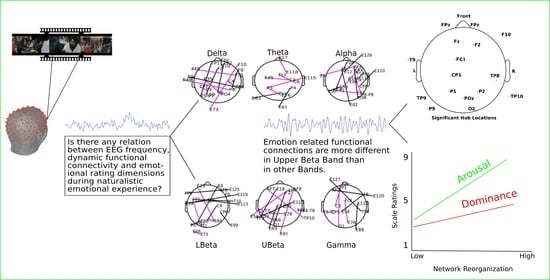
Dynamic Functional Connectivity of Emotion Processing in Beta Band with Naturalistic Emotion Stimuli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli and Apparatus
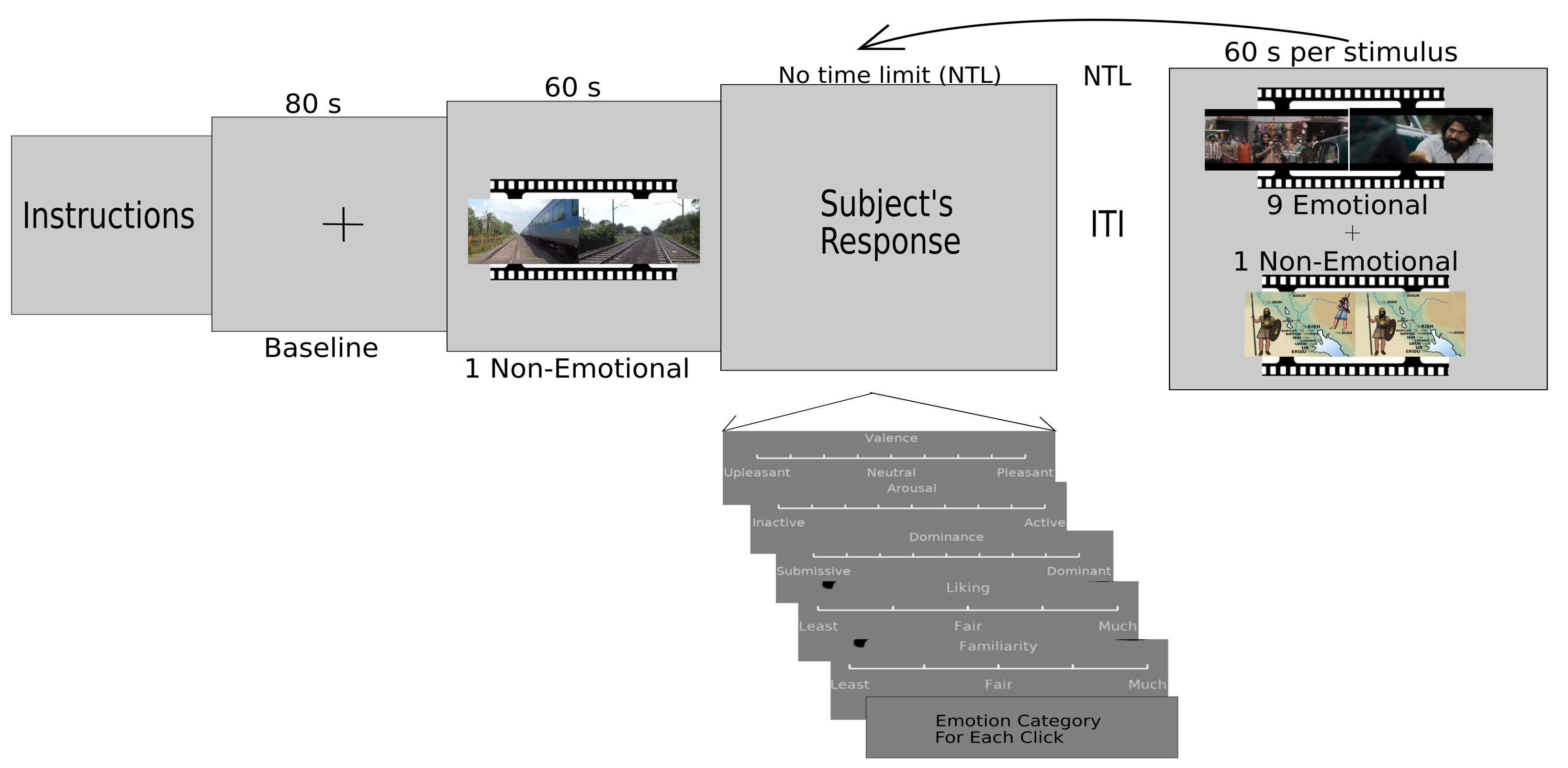
2.3. Experiment Paradigm
2.4. EEG Data Acquisition and Pre-Processing
2.5. Analysis
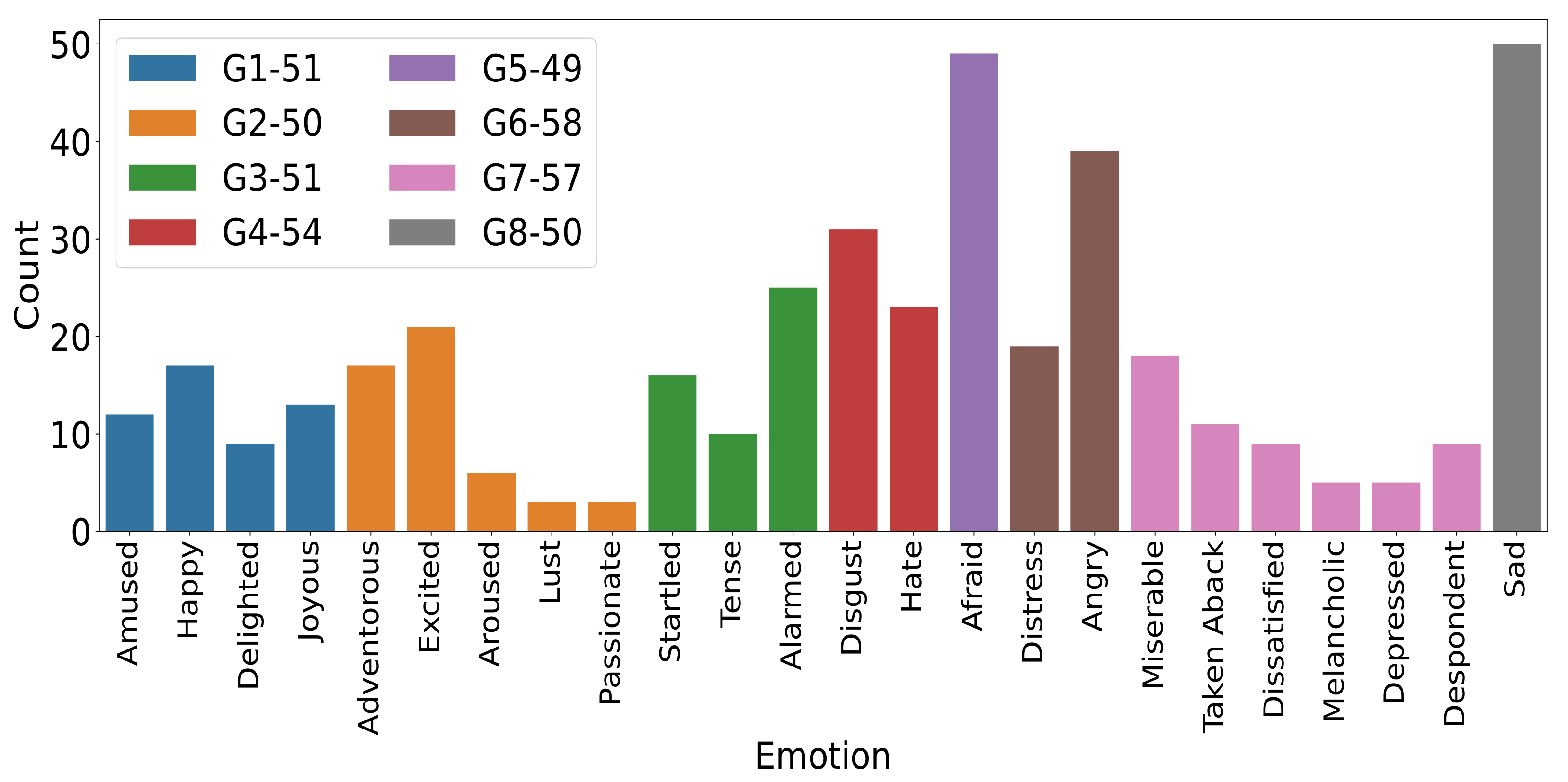
2.5.1. Emotion Grouping
2.5.2. Frequency Bands
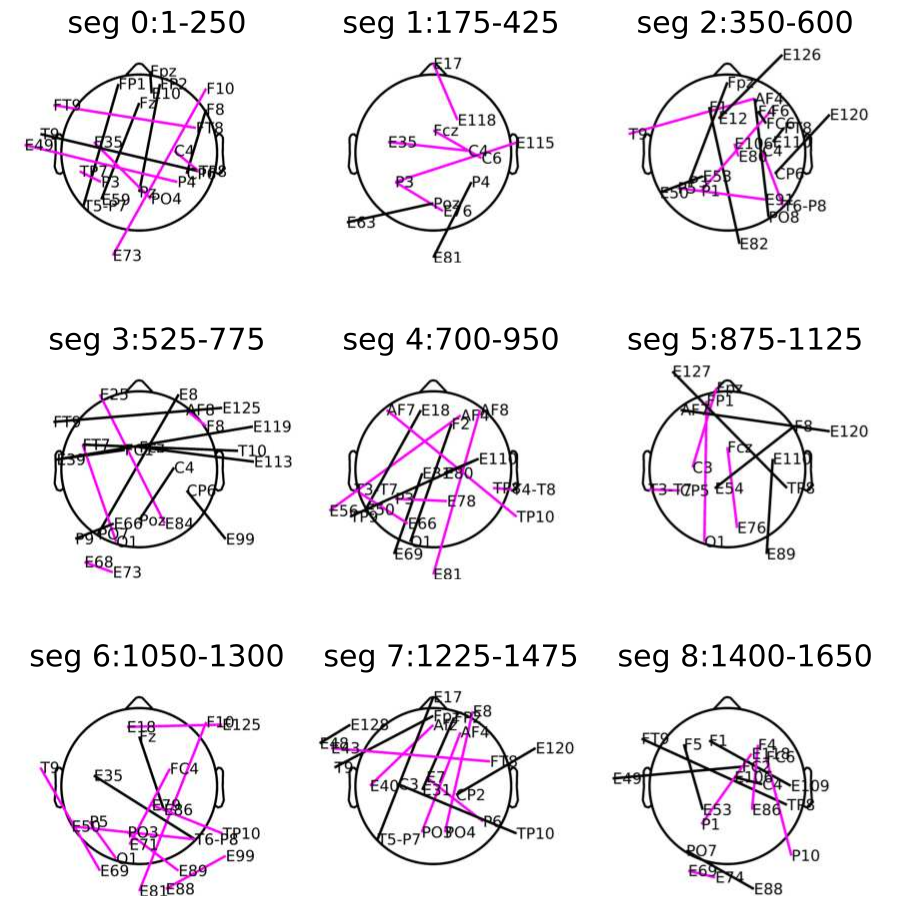
2.5.3. EEG Signal Segmentation
2.5.4. EEG Dynamic Functional Connectivity and Temporal Variability of the Functional Connectivity Profile
2.5.5. Hub Calculation
2.6. Code Accessibility
3. Results
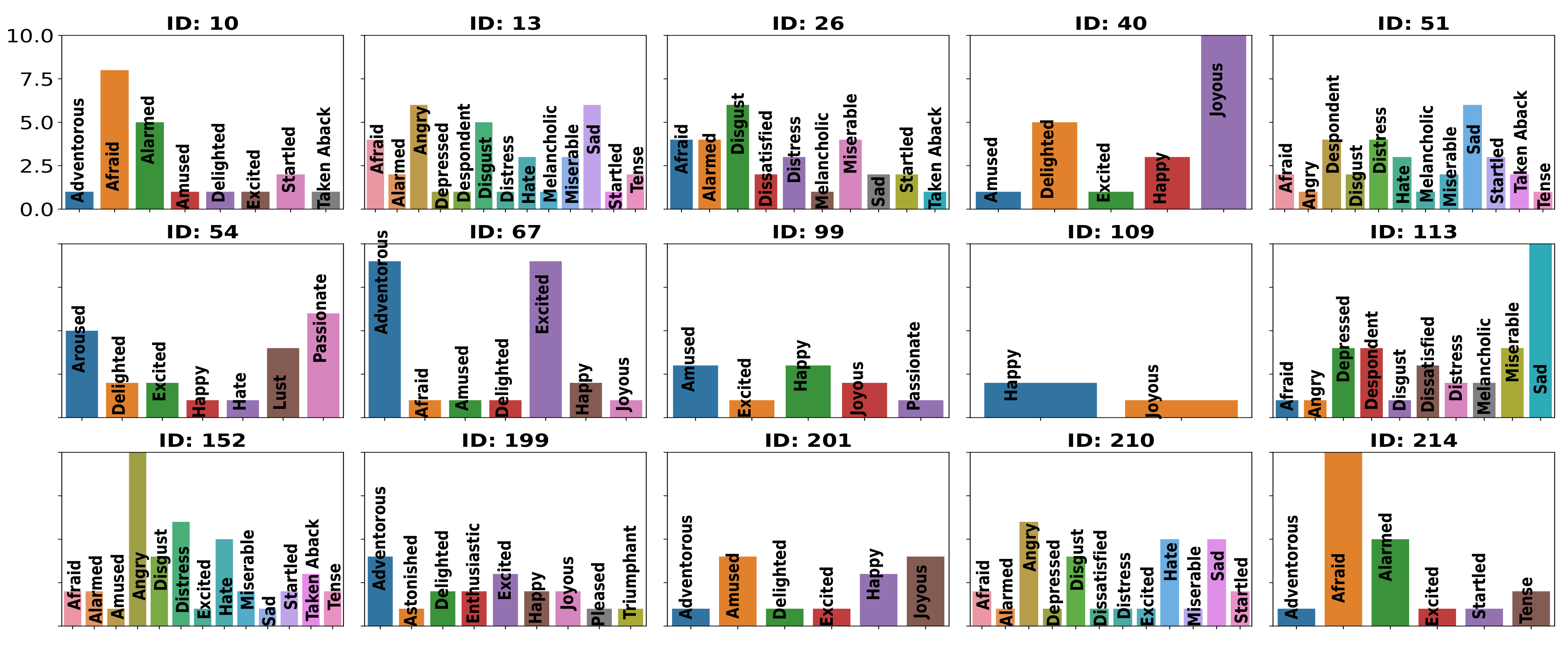
3.1. Distribution of Labeled Emotional Experiences
3.2. Band Specificity for Emotion Processing and Connectivity Networks for Different Emotion Groups
3.3. Temporal Variability of Emotional Functional Networks Are Correlated with Emotional Arousal and Dominance
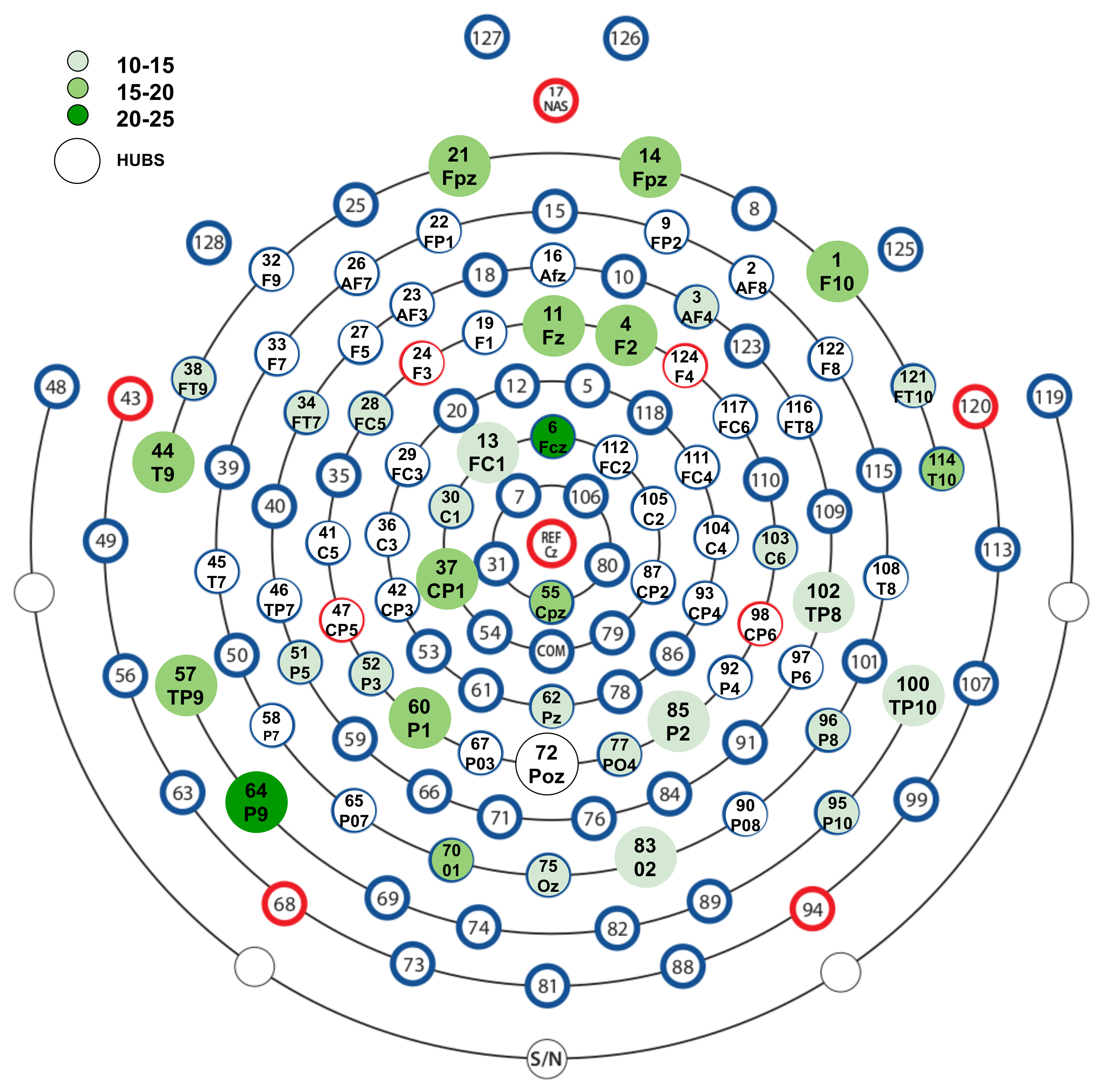
3.4. Nodes Contributing More in Temporal Dynamics of Emotional Functional Networks
4. Discussion
4.1. Emotion-Specific Activity in the Beta Band
4.2. Temporal Variability in Dynamic Functional Connectivity
4.3. Hub Activity in Right Frontal and Bilateral Posterior Brain Regions
4.4. Limitations
5. Conclusions
6. Data Statement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFC | dynamic functional connectivity |
| tvDFC | temporal variability in DFC |
| IAPS | International Affective Picture System |
| tISFC | time-varying inter-subject brain synchronization |
| PLV | Phase Locking Value |
References
- Ekman, P.; Friesen, W.V. Constants across cultures in the face and emotion. J. Personal. Soc. Psychol. 1971, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Squires, N.K.; Squires, K.C.; Hillyard, S.A. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr. Clin. Neurophysiol. 1975, 38, 387–401. [Google Scholar] [CrossRef]
- Saarimäki, H. Naturalistic stimuli in affective neuroimaging: A review. Front. Hum. Neurosci. 2021, 15, 318. [Google Scholar] [CrossRef]
- Jääskeläinen, I.P.; Sams, M.; Glerean, E.; Ahveninen, J. Movies and narratives as naturalistic stimuli in neuroimaging. NeuroImage 2021, 224, 117445. [Google Scholar] [CrossRef]
- Miskovic, V.; Schmidt, L.A. Cross-regional cortical synchronization during affective image viewing. Brain Res. 2010, 1362, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Başar, E. Event-related beta oscillations are affected by emotional eliciting stimuli. Neurosci. Lett. 2010, 483, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, J.; Liu, X.; Fang, H.; Li, H.; Wang, D.; Shen, J. Event-related synchronization of delta and beta oscillations reflects developmental changes in the processing of affective pictures during adolescence. Int. J. Psychophysiol. 2013, 90, 334–340. [Google Scholar] [CrossRef]
- Jessen, S.; Kotz, S.A. The temporal dynamics of processing emotions from vocal, facial, and bodily expressions. Neuroimage 2011, 58, 665–674. [Google Scholar] [CrossRef]
- Wang, X.-W.; Nie, D.; Lu, B.-L. Emotional state classification from eeg data using machine learning approach. Neurocomputing 2014, 129, 94–106. [Google Scholar] [CrossRef]
- Soleymani, M.; Lichtenauer, J.; Pun, T.; Pantic, M. A multimodal database for affect recognition and implicit tagging. IEEE Trans. Affect. Comput. 2011, 3, 42–55. [Google Scholar] [CrossRef]
- Koelstra, S.; Muhl, C.; Soleymani, M.; Lee, J.-S.; Yazdani, A.; Ebrahimi, T.; Pun, T.; Nijholt, A.; Patras, I. Deap: A database for emotion analysis; using physiological signals. IEEE Trans. Affect. Comput. 2011, 3, 18–31. [Google Scholar] [CrossRef]
- Woodruff, C.C.; Daut, R.; Brower, M.; Bragg, A. Electroencephalographic α-band and β-band correlates of perspective-taking and personal distress. Neuroreport 2011, 22, 744–748. [Google Scholar] [CrossRef]
- Kheirkhah, M.; Baumbach, P.; Leistritz, L.; Brodoehl, S.; Götz, T.; Huonker, R.; Witte, O.W.; Klingner, C.M. The temporal and spatial dynamics of cortical emotion processing in different brain frequencies as assessed using the cluster-based permutation test: An meg study. Brain Sci. 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Aftanas, L.I.; Varlamov, A.A.; Pavlov, S.V.; Makhnev, V.P.; Reva, N.V. Time-dependent cortical asymmetries induced by emotional arousal: Eeg analysis of event-related synchronization and desynchronization in individually defined frequency bands. Int. J. Psychophysiol. 2002, 44, 67–82. [Google Scholar] [CrossRef]
- Kim, H.; Seo, P.; Choi, J.W.; Kim, K.H. Emotional arousal due to video stimuli reduces local and inter-regional synchronization of oscillatory cortical activities in alpha-and beta-bands. PLoS ONE 2021, 16, e0255032. [Google Scholar] [CrossRef]
- Tay, R.Y.L.; Ng, B.C. Effects of affective priming through music on the use of emotion words. PLoS ONE 2019, 14, e0214482. [Google Scholar]
- Medaglia, J.D.; Lynall, M.-E.; Bassett, D.S. Cognitive network neuroscience. J. Cogn. Neurosci. 2015, 27, 1471–1491. [Google Scholar] [CrossRef]
- Hao, Y.; Yao, L.; Smith, D.M.; Sorel, E.; Anderson, A.K.; Schumacher, E.H.; Evans, G.W. Prefrontal-posterior coupling mediates transitions between emotional states and influences executive functioning. Sci. Rep. 2019, 9, 8252. [Google Scholar] [CrossRef]
- Dasdemir, Y.; Yildirim, E.; Yildirim, S. Analysis of functional brain connections for positive–negative emotions using phase locking value. Cogn. Neurodyn. 2017, 11, 487–500. [Google Scholar] [CrossRef]
- Markett, S.; Wudarczyk, O.A.; Biswal, B.B.; Jawinski, P.; Montag, C. Affective network neuroscience. Front. Neurosci. 2018, 12, 895. [Google Scholar] [CrossRef]
- Cohen, J.R. The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. NeuroImage 2018, 180, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Garrett, D.D.; Samanez-Larkin, G.R.; MacDonald, S.W.; Lindenberger, U.; McIntosh, A.R.; Grady, C.L. Moment-to-moment brain signal variability: A next frontier in human brain mapping? Neurosci. Biobehav. Rev. 2013, 37, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, V.D.; Miller, R.; Pearlson, G.; Adalı, T. The chronnectome: Time-varying connectivity networks as the next frontier in fmri data discovery. Neuron 2014, 84, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Shou, G.; Yuan, H.; Li, C.; Chen, Y.; Chen, Y.; Ding, L. Whole-brain electrophysiological functional connectivity dynamics in resting-state eeg. J. Neural Eng. 2020, 17, 026016. [Google Scholar] [CrossRef]
- Chen, J.E.; Chang, C.; Greicius, M.D.; Glover, G.H. Introducing co-activation pattern metrics to quantify spontaneous brain network dynamics. Neuroimage 2015, 111, 476–488. [Google Scholar] [CrossRef]
- Elton, A.; Gao, W. Task-related modulation of functional connectivity variability and its behavioral correlations. Hum. Brain Mapp. 2015, 36, 3260–3272. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, W.; Liu, Z.; Zhang, K.; Lei, X.; Yao, Y.; Becker, B.; Liu, Y.; Kendrick, K.M.; Lu, G.; et al. Neural, electrophysiological and anatomical basis of brain-network variability and its characteristic changes in mental disorders. Brain 2016, 139, 2307–2321. [Google Scholar] [CrossRef]
- Núñez, P.; Poza, J.; Gómez, C.; Rodríguez-González, V.; Hillebrand, A.; Tola-Arribas, M.A.; Cano, M.; Hornero, R. Characterizing the fluctuations of dynamic resting-state electrophysiological functional connectivity: Reduced neuronal coupling variability in mild cognitive impairment and dementia due to alzheimer’s disease. J. Neural Eng. 2019, 16, 056030. [Google Scholar] [CrossRef]
- Young, C.B.; Raz, G.; Everaerd, D.; Beckmann, C.F.; Tendolkar, I.; Hendler, T.; Fernández, G.; Hermans, E.J. Dynamic shifts in large-scale brain network balance as a function of arousal. J. Neurosci. 2017, 37, 281–290. [Google Scholar] [CrossRef]
- Raz, G.; Touroutoglou, A.; Wilson-Mendenhall, C.; Gilam, G.; Lin, T.; Gonen, T.; Jacob, Y.; Atzil, S.; Admon, R.; Bleich-Cohen, M.; et al. Functional connectivity dynamics during film viewing reveal common networks for different emotional experiences. Cogn. Affect. Behav. Neurosci. 2016, 16, 709–723. [Google Scholar] [CrossRef]
- Sachs, M.E.; Habibi, A.; Damasio, A.; Kaplan, J.T. Dynamic intersubject neural synchronization reflects affective responses to sad music. NeuroImage 2020, 218, 116512. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Handjaras, G.; Setti, F.; Cappello, E.M.; Bruno, V.; Diano, M.; Leo, A.; Ricciardi, E.; Pietrini, P.; Cecchetti, L. Default and control network connectivity dynamics track the stream of affect at multiple timescales. Soc. Cogn. Affect. Neurosci. 2022, 17, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Tiwary, U.S.; Srinivasan, N. Affective Film Dataset from India (AFDI) Films. 2022. Available online: https://osf.io/tgcj8 (accessed on 25 July 2021).
- Mishra, S.; Srinivasan, N.; Tiwary, U.S. Material OSF. 2022. Available online: https://osf.io/gzqj3 (accessed on 1 August 2022).
- Mishra, S.; Srinivasan, N.; Tiwary, U.S.; Asif, M. Affective Film Dataset from India (Afdi): Creation and Validation with an Indian Sample. 2021. Available online: https://psyarxiv.com/yajsk/ (accessed on 11 January 2021).
- Dictionary, O. Oxford Lexico. 2019. Available online: https://www.lexico.com/ (accessed on 8 October 2020).
- Russell, J.A. Mehrabian, A. Evidence for a three-factor theory of emotions. J. Res. Personal. 1977, 11, 273–294. [Google Scholar] [CrossRef]
- Ferree, T.; Luu, P.; Russell, G.; Tucker, D. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001, 112, 536–544. [Google Scholar] [CrossRef]
- Nobre, A.; Nikolaev, A.; Wagemans, J. High-density scalp EEG data acquired in an inattentional blindness paradigm with background Gestalt stimuli. Data Brief 2020, 28, 104901. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, C.; Dickinson, A.; Baker, E.; Jeste, S. EEG data collection in children with ASD: The role of state in data quality and spectral power. Res. Autism Spectr. Disord. 2019, 57, 132–144. [Google Scholar] [CrossRef]
- Shen, G.; Gao, K.; Zhao, N.; Wang, Z.; Jiang, C.; Liu, J. A Fully Flexible Hydrogel Electrode for Daily EEG Monitoring. IEEE Sens. J. 2022, 22, 12522–12529. [Google Scholar] [CrossRef]
- Symeonidou, E.; Nordin, A.; Hairston, W.; Ferris, D. Effects of cable sway, electrode surface area, and electrode mass on electroencephalography signal quality during motion. Sensors 2018, 18, 1073. [Google Scholar] [CrossRef]
- Pion-Tonachini, L.; Kreutz-Delgado, K.; Makeig, S. Iclabel: An automated electroencephalographic independent component classifier, dataset, and website. NeuroImage 2019, 198, 181–197. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Lin, Y.; Onton, J.; Jung, T.-P.; Makeig, S. Unsupervised learning of brain state dynamics during emotion imagination using high-density eeg. NeuroImage 2022, 249, 118873. [Google Scholar] [CrossRef]
- Mishra, S.; Srinivasan, N.; Tiwary, U. Cardiac–Brain Dynamics Depend on Context Familiarity and Their Interaction Predicts Experience of Emotional Arousal. Brain Sci. 2022, 12, 702. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.K.; Tiwary, U.S. Multimodal fusion framework: A multiresolution approach for emotion classification and recognition from physiological signals. NeuroImage 2014, 102, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Aydore, S.; Pantazis, D.; Leahy, R.M. A note on the phase locking value and its properties. Neuroimage 2013, 74, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.M.; Schoffelen, J.-M. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front. Syst. Neurosci. 2016, 9, 175. [Google Scholar] [CrossRef]
- Schoffelen, J.-M.; Gross, J. Source connectivity analysis with meg and eeg. Hum. Brain Mapp. 2009, 30, 1857–1865. [Google Scholar] [CrossRef]
- Frackowiak, R.S.; Friston, K.J.; Frith, C.D.; Dolan, R.J.; Price, C.J.; Zeki, S.; Ashburner, J.T.; Penny, W.D. (Eds.) Chapter 46—Nonparametric Permutation Tests for Functional Neuroimaging, 2nd ed.; Academic Press: Cambridge, MA, USA, 2004; pp. 887–910. [Google Scholar]
- Holmes, A.P.; Blair, R.; Watson, J.; Ford, I. Nonparametric analysis of statistic images from functional mapping experiments. J. Cereb. Blood Flow Metab. 1996, 16, 7–22. [Google Scholar] [CrossRef]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef]
- Khambhati, A.N.; Medaglia, J.D.; Karuza, E.A.; Thompson-Schill, S.L.; Bassett, D.S. Subgraphs of functional brain networks identify dynamical constraints of cognitive control. PLoS Comput. Biol. 2018, 14, e1006234. [Google Scholar]
- Rolls, E.T.; Cheng, W.; Feng, J. Brain dynamics: The temporal variability of connectivity, and differences in schizophrenia and adhd. Transl. Psychiatry 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Betti, V.; Corbetta, M.; de Pasquale, F.; Wens, V.; Della Penna, S. Topology of functional connectivity and hub dynamics in the beta band as temporal prior for natural vision in the human brain. J. Neurosci. 2018, 38, 3858–3871. [Google Scholar] [CrossRef]
- Fang, F.; Potter, T.; Nguyen, T.; Zhang, Y. Dynamic reorganization of the cortical functional brain network in affective processing and cognitive reappraisal. Int. J. Neural Syst. 2020, 30, 2050051. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, G.; Margulies, D.S.; Horstmann, A.; Pleger, B.; Lepsien, J.; Goldhahn, D.; Schloegl, H.; Stumvoll, M.; Villringer, A.; Turner, R. Eigenvector centrality mapping for analyzing connectivity patterns in fmri data of the human brain. PloS ONE 2010, 5, e10232. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Srinivasan, N.; Tiwary, U.S. Analysis OSF. 2022. Available online: https://osf.io/q4jvh (accessed on 29 April 2022).
- Barrett, L.F. The theory of constructed emotion: An active inference account of interoception and categorization. Soc. Cogn. Affect. Neurosci. 2017, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Leung, R.C.; Ye, A.X.; Wong, S.M.; Taylor, M.J.; Doesburg, S.M. Reduced beta connectivity during emotional face processing in adolescents with autism. Mol. Autism 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Spitzer, B.; Haegens, S. Beyond the status quo: A role for beta oscillations in endogenous content (re) activation. eneuro 2017, 4, ENEURO.0170-17.2017. [Google Scholar] [CrossRef]
- Kopell, N.; Whittington, M.A.; Kramer, M.A. Neuronal assembly dynamics in the beta1 frequency range permits short-term memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3779–3784. [Google Scholar] [CrossRef]
- Sonkusare, S.; Breakspear, M.; Guo, C. Naturalistic stimuli in neuroscience: Critically acclaimed. Trends Cogn. Sci. 2019, 23, 699–714. [Google Scholar] [CrossRef]
- Bastos, A.M.; Vezoli, J.; Bosman, C.A.; Schoffelen, J.-M.; Oostenveld, R.; Dowdall, J.R.; De Weerd, P.; Kennedy, H.; Fries, P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 2015, 85, 390–401. [Google Scholar] [CrossRef]
- Bouret, S.; Sara, S.J. Network reset: A simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005, 28, 574–582. [Google Scholar] [CrossRef]
- Tardiff, N.; Medaglia, J.D.; Bassett, D.S.; Thompson-Schill, S.L. The modulation of brain network integration and arousal during exploration. NeuroImage 2021, 240, 118369. [Google Scholar] [CrossRef]
- Lee, K.; Horien, C.; O’Connor, D.; Garand-Sheridan, B.; Tokoglu, F.; Scheinost, D.; Lake, E.M.; Constable, R.T. Arousal impacts distributed hubs modulating the integration of brain functional connectivity. NeuroImage 2022, 258, 119364. [Google Scholar] [CrossRef]
- Rack-Gomer, A.L.; Liu, T.T. Caffeine increases the temporal variability of resting-state bold connectivity in the motor cortex. Neuroimage 2012, 59, 2994–3002. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, M.E.; Yee, C.M.; Heller, W.; Miller, G.A.; Spielberg, J.M. Reconfiguration of brain networks supporting inhibition of emotional challenge. NeuroImage 2019, 186, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A.; Mehrabian, A. Distinguishing anger and anxiety in terms of emotional response factors. J. Consult. Clin. Psychol. 1974, 42, 79. [Google Scholar] [CrossRef]
- Lindquist, K.; Wager, T.; Kober, H.; Bliss-Moreau, E.; Barrett, L. The brain basis of emotion: A meta-analytic review. Behav. Brain Sci. 2012, 35, 121. [Google Scholar] [CrossRef]
- Gainotti, G. Emotional behavior and hemispheric side of the lesion. Cortex 1972, 8, 41–55. [Google Scholar] [CrossRef]
- Gainotti, G. Unconscious processing of emotions and the right hemisphere. Neuropsychologia 2012, 50, 205–218. [Google Scholar] [CrossRef]
- Mandal, M.K.; Ambady, N. Laterality of facial expressions of emotion: Universal and culture-specific influences. Behav. Neurol. 2004, 15, 23–34. [Google Scholar] [CrossRef]
- Reznik, S.J.; Allen, J.J. Frontal asymmetry as a mediator and moderator of emotion: An updated review. Psychophysiology 2018, 55, e12965. [Google Scholar] [CrossRef]
- Spitzer, B.; Wacker, E.; Blankenburg, F. Oscillatory correlates of vibrotactile frequency processing in human working memory. J. Neurosci. 2010, 30, 4496–4502. [Google Scholar] [CrossRef]
- Huebl, J.; Brücke, C.; Merkl, A.; Bajbouj, M.; Schneider, G.-H.; Kühn, A.A. Processing of emotional stimuli is reflected by modulations of beta band activity in the subgenual anterior cingulate cortex in patients with treatment resistant depression. Soc. Cogn. Affect. Neurosci. 2016, 11, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Geerligs, L.; Akyürek, E.G. Temporal integration depends on increased prestimulus beta band power. Psychophysiology 2012, 49, 1632–1635. [Google Scholar] [CrossRef] [PubMed]
- Fransson, P.; Thompson, W.H. Temporal flow of hubs and connectivity in the human brain. Neuroimage 2020, 223, 117348. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Dolan, R.J.; Rugg, M.D. Event-related potential correlates of the retrieval of emotional and nonemotional context. J. Cogn. Neurosci. 2004, 16, 760–775. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Yu, M.; Zhao, G.; Song, J.; Ge, Y.; Shi, Y. Real-time movie-induced discrete emotion recognition from eeg signals. IEEE Trans. Affect. Comput. 2017, 9, 550–562. [Google Scholar] [CrossRef]
- Pollatos, O.; Kirsch, W.; Schandry, R. Brain structures involved in interoceptive awareness and cardioafferent signal processing: A dipole source localization study. Hum. Brain Mapp. 2005, 26, 54–64. [Google Scholar] [CrossRef]
- Herbert, B.M.; Pollatos, O.; Schandry, R. Interoceptive sensitivity and emotion processing: An eeg study. Int. J. Psychophysiol. 2007, 65, 214–227. [Google Scholar] [CrossRef]
- Marshall, A.C.; Gentsch, A.; Schütz-Bosbach, S. Interoceptive cardiac expectations to emotional stimuli predict visual perception. Emotion 2020, 20, 1113. [Google Scholar] [CrossRef]
- Craig, A.D.; Craig, A. How do you feel–now? the anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Das, A.; Myers, J.; Mathura, R.; Shofty, B.; Metzger, B.A.; Bijanki, K.; Wu, C.; Jacobs, J.; Sheth, S.A. Spontaneous neuronal oscillations in the human insula are hierarchically organized traveling waves. Elife 2022, 11, e76702. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Ge, Y. Frontal eeg asymmetry and middle line power difference in discrete emotions. Front. Behav. Neurosci. 2018, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Van Bochove, M.E.; Ketel, E.; Wischnewski, M.; Wegman, J.; Aarts, E.; de Jonge, B.; Medendorp, W.P.; Schutter, D.J. Posterior resting state eeg asymmetries are associated with hedonic valuation of food. Int. J. Psychophysiol. 2016, 110, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Schutter, D.J.; Putman, P.; Hermans, E.; van Honk, J. Parietal electroencephalogram beta asymmetry and selective attention to angry facial expressions in healthy human subjects. Neurosci. Lett. 2001, 314, 13–16. [Google Scholar] [CrossRef]
- Moran, J.; Michail, G.; Heinz, A.; Keil, J.; Senkowski, D. Long-range temporal correlations in resting state beta oscillations are reduced in schizophrenia. Front. Psychiatry 2019, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Ebner, N.; Fischer, H. Emotion and aging: Evidence from brain and behavior. Front. Psychol. 2014, 5, 996. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chang, L.; Yang, M.; Huo, M.; Zhou, R. Gender differences in emotional response: Inconsistency between experience and expressivity. PLoS ONE 2016, 11, e0158666. [Google Scholar] [CrossRef]
- Mishra, S.; Asif, M.; Tiwary, U.S.; Srinivasan, N. Dataset on Emotion with Naturalistic Stimuli (Dens). 2021. Available online: https://openneuro.org/datasets/ds003751/versions/1.0.3 (accessed on 13 April 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.; Srinivasan, N.; Tiwary, U.S. Dynamic Functional Connectivity of Emotion Processing in Beta Band with Naturalistic Emotion Stimuli. Brain Sci. 2022, 12, 1106. https://doi.org/10.3390/brainsci12081106
Mishra S, Srinivasan N, Tiwary US. Dynamic Functional Connectivity of Emotion Processing in Beta Band with Naturalistic Emotion Stimuli. Brain Sciences. 2022; 12(8):1106. https://doi.org/10.3390/brainsci12081106
Chicago/Turabian StyleMishra, Sudhakar, Narayanan Srinivasan, and Uma Shanker Tiwary. 2022. "Dynamic Functional Connectivity of Emotion Processing in Beta Band with Naturalistic Emotion Stimuli" Brain Sciences 12, no. 8: 1106. https://doi.org/10.3390/brainsci12081106
APA StyleMishra, S., Srinivasan, N., & Tiwary, U. S. (2022). Dynamic Functional Connectivity of Emotion Processing in Beta Band with Naturalistic Emotion Stimuli. Brain Sciences, 12(8), 1106. https://doi.org/10.3390/brainsci12081106