1.93, df: 3, p > 0.05), (−8, −18, 20) (bias: 1.36, t: 0.32, df: 3, p > 0.05)). Funnel plots are shown in Figure 4.
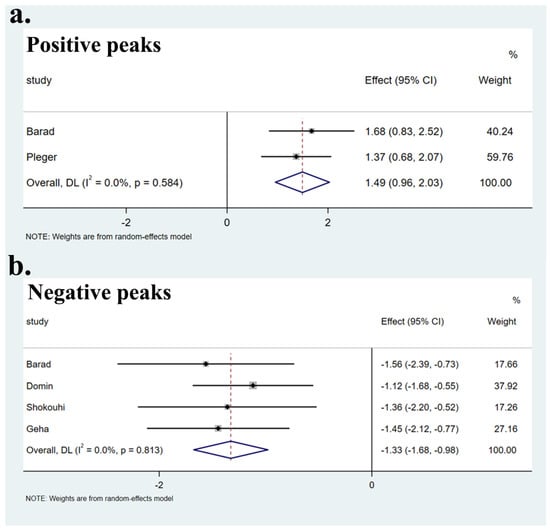
Figure 3.
Forest plots of heterogeneity of main results. Heterogeneity assessment of the main results showed no heterogeneity in both positive peaks (a) and negative peaks (b) (p > 0.05).
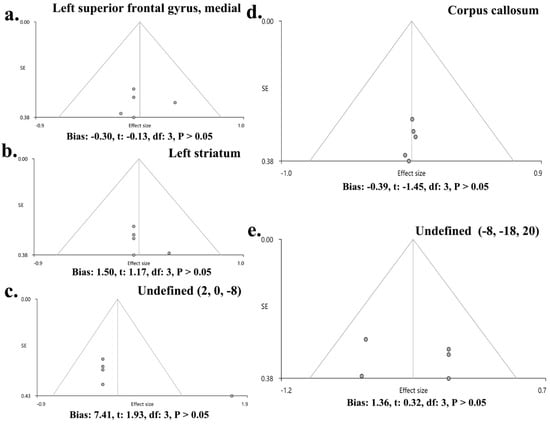
Figure 4.
Funnel plots of publication bias in the main results. The main results showed no publication bias in SFGmedial (bias: −0.30, t: −0.13, df: 3, p > 0.05) (a), left striatum (bias: 1.50, t: 1.17, df: 3, p > 0.05) (b), undefined area (2, 0, −8) (bias: 7.41, t: 1.93, df: 3, p > 0.05) (c), CC (bias: −0.39, t: −1.45, df: 3, p > 0.05) (d), and undefined area (−8, −18, 20) (bias: 1.36, t: 0.32, df: 3, p > 0.05) (e).
3.4. Meta-Regression Analysis
By performing a meta-regression analysis, the disease duration (month) (r = 0.5089, p < 0.0005) and the percentage of female patients (r = 0.9602, p < 0.0005) showed a negative relationship with the increased GMV of the SFGmedial.L in CRPS patients (Figure 5), whereas the age and pain score (VAS) showed no significant relationship with CRPS.
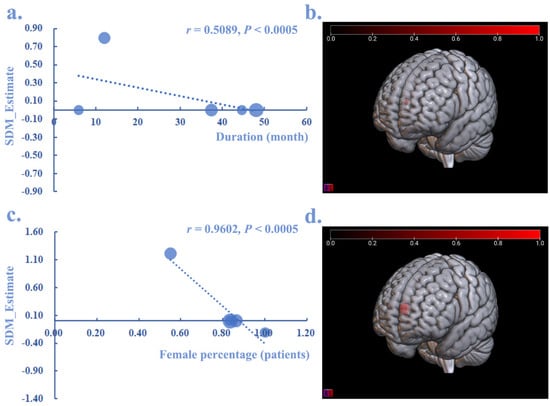
Figure 5.
The meta-regression analysis between GMV alteration in CRPS and disease duration, as well as the percentage of female patients. Both disease duration (r = 0.5089, p < 0.0005) (a,b) and the percentage of female patients (r = 0.9602, p < 0.0005) (c,d) showed a negative relationship with the increased (red) GMV in SFGmedial.L.
4. Discussion
In this study, a systematic review and meta-analysis of GMV abnormalities was first performed within CRPS patients. The main analysis indicated that increased GMV in the SFGmedial.L, left striatum, and an undefined area (2, 0, −8) was shown in CRPS patients, whereas decreased GMV was found in the CC area (extending to the SMA.R and MCC.R) and an undefined area (extending to the CAU.R and THA.R). In addition, the meta-regression analysis suggested that disease duration and the percentage of female patients might have a negative effect on the increased GMV in the SFGmedial.L in CRPS patients.
Interestingly, our meta-analysis results found higher GMV in the SFGmedial.L in CRPS patients than HCs, which demonstrated a negative relationship with the disease duration and percentage of female patients. Previous systematic review and meta-analysis studies reported significant gray matter (GM) deficits in the SFG in several neuropsychological disorders [23,24]. It is well known that the SFG in humans plays a crucial role in cognitive function, especially in the working memory (WM) [25,26]. The SFGmedial is associated with various neuropsychological disorders, including schizophrenia (SCZ), obsessive compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), and bipolar disorder [27,28,29]. Combined with the evidence that long-term chronic pain stimulation can also induce a series of emotional disorders (e.g., anxiety, depression) [30,31] and the female predominance in CRPS, we speculated that the increased GMV in the SFGmedial may be a functional compensation in patients with CRPS, which may be attenuated by the disease duration and female ratio. Our meta-analysis also showed a larger GMV in the left striatum, which was regarded as one of the most important nuclei in regulating motor function [32]. It has also been reported that the increased GMV of the left striatum is significantly involved in the process of essential tremor and functional movement disorders [33,34]. Taken together, we can hypothesize that the possible mechanism of persistent pain stimulation from the extremities impairs the motor function in patients with CRPS. According to the neuroimaging evidence, we found that the undefined area (2, 0, −8) may be in the hypothalamic area [35,36]. Interestingly, the clinical and neuroimaging studies showed the altered neurotransmitter system involved in the hypothalamus and functional connectivity alterations between the hypothalamus and distal nuclei may be an incentive for pain-related aversions (e.g., fatigue and emotional alterations), as well as a biomarker for migraine occurrence [37]. Therefore, the brain structure alteration within the hypothalamus may be a potential mechanism for CRPS-related clinical or neurophysiological symptoms.
In our study, the GMV of the SMA.R and THA.R was significantly decreased, which was consistent with the previous meta-analyses of neuropathic pain [38,39]. Krainik et al. identified the important role of the SMA in motor development in patients undergoing medial frontal lobectomy [40]. Motor skill impairment has also been found in healthy adults receiving SMA.R-guided transcranial magnetic stimulation (nTMS) [41]. Taken together, these findings suggest CRPS may induce the motor dysfunction of patients by impairing the brain structure in the SMA.R. Interestingly, patients with hypoactivation of the SMA showed the same motor dysfunction as medial frontal lobectomy patients, which suggested the possible functional compensation of greater GMV in the SFGmedial to withstand motor dysfunction [40]. Furthermore, our study also found decreased GMV in the THA.R, which may play a dual role in both sensory and motor dysfunction in CRPS patients. For instance, the THA was generally considered to be a very important relay of peripheral sensory information to the cortex [42]. It also plays a key role in generating and monitoring movement by establishing direct contact with the movement-related cortex (e.g., SMA) [43]; the motor THA has also been an important target in treating tremors [44]. In addition, GM atrophy in the CAU was consistent with a previous neuroimaging study in the elderly with slower walking speeds [45]. The clinical and animal studies also identified the crucial role of the CAU in both posture and motor function maintenance [46]. Anatomical evidence revealed CAU lesions were significantly involved in motor apraxia [47]. The CRPS patients also showed a lower GMV in the MCC.R in our study, which is involved in the process of first-episode schizophrenia [48]. Interestingly, previous systematic reviews and meta-analyses of chronic migraine and fibromyalgia showed significantly decreased GMV in the bilateral anterior cingulate/paracingulate cortex (ACC) [49,50], which is involved in bipolar disorder [51]. Therefore, we may hypothesize that different types of chronic pain can impair the different subregions of the cingulate/paracingulate cortex to induce various psychological disorders.
This meta-regression analysis showed the female percentage of CRPS patients might negatively affect the increased GMV in the SFGmedial, which was consistent with the fact that female patients were more prone to CRPS (female:male = 4:1) [52]. Additionally, the disease duration negatively correlated with an increased GMV in the SFGmedial, suggesting long-term pain stimulation might induce worse impairments in the brain structure. Taken together, the potential effects of sex differences and disease duration should be considered in future CRPS studies. Furthermore, it was suggested to explore other risks (e.g., age and pain severity score) of the disease to further expand these datasets. It must also be pointed out that the sociodemographic factors, prior interventions (e.g., medication), prior behavioral health history/comorbidities (prevalence of anxiety/depression), and other potential confounding variables that were unavailable in the included studies might also have an effect on the brain structural alterations in patients with CRPS.
5. Limitations
This study had some limitations, which are declared as follows: Firstly, the study only extracted the available peak coordinate information, which is a common drawback in neuroimaging meta-analyses. Secondly, only five studies from three databases were included and, because of this, a subgroup analysis of CRPS (type I and type II) could not be performed. Thirdly, the meta-regression results should be treated cautiously due to the small size of the datasets, as well as the risk factors that were suggested for comprehensive exploration with larger datasets. Fourthly, the reliability of the heterogeneity, sensitivity, and publication bias assessment may be limited, which can be enhanced by including more studies in the future. Fifthly, the lesser studies included attenuated the reliability of the main findings in this study.
6. Conclusions
In conclusion, patients with CRPS may be susceptible to GMV impairments in sensorimotor cerebral areas. The increased GMV of the SFGmedial may carry out a functional compensation due to motor function deficits. In the process of CRPS, the SFGmedial and THA may play dual roles in motor and neuropsychological disorders, whereas the SMA, CAU, and MCC affect motor and emotional dysfunction. In short, our study provided a reference for a better understanding and exploration of CRPS.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/brainsci12081115/s1, Table S1: PRISMA checklists for systemic review and meta-analysis, Table S2: Systemically search strategy of 3 databases, Table S3: Studies quality assessment by 12-point checklist, Table S4: Heterogeneity assessment of main results by Q statistics.
Author Contributions
Article framework design, L.-F.Y., G.-B.C. and J.-L.L.; funding support, L.-F.Y. and G.-B.C.; systematic search, study selection, and data analysis, T.M. and Z.-Y.L.; review of methods and results, search for reference materials, and contribution to the interpretation of findings, Y.Y. (Ying Yu), Y.Y. (Yang Yang), M.-H.N., H.X., W.W. and Y.-X.H.; writing—original draft preparation, T.M., Z.-Y.L. and Y.Y. (Ying Yu); writing—review and editing, L.-F.Y., G.-B.C. and J.-L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No.81771815, G.-B.C.), the Military Medical Enhancement Program of the Air Force Medical University (No.2018HKPY03, G.-B.C.; No.2018JSTS13, L.-F.Y.), and the Basal Application Research Project of Medical Technology Youth Incubation Programme (No. 21QNPY075, L.-F.Y.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pleger, B.; Draganski, B.; Schwenkreis, P.; Lenz, M.; Nicolas, V.; Maier, C.; Tegenthoff, M. Complex regional pain syndrome type I affects brain structure in prefrontal and motor cortex. PLoS ONE 2014, 9, e85372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruehl, S. Complex regional pain syndrome. BMJ 2015, 351, h2730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, H.; Rose, J.; Halle, S.; Shekane, P. Complex regional pain syndrome: A narrative review for the practising clinician. Br. J. Anaesth. 2019, 123, e424–e433. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; McDonnell, P.; Gershwin, M.E. Complex regional pain syndrome—False hopes and miscommunications. Autoimmun. Rev. 2019, 18, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Halicka, M.; Vittersø, A.D.; Proulx, M.J.; Bultitude, J.H. Neuropsychological Changes in Complex Regional Pain Syndrome (CRPS). Behav. Neurol. 2020, 2020, 4561831. [Google Scholar] [CrossRef] [PubMed]
- Becerra, L.; Schwartzman, R.J.; Kiefer, R.T.; Rohr, P.; Moulton, E.A.; Wallin, D.; Pendse, G.; Morris, S.; Borsook, D. CNS Measures of Pain Responses Pre- and Post-Anesthetic Ketamine in a Patient with Complex Regional Pain Syndrome. Pain Med. 2009, 16, 2368–2385. [Google Scholar] [CrossRef]
- Diers, M. Neuroimaging the pain network—Implications for treatment. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101418. [Google Scholar] [CrossRef]
- Marinus, J.; Moseley, G.L.; Birklein, F.; Baron, R.; Maihöfner, C.; Kingery, W.S.; van Hilten, J.J. Clinical features and pathophysiology of Complex Regional Pain Syndrome—Current state of the art. Lancet Neurol. 2011, 10, 637–648. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.; Nelson, S.; Lewis, J.; McCabe, C.S. Imaging and clinical evidence of sensorimotor problems in CRPS: Utilizing novel treatment approaches. J. Neuroimmune Pharmacol. 2013, 8, 564–575. [Google Scholar] [CrossRef] [Green Version]
- Ashburner, J.; Friston, K.J. Voxel-Based Morphometry—The Methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef] [Green Version]
- Geha, P.Y.; Baliki, M.N.; Harden, R.N.; Bauer, W.R.; Parrish, T.B.; Apkarian, A.V. The Brain in Chronic CRPS Pain: Abnormal Gray-White Matter Interactions in Emotional and Autonomic Regions. Neuron 2008, 60, 570–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokouhi, M.; Clarke, C.; Morley-Forster, P.; Moulin, D.E.; Davis, K.D.; St. Lawrence, K. Structural and Functional Brain Changes at Early and Late Stages of Complex Regional Pain Syndrome. J. Pain 2018, 19, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Barad, M.J.; Ueno, T.; Younger, J.; Chatterjee, N.; Mackey, S. Complex Regional Pain Syndrome Is Associated with Structural Abnormalities in Pain-Related Regions of the Human Brain. J. Pain 2014, 15, 197–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domin, M.; Strauss, S.; McAuley, J.H.; Lotze, M. Complex Regional Pain Syndrome: Thalamic GMV Atrophy and Associations of Lower GMV with Clinical and Sensorimotor Performance Data. Front. Neurol. 2021, 12, 722334. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Luo, C.; Li, Q.; Hu, N.; Xiao, Y.; Liu, N.; Lui, S.; Gong, Q. White Matter Abnormalities in Patients with Parkinson’s Disease: A Meta-Analysis of Diffusion Tensor Imaging Using Tract-Based Spatial Statistics. Front. Aging Neurosci. 2020, 12, 610962. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Chen, Z.; Long, J.; Dai, J.; Huang, X.; Lui, S.; Radua, J.; Vieta, E.; Kemp, G.J.; et al. Meta-analysis of cortical thickness abnormalities in medication-free patients with major depressive disorder. Neuropsychopharmacology 2020, 45, 703–712. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, B.; Luo, Q.; Qiu, L.; Wang, S. Gray Matter Structural Alterations in Social Anxiety Disorder: A Voxel-Based Meta-Analysis. Front. Psychiatry 2018, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Wollman, S.C.; Alhassoon, O.M.; Hall, M.G.; Stern, M.J.; Connors, E.J.; Kimmel, C.L.; Allen, K.E.; Stephan, R.A.; Radua, J. Gray matter abnormalities in opioid-dependent patients: A neuroimaging meta-analysis. Am. J. Drug Alcohol Abus. 2017, 43, 505–517. [Google Scholar] [CrossRef]
- Radua, J.; Rubia, K.; Canales-Rodríguez, E.J.; Pomarol-Clotet, E.; Fusar-Poli, P.; Mataix-Cols, D. Anisotropic Kernels for Coordinate-Based Meta-Analyses of Neuroimaging Studies. Front. Psychiatry 2014, 5, 13. [Google Scholar] [CrossRef]
- Radua, J.; Mataix-Cols, D.; Phillips, M.L.; El-Hage, W.; Kronhaus, D.M.; Cardoner, N.; Surguladze, S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur. Psychiatry 2012, 27, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lai, H.; Li, J.; Becker, B.; Zhao, Y.; Cheng, B.; Wang, S. Gray matter structures associated with neuroticism: A meta-analysis of whole-brain voxel-based morphometry studies. Hum. Brain Mapp. 2021, 42, 2706–2721. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Cheung, C.; Yu, K.; Yip, B.; Sham, P.; Li, Q.; Chua, S.; McAlonan, G. Gray Matter in First-Episode Schizophrenia Before and After Antipsychotic Drug Treatment. Anatomical Likelihood Estimation Meta-Analyses with Sample Size Weighting. Schizophr. Bull. 2016, 37, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Norman, L.J.; Carlisi, C.; Lukito, S.; Hart, H.; Mataix-Cols, D.; Radua, J.; Rubia, K. Structural and Functional Brain Abnormalities in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder. JAMA Psychiatry 2016, 73, 815–825. [Google Scholar] [CrossRef]
- Boisgueheneuc, F.D.; Levy, R.; Volle, E.; Seassau, M.; Duffau, H.; Kinkingnehun, S.; Samson, Y.; Zhang, S.; Dubois, B. Functions of the left superior frontal gyrus in humans: A lesion study. Brain 2006, 129, 3315–3328. [Google Scholar] [CrossRef] [Green Version]
- Leng, K.; Li, E.; Eser, R.; Piergies, A.; Sit, R.; Tan, M.; Neff, N.; Li, S.H.; Rodriguez, R.D.; Suemoto, C.K.; et al. Molecular characterization of selectively vulnerable neurons in Alzheimer’s disease. Nat. Neurosci. 2021, 24, 276–287. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, L.; Xie, W.; Yang, Z.Y.; Zhu, X.Z.; Cheung, E.F.; Sørensenci, T.A.; Raymond, A.M.; Chan, C.K. Altered grey matter volume and cortical thickness in patients with schizo-obsessive comorbidity. Psychiatry Res. Neuroimaging 2018, 276, 65–72. [Google Scholar] [CrossRef]
- Zou, H.; Yang, J. Temporal Variability-Based Functional Brain Lateralization Study in ADHD. J. Atten. Disord. 2021, 25, 839–847. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Howes, O.; Bechdolf, A.; Borgwardt, S. Mapping vulnerability to bipolar disorder: A systematic review and meta-analysis of neuroimaging studies. J. Psychiatry Neurosci. 2012, 37, 170–184. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef]
- Li, J. Pain and depression comorbidity: A preclinical perspective. Behav. Brain Res. 2015, 276, 92–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Nielsen, B.E.; Boxer, E.E.; Aoto, J.; Ford, C.P. Loss of nigral excitation of cholinergic interneurons contributes to parkinsonian motor impairments. Neuron 2021, 109, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Maurer, C.W.; LaFaver, K.; Limachia, G.S.; Capitan, G.; Ameli, R.; Sinclair, S.; Epstein, S.A.; Hallett, M.; Horovitz, S.G. Gray matter differences in patients with functional movement disorders. Neurology 2018, 91, e1870–e1879. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Hou, Y.; Shang, H. A Voxel-Wise Meta-Analysis of Gray Matter Abnormalities in Essential Tremor. Front. Neurol. 2018, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.J.; Stopyra, M.A.; Mönning, E.; Sailer, S.; Lavandier, N.; Kihm, L.P.; Bendszus, M.; Preissl, H.; Herzog, W.; Friederich, H.-C. Neuroimaging of hypothalamic mechanisms related to glucose metabolism in anorexia nervosa and obesity. J. Clin. Investig. 2020, 130, 4094–4103. [Google Scholar] [CrossRef] [Green Version]
- Billot, B.; Bocchetta, M.; Todd, E.; Dalca, A.V.; Rohrer, J.D.; Iglesias, J.E. Automated segmentation of the hypothalamus and associated subunits in brain MRI. Neuroimage 2020, 223, 117287. [Google Scholar] [CrossRef]
- May, A.; Burstein, R. Hypothalamic regulation of headache and migraine. Cephalalgia 2019, 39, 1710–1719. [Google Scholar] [CrossRef]
- Pan, P.L.; Zhong, J.G.; Shang, H.F.; Zhu, Y.L.; Xiao, P.R.; Dai, Z.Y.; Shi, H.C. Quantitative meta-analysis of grey matter anomalies in neuropathic pain. Eur. J. Pain 2015, 19, 1224–1231. [Google Scholar] [CrossRef]
- Wang, W.; Tang, S.; Li, C.; Chen, J.N.; Li, H.F.; Su, Y.L.; Ning, B. Specific Brain Morphometric Changes in Spinal Cord Injury: A Voxel-Based Meta-Analysis of White and Gray Matter Volume. J. Neurotraum. 2019, 36, 2348–2357. [Google Scholar] [CrossRef]
- Krainik, A.; Lehericy, S.; Duffau, H.; Vlaicu, M.; Poupon, F.; Capelle, L.; Cornu, P.; Clemenceau, S.; Sahel, M.; Valery, C.-A.; et al. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology 2001, 57, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Schramm, S.; Albers, L.; Ille, S.; Schröder, A.; Meyer, B.; Sollmann, N.; Krieg, S.M. Navigated transcranial magnetic stimulation of the supplementary motor cortex disrupts fine motor skills in healthy adults. Sci. Rep. 2019, 9, 17744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, S.M.; Guillery, R.W. The role of the thalamus in the flow of information to the cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 1695–1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, M.A. The role of the thalamus in motor control. Curr. Opin. Neurobiol. 2003, 13, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamani, C.; Dostrovsky, J.O.; Lozano, A.M. The motor thalamus in neurosurgery. Neurosurgery 2006, 58, 146–158. [Google Scholar] [CrossRef]
- Dumurgier, J.; Crivello, F.; Mazoyer, B.; Ahmed, I.; Tavernier, B.; Grabli, D.; François, C.; Tzourio-Mazoyer, N.; Tzourio, C.; Elbaz, A. MRI atrophy of the caudate nucleus and slower walking speed in the elderly. Neuroimage 2012, 60, 871–878. [Google Scholar] [CrossRef]
- Villablanca, J.R. Why do we have a caudate nucleus? Acta Neurobiol. Exp. 2010, 70, 95–105. [Google Scholar]
- Çırak, M.; Yağmurlu, K.; Kearns, K.N.; Ribas, E.C.; Urgun, K.; Shaffrey, M.E.; Kalani, M.Y.S. The Caudate Nucleus: Its Connections, Surgical Implications, and Related Complications. World Neurosurg. 2020, 139, e428–e438. [Google Scholar] [CrossRef]
- Shah, C.; Zhang, W.; Xiao, Y.; Yao, L.; Zhao, Y.; Gao, X.; Liu, L.; Liu, J.; Li, S.; Tao, B.; et al. Common pattern of gray-matter abnormalities in drug-naive and medicated first-episode schizophrenia: A multimodal meta-analysis. Psychol. Med. 2017, 47, 401–413. [Google Scholar] [CrossRef]
- Shi, H.; Yuan, C.; Dai, Z.; Ma, H.; Sheng, L. Gray matter abnormalities associated with fibromyalgia: A meta-analysis of voxel-based morphometric studies. Semin. Arthritis Rheum. 2016, 46, 330–337. [Google Scholar] [CrossRef]
- Dai, Z.; Zhong, J.; Xiao, P.; Zhu, Y.; Chen, F.; Pan, P.; Shi, H. Gray matter correlates of migraine and gender effect: A meta-analysis of voxel-based morphometry studies. Neuroscience 2015, 299, 88–96. [Google Scholar] [CrossRef]
- Fornito, A.; Malhi, G.S.; Lagopoulos, J.; Ivanovski, B.; Wood, S.J.; Velakoulis, D.; Saling, M.M.; McGorry, P.D.; Pantelis, C.; Yücel, M.; et al. In vivo evidence for early neurodevelopmental anomaly of the anterior cingulate cortex in bipolar disorder. Acta Psychiatr. Scand. 2007, 116, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, P.; Benrud-Larson, L.M.; McClelland, R.L.; Low, P.A. Complex regional pain syndrome type I: Incidence and prevalence in Olmsted County, a population-based study. Pain 2003, 103, 199–207. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).