Effectiveness of Somatosensory Stimulation for the Lower Limb and Foot to Improve Balance and Gait after Stroke: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Searching for Literature
2.2. Screening for Eligibility
2.3. Quality Assessment
2.4. Data Extraction
2.5. Data Analysis
3. Results
3.1. Selection of Studies and Data Collection and Management
3.2. Effects of Somatosensory Stimulation
Somatosensory Interventions
| Study | Study Design, Sample Size | Outcome Measurement | Study Group | No of Participants | Sex M/F | Side of Paresis L/R | Age Mean (SD) (Years) | Time post-Stroke; Mean (SD) | Type of Stroke: Infarct/ Haemorrhage | No. Finished Intervention |
|---|---|---|---|---|---|---|---|---|---|---|
| Bayouk et al. (2006) [41] | Matched pairs RCT, n = 16 | Before and after 8-week program, no follow-up | Task-orientated training (2×/wk for 8 wks), 30 min each session—different surfaces proprioception feet/ankles and/or vision manipulated (8 h, total 16 h) | 8 | 6/2 | 6/2 | 68.4 (7.1) | 7.10 (12.50) yrs | Not stated | 8 |
| Task-orientated training eyes open, hard surface (total 16 h.) | 8 | 3/5 | 4/4 | 62.0 (4.6) | 5.70 (6.90) yrs | Not stated | 8 | |||
| Cho et al. (2013) [34] | Randomized placebo-controlled trial, n = 42 | Before and after intervention, with follow-up next day | Physical therapy for 30 min prior to TENS, single session for 1 h (total 90 min) | 22 | 14/8 | Not stated | 55.2 (11.5) | 15.00 (4.90) months | 15/7 | 22 |
| Physical therapy, 30 min prior to placebo TENS (total 90 min) | 20 | 13/7 | Not stated | 55.7 (8.6) | 13.90 (5.10) months | 14/6 | 20 | |||
| Ferreira et al. (2018) [42] | RCT, n = 24 | Before wearing insoles and after 3 months of insole use | Postural insoles influencing muscle proprioception (3 months of insole use, unclear how long they were worn each day) | 12 | 11/1 | 6/6 | 59.2 (10.4) | 3.90 (1.50) yrs | 10/2 | 12 |
| Placebo insoles, no corrective elements. | 12 | 5/3 | 6/2 | 60.3 (13.3) | 3.30 (1.10) yrs | 6/2 | 8 | |||
| Goliwas et al. (2015) [39] | Single-blinded RCT, n = 27 | On first and last day of stay in rehabilitation facility, no follow-up | Standard 5×/wk, 6-wk. rehabilitation program (30 min × 25 sessions, plus 15 min of sensorimotor foot stimulation (8.3 h, total 18.75 h) | 13 | 5/3 | 2/6 | 62.3 (9.4) | 4.40 (3.10) yrs | 8/0 | 8 |
| Standard therapeutic rehabilitation program (45 min × 25 sessions, total 18.75 h.) | 14 | 7/8 | 5/7 | 67.7 (9.2) | 4.10 (2.80) yrs | 12/0 | 12 | |||
| In et al. (2021) [46] | Double-blinded RCT, n = 40 | One day before and one day after sit-to-stand training, no follow-up | Sit-to-stand training, 30 min/day, 5x/wk, 6 wks + taping on tibialis anterior (total 15 h training + tape left in situ, changed every three days) | 20 | Not stated | 10/10 | 56.2 (10.4) | 7.05 (2.78) months | Not stated | 20 |
| Just sit-to stand training, no taping (total 15 h) | 20 | Not stated | 11/9 | 55.1 (9.9) | 6.80 (2.50) months | Not stated | 20 | |||
| Jung et al. (2017) [36] | Double-blinded RCT, n = 41 | Before and after 6-week training, no follow-up | 30 min TENS to peroneal nerve 5×/wk for 6 wks (15 h) + Sit-to-stand (STS) training, 15 min/day, 5×/wk for 6 wks (15 h) + therapy, 1 h a day, 5×/wk., for 6 wks, (total 52.5 h) | 20 | 11/9 | 10/10 | 56.2 (10.4) | 6.05 (2.70) months | 12/8 | 20 |
| Placebo TENS 30 min 5×/wk, 6 wks. (15 h) + STS training and therapy (total 52.5 h) | 21 | 12/8 | 11/9 | 56.3 (10.2) | 6.60 (2.50) months | 11/9 | 20 | |||
| Kluding and Santos (2008) [40] | Pilot RCT, n = 17 | Before and after 4-week training, no follow-up | 30 min, 2×/wk therapy for 4 wks functional training + contralesional ankle joint mobilizations (5 min) 2×/wk for 4 wks (40 min ankle mobilizations) (total 4.67 h.) | 8 | 4/4 | 4/4 | 55.5 (10.8) | 18.30 (11.8) months | Not stated | 8 |
| 2×/wk therapy (30 min) for 4 wks involving functional training (total 4 h) | 9 | 5/3 | 7/1 | 56.1 (13.7) | 24.60 (15.7) months | Not stated | 8 | |||
| Lynch et al. (2007) [14] | Pilot single-blind RCT, n = 21 | Prior to treatment and on completion of treatment, with 2-week follow-up | Daily 1-h group session+30–60 min/day individual therapy (according to need) +10, 30 min sensory retraining sessions for 2 wks (5 h sensory) (total 20 h.) | 10 | 7/3 | 5/5 | 61.0 (15.8) | 48.70 (31.1) days | 9/1 | 10 |
| Daily 1-h group session + 30–60 min/day individual therapy session + standing same time period (eyes closed) and 30 min of relaxation techniques (supine, eyes closed) (total 20 h) | 11 | 9/2 | 3/8 | 62.0 (12.3) | 47.80 (27.7) days | 9/2 | 11 | |||
| Ng and Hui-Chan (2009) [37] | Randomized, blinded placebo-controlled clinical trial (4 groups), n = 109 | At baseline, after 2 and 4 weeks of treatment, follow-up 4 weeks after | TENS + exercise (5×/wk (60 min) for 4 wks) (20 h TENS and 20 h exercise, total 40 h.) | 27 | 21/6 | 17/10 | 57.8 (7.3) | 4.70 (2.80) yrs | 11/16 | 26 |
| TENS (5×/wk. (60 min) for 4 wks) (total 20 h) | 28 | 24/4 | 18/10 | 56.5 (8.2) | 4.90 (3.90) yrs | 13/15 | 25 | |||
| Placebo stimulation + Exercise (total 40 h) | 25 | 20/5 | 13/12 | 56.9 (8.6) | 4.70 (3.40) yrs | 15/10 | 23 | |||
| Control (No treatment) | 29 | 20/9 | 20/9 | 55.5 (8.0) | 5.00 (3.00) yrs | 16/13 | 27 | |||
| Önal et al. (2022) [45] | RCT, n = 36 | At baseline, and post intervention, no follow-up | Conventional physical therapy (CPT) (5×/wk for 4 wks - three 45 min sessions and two 60 min CPT sessions), plus local vibration therapy (LVT) (80 Hz) to plantar region (both feet for 15 min 3×/wk) (3 h. LVT and 17 h. CPT) (total 20 h.) | 15 | 9/6 | 7/8 | 60(9) | 12(3–24) | 10/5 | 15 |
| CPT (5×/wk for 4 wks) (total 20 h) | 15 | 11/4 | 10/5 | 59(9) | 14 (6–39) | 7/8 | 15 | |||
| Paoloni et al. (2010) [44] | RCT, n = 44 | Before and after training, no follow-up | 50 min physical therapy session, (3 ×/wk for 4 wks + segmental muscle vibration 120 Hz (30 min each session) (Total 6 h vibration, 10 h physical therapy) (total 16 h) | 22 | 19/3 | 11/11 | 59.5 (13.3) | 1.90 (0.59) yrs | Not stated | 22 |
| 50 min physical therapy session, (3 ×/wk for 4 wks) (total 10 h) | 22 | 20/2 | 10/12 | 62.6 (9.5) | 1.86 (0.61) yrs | Not stated | 22 | |||
| Park et al. (2014) [32] | Single-blind RCT, n = 34 | One week before and one week after intervention, no follow-up | 30 min exercise with a physical therapist (5×/wk for 6 wks) + TENS (total 15 h TENS during 15 h exercise) (total 15 h) | 17 (but characteristics for 15) | 12/3 | 10/5 | 71.2 (3.46) | 18.70 (2.46) months | Not stated | 15 |
| 30 min exercise with physical therapist + placebo TENS (total 15 h placebo TENS during 15 h exercise) (total 15 h.) | 17 (but characteristics for 14) | 8/6 | 8/7 | 71.1(3.82) | 18.60 (1.70) months | Not stated | 14 | |||
| Suh et al. (2014) [38] | Single-blind RCT, n = 42 | Immediately before and one week after intervention, no follow-up | 30 min standard rehabilitation + electrical stimulation—60 min single session, interferential current (total 1 hr) | 21 | 15/6 | Not stated | 54.4 (12.1) | 15.05 (4.90) months | 14/6 | 21 |
| 30 min standard rehabilitation + sham electrical stimulation - one session, interferential current (total 1 h) | 21 | 14/7 | Not stated | 53.9 (12.4) | 13.90 (5.10) months | 15/5 | 21 | |||
| Wang et al. (2021) [43] | Single blind randomized clinical trial, n = 50 | At baseline, and 4 weeks from baseline, no follow-up | Conventional gait training (40 min once a day 5×/wk for 4 wks) + customized insoles (worn for a minimum of 1 hr daily) | 25 | 19/6 | 17/8 | 56.0 (range 49.5–66.5) | 130.36 (64.87) days | 13/12 | 25 |
| Conventional training (40 min once a day 5×/wk for 4 wks) (total 13.3 h) | 25 | 18/7 | 18/7 | 60.0 (range 54.0–65.0) | 123.08 (54.06) days | 16/9 | 25 | |||
| Yan and Hui-Chan (2009) [35] | Single blind stratified RCT, n = 62 | Prior to treatment, weekly during 3-week treatment, follow-up 8 weeks post-stroke | Standard rehabilitation (OT and PT) each 60 min + transcutaneous electrical stimulation (5×/wk. for 3 wks.) (TES) (total 15 h) | 21 | 9/10 | 11/8 | 68.4 (9.6) | 9.20 (4.40) days | 16/3 | 19 |
| Standard rehabilitation (OT and PT) each 60 min + Placebo stimulation (5×/wk for 3 wks) (total 15 h) | 21 | 10/9 | 11/8 | 72.8 (7.4) | 9.90 (2.60) days | 16/3 | 19 | |||
| Standard rehabilitation (OT and PT) each lasting for 60 min (5×/wk. for 3 wks. (total 15 h) | 20 | 9/9 | 11/7 | 70.4 (7.6) | 8.70 (3.30) days | 15/3 | 18 | |||
| Yen et al. (2019) [33] | Prospective, assessor-blinded pilot RCT, n = 42 | At baseline, at end of two-week intervention, with follow-up two weeks later | Standard rehabilitation (30 min 5×/wk. for 2 wks. +TENS (total 5 h) | 14 | 7/6 | Not stated | 58.4 (13.5) | 1.54 (0.78) days | 7/6 | 13 |
| Standard rehabilitation + NMES † (total 5 h) | 14 | 7/6 | Not stated | 61.6 (9.3) | 1.38 (0.51) days | 6/7 | 13 | |||
| Standard rehabilitation (30 min 5×/wk for 2 wks) (total 5 h) | 14 | 9/5 | Not stated | 61.4 (12.6) | 1.36 (0.50) days | 6/8 | 14 |
| Study | Outcome Measure | Group (n) | Baseline; Mean (SD) | Post-Treatment; Mean (SD) | Mean Difference (95% Confidence Interval) * | Standardized Effect Size |
|---|---|---|---|---|---|---|
| Manipulation of the surface beneath the feet to alter proprioceptive input | ||||||
| Bayouk et al. (2006) [29] | Ten-meter walk test (s) | 1. Experimental—task-orientated training on different surfaces (8) | 20.8 (8.3) | 18.3 (6.5) | –1.4 (–11.95, 9.15) $ # | d = 0.123 # |
| 2. Control—task orientated training hard surface (8) | 22.4 (13.8) | 19.7 (12.3) | ||||
| Ferreira et al. (2018) [31] | Mean velocity (m/s) | 1. Experimental—postural insoles (12) | 0.57 (0.15) | 0.57 (0.19) | 0.00 (–0.18, 0.18) | d = 0.000 |
| 2. Control—placebo insoles (8) | 0.61 (0.30) | 0.57 (0.19) | ||||
| Wang et al. (2021) [41] | Six-minute walk test (m) | 1. Experimental—conventional gait training and customized insoles (25; 24 analyzed) | Data unavailable | 64.68 (32.12) | 16.8 (–1.95, 35.55) $ | d = 0.527 |
| 2. Control—conventional gait training (25; 23 analyzed) | Data unavailable | 47.88 (31.67) | ||||
| Sensory retraining including sensorimotor foot stimulation and ankle mobilizations | ||||||
| Goliwas et al. (2015) [32] | Difference in weight distribution (eyes closed) (%) | 1. Experimental—standard rehabilitation and sensorimotor foot stimulation (8) | 26.9 (16.9) | 18.1 (17.3) | 1.60 (–15.88, 19.08) $ | d = 0.084 |
| 2. Control—standard rehabilitation (12) | 18.9 (20.9) | 16.5 (18.8) | ||||
| Kluding and Santos (2008) [35] | Peak weight bearing difference in sit-to-stand (%) | 1. Experimental—functional training and ankle joint mobilizations (8) | 20.59 (11.67) | 23.96 (13.04) | 7.19 (–7.00, 21.38) | d = 0.543 |
| 2. Control—functional training (9; 8 analyzed) | 26.28 (14.67) | 16.77 (13.42) | ||||
| Lynch et al. (2007) [13] | Ten-meter walk test (s) ** | 1. Experimental—group session and individual therapy plus sensory retraining (10) | 35 | 23 | 2 | — |
| 2. Control—group session and individual therapy and relaxation (11) | 26 | 21 | ||||
| Focal muscle vibration | ||||||
| Paoloni et al. (2010) [38] | Gait speed (m/s) | 1. Experimental—physical therapy and segmental muscle vibration (22) | 0.44 (0.13) | 0.53 (0.13) | 0.07 (–0.04, 0.18) $ | d = 0.400 |
| 2. Control—physical therapy (22) | 0.44 (0.21) | 0.46 (0.21) | ||||
| Önal et al. (2022) [37] | Ten-meter walk test (s) | 1. Experimental—plantar vibration therapy (18; 15 analyzed) | 27.83 (30.69) | 20.15 (18.74) | 3.38 (–8.15, 14.91) $ | d = 0.219 |
| 2. Control—conventional physical therapy (18; 15 analyzed) | 18.15 (11.07) | 16.77 (11.15) | ||||
| Taping | ||||||
| In et al. (2021) [33] | Ten-meter walk test (s) | 1. Experimental—sit-to-stand training and taping (20) | 25.74 (4.62) | 20.11 (4.40) | –3.11 (–6.01, 0.21) $ | d = 0.687 |
| 2. Control—sit-to-stand training (20) | 25.01 (4.40) | 23.22 (4.65) | ||||
| Electrical stimulation (TENS or interferential therapy) | ||||||
| Cho et al. (2013) [30] | Postural sway (eyes closed), (cm) | 1. Experimental—physical therapy and TENS (22) | 89.79 (21.78) | 69.05 (71.11) | –9.15 (–41.98, 23.68) $ | d = 0.178 |
| 2. Control—physical therapy and placebo TENS (20) | 85.31 (16.30) | 78.20 (15.17) | ||||
| Jung et al. (2017) [34] | Postural sway (eyes closed), (cm) | 1. Experimental—conventional therapy, sit-to-stand training and TENS (20) | 104.1 (35.9) | 77.6 (24.7) | –27.00 (–52.04, 1.96) $ | d = 0.690 |
| 2. Control—conventional therapy, sit-to-stand training and placebo TENS (21; 20 analyzed) | 117.7 (50.9) | 104.6 (49.5) | ||||
| Ng and Hui-Chan (2009) [36] | Gait velocity (cm/s) | 1. TENS (28; 25 analyzed) | 57.7 (26.3) | 60.9 (24.8) | 0.00 (–13.83, 13.83) 1 vs. 4 | f = 0.049 |
| 2. TENS + exercise (27; 26 analyzed) | 47.9 (26.8) | 66.6 (32.5) | 6.00 (–11.98, 23.98) 2 vs. 3$ | |||
| 3. Placebo stimulation + exercise (25; 23 analyzed) | 50.7 (24.5) | 60.6 (29.7) | ||||
| 4. Control (29; 27 analyzed) | 58.9 (24.9) | 60.9 (24.8) | ||||
| Park et al. (2014) [39] | Gait velocity (cm/s) | 1. Experimental—physical therapy and TENS (17; 15 analyzed) | 45.81 (15.22) | 52.89 (17.43) | 3.49 (–10.97, 17.95) $ | d = 0.183 |
| 2. Placebo—physical therapy and placebo TENS (17; 14 analyzed) | 46.85 (20.07) | 49.40 (20.50) | ||||
| Suh et al. (2014) [40] | Ten-meter walk test (s) | 1. Experimental—standard rehabilitation and interferential current (21) | 44.75 (18.40) | 37.74 (15.70) | –6.22 (–14.95, 2.51) $ | d = 0.446 |
| 2. Placebo—standard rehab and sham stimulation (21) | 45.93 (13.22) | 43.96 (12.04) | ||||
| Yan and Hui-Chan (2009) [42] | Timed up-and-go (s) | 1. Experimental—standard rehabilitation and TENS (21) | Data unavailable | 30.0 (13.5) | –11.10 (–30.59, 8.39) 1 vs. 2 $ | f = 0.181 |
| 2. Placebo—standard rehabilitation and placebo TENS (21) | Data unavailable | 41.1 (27.9) | –25.40 (–56.54, 5.74) 1 vs. 3 $ | |||
| 3. Control—standard rehabilitation (20) | Data unavailable | 55.4 (47.1) | ||||
| Yen et al. (2019) [43] | Postural Assessment Scale for Stroke | 1. TENS—standard rehabilitation and TENS (14; 13 analyzed) | 3.77 (2.35) | 31.38 (5.39) | 7.46 (1.50, 13.42) 1 vs. 2 $† | f = 1.984 |
| 2. NMES - standard rehabilitation and NMES (14; 13 analyzed) | 2.77 (1.01) | 23.92 (8.91) | 13.38 (7.61, 19.15) 1 vs. 3 $† | |||
| 3. Control—standard rehabilitation (14) | 3.21 (1.12) | 18.00 (8.65) | ||||
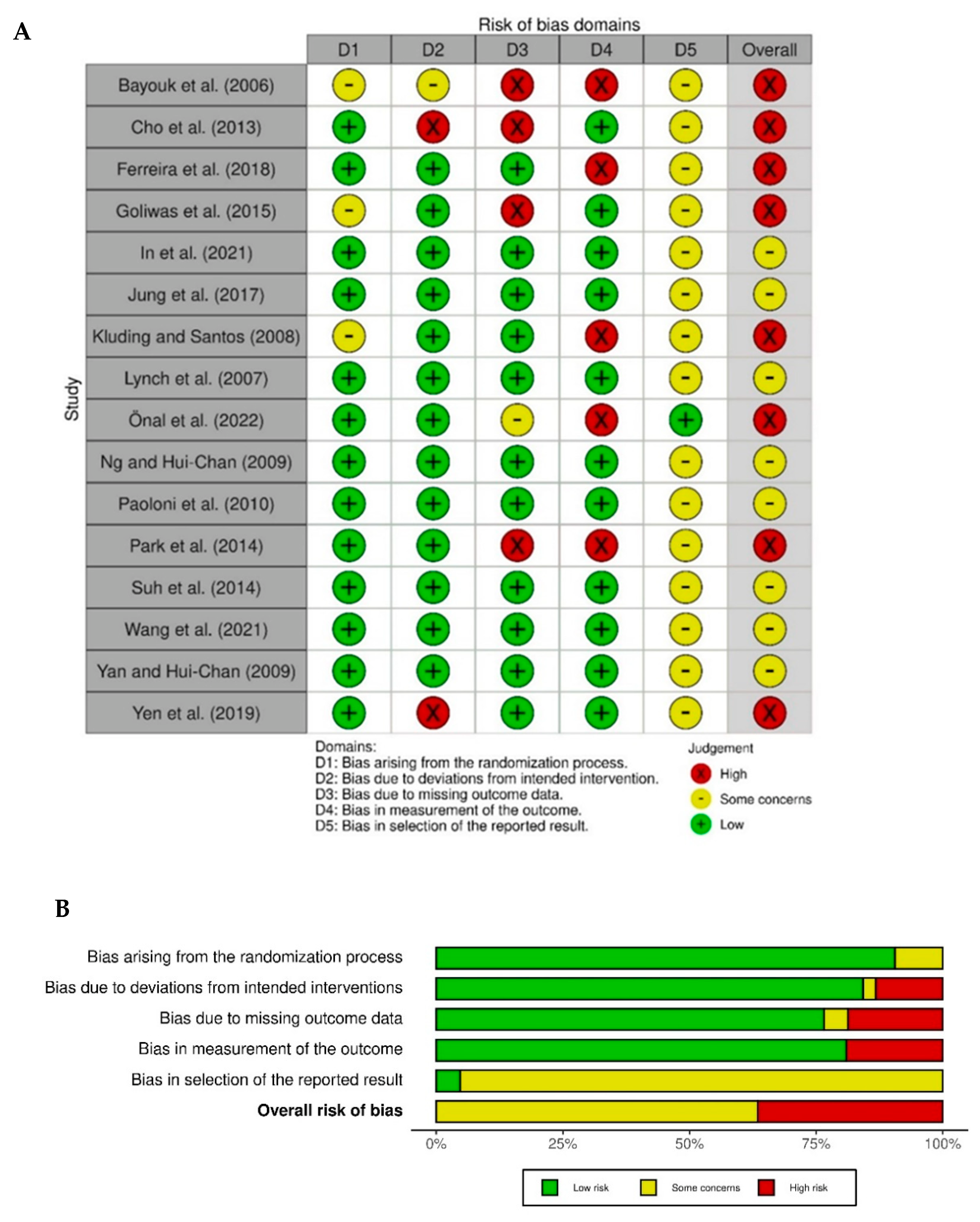
3.3. Quality Assessment
4. Discussion
Strengths and Limitations of Our Systematic Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Townsend, N.; Wickramasinghe, K.; Bhatnagar, P.; Smolina, K.; Nichols, M.; Leal, J.; Luengo-Fernandez, R.; Rayner, M. Coronary Heart Disease Statistics a Compendium of Health Statistics 2012 Edition. Available online: https://www.bhf.org.uk/informationsupport/publications/statistics/coronary-heart-disease-statistics-2012. (accessed on 5 July 2022).
- Kim, J.S.; Choi-Kwon, S. Discriminative sensory dysfunction after unilateral stroke. Stroke 1996, 27, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Gorst, T.; Rogers, A.; Morrison, S.C.; Cramp, M.; Paton, J.; Freeman, J.; Marsden, J. The prevalence, distribution, and functional importance of lower limb somatosensory impairments in chronic stroke survivors: A cross sectional observational study. Disabil. Rehabil. 2019, 41, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Tyson, S.F.; Crow, J.L.; Connell, L.; Winward, C.; Hillier, S. Sensory impairments of the lower limb after stroke: A pooled analysis of individual patient data. Top. Stroke Rehabil. 2013, 20, 441–449. [Google Scholar] [CrossRef]
- Chersi, F.; Ferrari, P.F.; Fogassi, L. Neuronal chains for actions in the parietal lobe: A computational model. PLoS ONE 2011, 6, e27652. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laaksonen, K.; Kirveskari, E.; Mäkelä, J.P.; Kaste, M.; Mustanoja, S.; Nummenmaa, L.; Tatlisumak, T.; Forss, N. Effect of afferent input on motor cortex excitability during stroke recovery. Clin. Neurophysiol. 2012, 123, 2429–2436. [Google Scholar] [CrossRef]
- Rossignol, S.; Dubuc, R.; Gossard, J.-P. Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 2006, 86, 89–154. [Google Scholar] [CrossRef]
- Patel, A.T.; Duncan, P.W.; Lai, S.; Studenski, S. The relation between impairments and functional outcomes poststroke. Arch. Phys. Med. Rehabil. 2000, 81, 1357–1363. [Google Scholar] [CrossRef]
- Annino, G.; Palazzo, F.; Lebone, P.; Caronti, A.; Lombardo, M.; Campoli, F.; Padua, E.; Iellamo, F. The efficacy of plantar stimulation on human balance control. Somatosens. Mot. Res. 2015, 32, 200–205. [Google Scholar] [CrossRef]
- Chien, J.H.; Ambati, V.N.P.; Huang, C.-K.; Mukherjee, M. Tactile stimuli affect long-range correlations of stride interval and stride length differently during walking. Exp. Brain Res. 2017, 235, 1185–1193. [Google Scholar] [CrossRef]
- Pillette, L.; Lotte, F.; N’Kaoua, B.; Joseph, P.-A.; Jeunet, C.; Glize, B. Why we should systematically assess, control and report somatosensory impairments in BCI-based motor rehabilitation after stroke studies. NeuroImage Clin. 2020, 28, 102417. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.M.; Matyas, T.A.; Oke, L.E. Sensory loss in stroke patients: Effective training of tactile and proprioceptive discrimination. Arch. Phys. Med. Rehabil. 1993, 74, 602–611. [Google Scholar] [CrossRef]
- Lynch, E.A.; Hillier, S.L.; Stiller, K.; Campanella, R.R.; Fisher, P.H. Sensory retraining of the lower limb after acute stroke: A randomized controlled pilot trial. Arch. Phys. Med. Rehabil. 2007, 88, 1101–1107. [Google Scholar] [CrossRef]
- Dechaumont-Palacin, S.; Marque, P.; De Boissezon, X.; Castel-Lacanal, E.; Carel, C.; Berry, I.; Pastor, J.; Albucher, J.F.; Chollet, F.; Loubinoux, I. Neural correlates of proprioceptive integration in the contralesional hemisphere of very impaired patients shortly after a subcortical stroke: An FMRI study. Neurorehabil. Neural Repair 2008, 22, 154–165. [Google Scholar] [CrossRef]
- Calafiore, D.; Negrini, F.; Tottoli, N.; Ferraro, F.; Ozyemisci-Taskiran, O.; de Sire, A. Efficacy of robotic exoskeleton for gait rehabilitation in patients with subacute stroke: A systematic review. Eur. J. Phys. Rehabil. Med. 2022, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.M.; Johansen-Berg, H.; Ward, N.; Kennedy, N.C.; Chandler, E.; Weir, C.J.; Rothwell, J.; Wing, A.M.; Grey, M.J.; Barton, G.; et al. Functional strength training and movement performance therapy for upper limb recovery early poststroke-efficacy, neural correlates, predictive markers, and cost-effectiveness: FAST-INdiCATE trial. Front. Neurol. 2018, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.M. Somatosensory loss after stroke. Crit. Rev. Phys. Rehabil. Med. 1995, 7, 51–91. [Google Scholar] [CrossRef]
- Schabrun, S.M.; Hillier, S. Evidence for the retraining of sensation after stroke: A systematic review. Clin. Rehabil. 2009, 23, 27–39. [Google Scholar] [CrossRef]
- Peters, D.M.; Fridriksson, J.; Stewart, J.C.; Richardson, J.D.; Rorden, C.; Bonilha, L.; Middleton, A.; Gleichgerrcht, E.; Fritz, S.L. Cortical disconnection of the ipsilesional primary motor cortex is associated with gait speed and upper extremity motor impairment in chronic left hemispheric stroke. Hum. Brain Mapp. 2018, 39, 120–132. [Google Scholar] [CrossRef]
- Schneider, W.; Shiffrin, R.M. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol. Rev. 1977, 84, 1–66. [Google Scholar] [CrossRef]
- Porter, R.; Lemon, R. Corticospinal Function and Voluntary Movement; Clarendon Press: Oxford, UK, 1995. [Google Scholar]
- Chia, F.S.F.; Kuys, S.; Low Choy, N. Sensory retraining of the leg after stroke: Systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M. Doing new research? Don’t forget the old. PLoS Med. 2004, 1, e35. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Akobeng, A.K. Principles of evidence based medicine. Arch. Dis. Child. 2005, 90, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Boland, A.; Cherry, M.G.; Dickson, R. Doing a Systematic Review. A students Guide, 2nd ed.; Sage: Thousand Oaks, CA, USA, 2017. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Park, J.; Seo, D.; Choi, W.; Lee, S. The effects of exercise with TENS on spasticity, balance, and gait in patients with chronic stroke: A randomized controlled trial. Med. Sci. Monit. 2014, 20, 1890–1896. [Google Scholar] [CrossRef]
- Yen, H.-C.; Chen, W.-S.; Jeng, J.-S.; Luh, J.-J.; Lee, Y.-Y.; Pan, G.-S. Standard early rehabilitation and lower limb transcutaneous nerve or neuromuscular electrical stimulation in acute stroke patients: A randomized controlled pilot study. Clin. Rehabil. 2019, 33, 1344–1354. [Google Scholar] [CrossRef]
- Cho, H.-Y.; In, T.S.; Cho, K.H.; Song, C.H. A single trial of transcutaneous electrical nerve stimulation (TENS) improves spasticity and balance in patients with chronic stroke. Tohoku J. Exp. Med. 2013, 229, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Hui-Chan, C. Transcutaneous electrical stimulation on acupuncture points improves muscle function in subjects after acute stroke: A randomized controlled trial. J. Rehabil. Med. 2009, 41, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-S.; In, T.-S.; Cho, H.-Y. Effects of sit-to-stand training combined with transcutaneous electrical stimulation on spasticity, muscle strength and balance ability in patients with stroke: A randomized controlled study. Gait Posture 2017, 54, 183–187. [Google Scholar] [CrossRef]
- Ng, S.S.M.; Hui-Chan, C.W.Y. Does the use of TENS increase the effectiveness of exercise for improving walking after stroke? A randomized controlled clinical trial. Clin. Rehabil. 2009, 23, 1093–1103. [Google Scholar] [CrossRef]
- Suh, H.R.; Han, H.C.; Cho, H.-y. Immediate therapeutic effect of interferential current therapy on spasticity, balance, and gait function in chronic stroke patients: A randomized control trial. Clin. Rehabil. 2014, 28, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Goliwas, M.; Kocur, P.; Furmaniuk, L.; Majchrzycki, M.; Wiernicka, M.; Lewandowski, J. Effects of sensorimotor foot training on the symmetry of weight distribution on the lower extremities of patients in the chronic phase after stroke. J. Phys. Ther. Sci. 2015, 27, 2925–2930. [Google Scholar] [CrossRef][Green Version]
- Kluding, P.M.; Santos, M. Effects of ankle joint mobilizations in adults poststroke: A pilot study. Arch. Phys. Med. Rehabil. 2008, 89, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Bayouk, J.; Boucher, J.P.; Leroux, A. Balance training following stroke: Effects of task-oriented exercises with and without altered sensory input. Int. J. Rehabil. Res. 2006, 29, 51–59. [Google Scholar] [CrossRef]
- Ferreira, L.A.B.; Cimolin, V.; Neto, H.P.; Grecco, L.; Lazzari, R.D.; Dumont, A.J.L.; Galli, M.; Oliveira, C.S. Effect of postural insoles on gait pattern in individuals with hemiparesis: A randomized controlled clinical trial. J. Bodyw. Mov. Ther. 2018, 22, 792–797. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, L.; Yu, L.; Wang, Y.; Taiar, R.; Zhang, Y.; Fu, W. Effect of customized insoles on gait in post-stroke hemiparetic individuals: A randomized controlled trial. Biology 2021, 10, 1187. [Google Scholar] [CrossRef]
- Paoloni, M.; Mangone, M.; Scettri, P.; Procaccianti, R.; Cometa, A.; Santilli, V. Segmental muscle vibration improves walking in chronic stroke patients with foot drop: A randomized controlled trial. Neurorehabil. Neural Repair 2010, 24, 254–262. [Google Scholar] [CrossRef]
- Önal, B.; Sertel, M.; Karaca, G. Effect of plantar vibration on static and dynamic balance in stroke patients: A randomised controlled study. J. Physiother. 2022, 116, 1–8. [Google Scholar] [CrossRef] [PubMed]
- In, T.-S.; Jung, J.-H.; Jung, K.-S.; Cho, H.-Y. Effect of sit-to-stand training combined with taping on spasticity, strength, gait speed and quality of life in patients with stroke: A randomized controlled trial. Life 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Lannin, N.A.; Borschmann, K.; English, C.; Ali, M.; Churilov, L.; Saposnik, G.; Winstein, C.; van Wegen, E.E.H.; Wolf, S.L.; et al. Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabil. Neural Repair 2017, 31, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Serrada, I.; Hordacre, B.; Hillier, S.L. Does sensory retraining improve sensation and sensorimotor function following stroke: A systematic review and meta-analysis. Front. Neurosci. 2019, 13, 402. [Google Scholar] [CrossRef]
- Sharififar, S.; Shuster, J.J.; Bishop, M.D. Adding electrical stimulation during standard rehabilitation after stroke to improve motor function. A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2018, 61, 339–344. [Google Scholar] [CrossRef]
- Laufer, Y.; Elboim-Gabyzon, M. Does sensory transcutaneous electrical stimulation enhance motor recovery following a stroke? A systematic review. Neurorehabil. Neural Repair 2011, 25, 799–809. [Google Scholar] [CrossRef]
- Marchal, G.; Serrati, C.; Rioux, P.; Petit-Taboué, M.C.; Viader, F.; de la Sayette, V.; Le Doze, F.; Lochon, P.; Derlon, J.M.; Orgogozo, J.M.; et al. PET imaging of cerebral perfusion and oxygen consumption in acute ischaemic stroke: Relation to outcome. Lancet 1993, 341, 925–927. [Google Scholar] [CrossRef]
- Tilson, J.K.; Sullivan, K.J.; Cen, S.Y.; Rose, D.K.; Koradia, C.H.; Azen, S.P.; Duncan, P.W. Meaningful gait speed improvement during the first 60 days poststroke: Minimal clinically important difference. Phys. Ther. 2010, 90, 196–208. [Google Scholar] [CrossRef]
- Perry, J.; Garrett, M.; Gronley, J.K.; Mulroy, S.J. Classification of walking handicap in the stroke population. Stroke 1995, 26, 982–989. [Google Scholar] [CrossRef]
- Hunter, S.M.; Crome, P.; Sim, J.; Pomeroy, V.M. Effects of mobilization and tactile stimulation on recovery of the hemiplegic upper limb: A series of replicated single-system studies. Arch. Phys. Med. Rehabil. 2008, 89, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.M.; Crome, P.; Sim, J.; Hunter, S.M. Effects of mobilization and tactile stimulation on chronic upper-limb sensorimotor dysfunction after stroke. Arch. Phys. Med. Rehabil. 2013, 94, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.M.; Hammett, L.; Ball, S.; Smith, N.; Anderson, C.; Clark, A.; Tallis, R.; Rudd, A.; Pomeroy, V.M. Dose-response study of mobilisation and tactile stimulation therapy for the upper extremity early after stroke: A phase I trial. Neurorehabil. Neural Repair 2011, 25, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, J.; Hayward, K.S.; Dancause, N.; Lannin, N.A.; Ward, N.S.; Nudo, R.J.; Farrin, A.; Churilov, L.; Boyd, L.A.; Jones, T.A.; et al. A stroke recovery trial development framework: Consensus-based core recommendations from the second Stroke Recovery and Rehabilitation Roundtable. Neurorehabil. Neural Repair 2019, 33, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Hummelsheim, H.; Hauptmann, B.; Neumann, S. Influence of physiotherapeutic facilitation techniques on motor evoked potentials in centrally paretic hand extensor muscles. Electroencephalogr. Clin. 1995, 97, 18–28. [Google Scholar] [CrossRef]
- Ali, M.; Ashburn, A.; Bowen, A.; Brodie, E.; Corr, S.; Drummond, A.; Edmans, J.; Gladman, J.; Kalra, L.; Langhorne, P.; et al. VISTA-Rehab: A resource for stroke rehabilitation trials. Int. J. Stroke 2010, 5, 447–452. [Google Scholar] [CrossRef]
- Aries, A.M.; Pomeroy, V.M.; Sim, J.; Read, S.; Hunter, S.M. Sensory stimulation of the foot and ankle early post-stroke: A pilot and feasibility study. Front. Neurol. 2021, 12, 675106. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Aveyard, H. Doing a Literature Review in Health and Social Care. A Practical Guide, 4th ed.; Open University Press: London, UK, 2018. [Google Scholar]

| Aspect | Keywords and Boolean Operators |
|---|---|
| Population | “stroke” OR “cerebrovascular accident” OR CVA OR “acquired brain injury” OR “traumatic brain injury” OR “head injury” OR “TBI” OR “ABI” OR hemiplegia OR hemiparesis OR “upper motor neuron lesion” |
| AND | |
| Intervention | Sens* OR stimulat* OR somatosens* OR propriocept* OR afferent OR mobilisation OR mobilization OR manipulat* |
| AND | |
| Site | Foot OR leg OR “lower limb” OR “lower extremity” |
| AND | |
| Outcome of interest | Walk* OR gait OR mobil* OR step OR stance OR ambulat* OR “weight bear* |
| AND | |
| Type of study | Randomised controlled trial OR “randomised controlled trial” OR randomized controlled trial OR “randomized controlled trial” |
| NOT | |
| Main exclusion (to focus the literature search) | “Functional electrical stimulation” OR functional electrical stimulation OR FES |
| Inclusion criteria |
|---|
| Adult stroke survivors aged ≥18 years |
| Somatosensory intervention involving sensory stimulation (mechanical or tactile, thermal, electrical for the purpose of sensory stimulation only, and proprioception) of the contralesional lower limb and/or foot |
| An appropriate control/placebo intervention |
| Gait and/or balance outcome measure |
| Randomized controlled trial |
| Published in English between 1 January 2002 and 31 March 2022 |
| Appropriate ethical approval |
| Exclusion criteria |
| Any other condition, or inability to separate a cohort of stroke participants from other reported conditions |
| Active or active-assisted movement, as part of the specific sensory intervention; e.g., proprioceptive neuromuscular facilitation (if a separate intervention was delivered equally to all groups, such as conventional therapy or task-orientated training, in addition to specific sensory training in one group, the study was considered appropriate for inclusion) |
| Acupuncture |
| Transcranial magnetic stimulation or transcranial direct-current stimulation |
| Visual or auditory stimulation or feedback only, including visual biofeedback |
| Conference abstracts or other ‘grey’ literature, including unpublished studies and theses |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aries, A.M.; Downing, P.; Sim, J.; Hunter, S.M. Effectiveness of Somatosensory Stimulation for the Lower Limb and Foot to Improve Balance and Gait after Stroke: A Systematic Review. Brain Sci. 2022, 12, 1102. https://doi.org/10.3390/brainsci12081102
Aries AM, Downing P, Sim J, Hunter SM. Effectiveness of Somatosensory Stimulation for the Lower Limb and Foot to Improve Balance and Gait after Stroke: A Systematic Review. Brain Sciences. 2022; 12(8):1102. https://doi.org/10.3390/brainsci12081102
Chicago/Turabian StyleAries, Alison M., Poppy Downing, Julius Sim, and Susan M. Hunter. 2022. "Effectiveness of Somatosensory Stimulation for the Lower Limb and Foot to Improve Balance and Gait after Stroke: A Systematic Review" Brain Sciences 12, no. 8: 1102. https://doi.org/10.3390/brainsci12081102
APA StyleAries, A. M., Downing, P., Sim, J., & Hunter, S. M. (2022). Effectiveness of Somatosensory Stimulation for the Lower Limb and Foot to Improve Balance and Gait after Stroke: A Systematic Review. Brain Sciences, 12(8), 1102. https://doi.org/10.3390/brainsci12081102






