Voxel-Mirrored Homotopic Connectivity Is Altered in Meibomian Gland Dysfunction Patients That Are Morbidly Obese
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. MRI Data Collections
2.3. fMRI Data Preconditioning
2.4. VMHC Data Analyses
2.5. Statistical Analyses
3. Results
3.1. Basic Information
3.2. VMHC Analysis Results
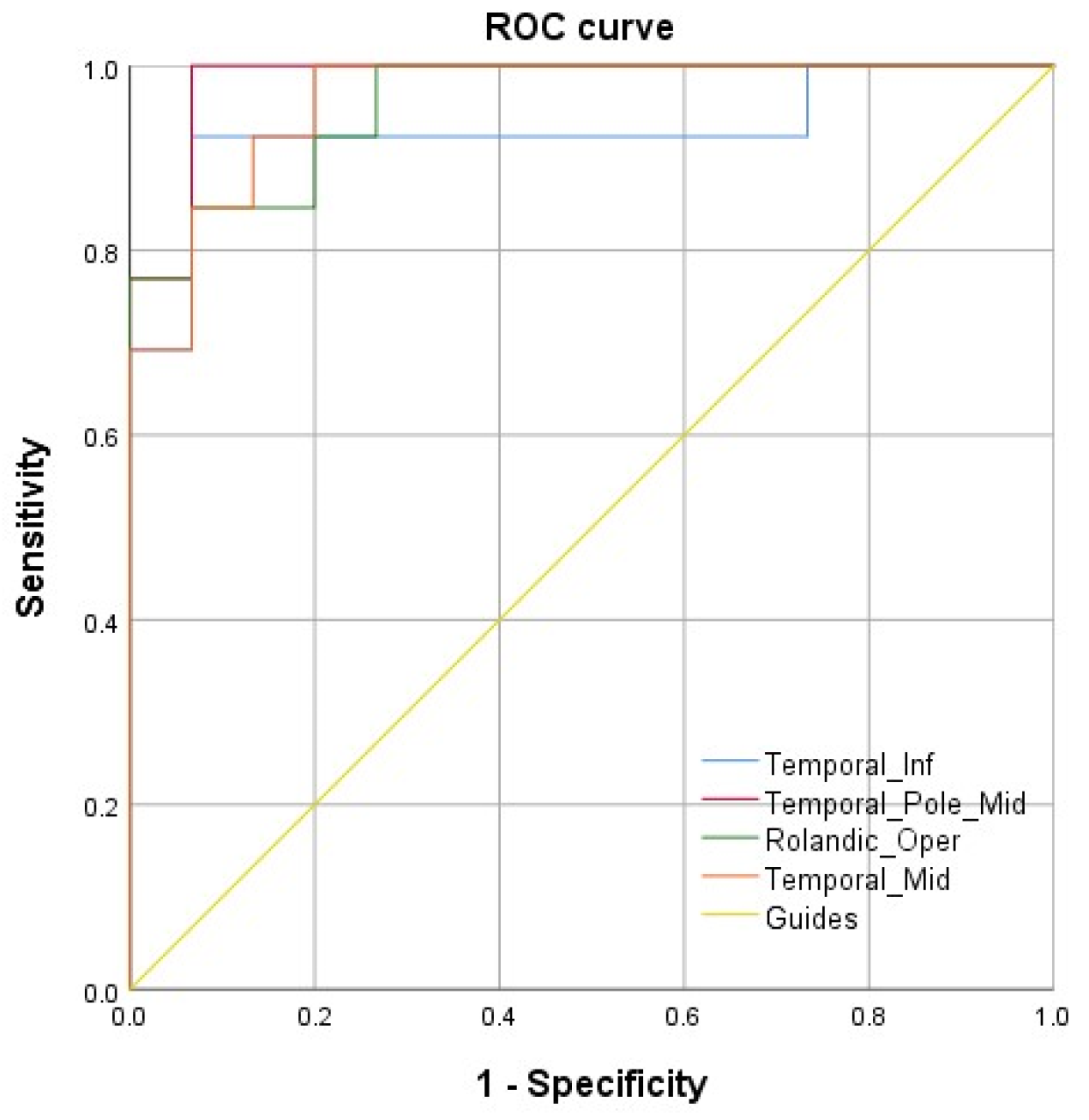
3.3. ROC Analyses
3.4. Correlation Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demaria, E.J. Bariatric surgery for morbid obesity. N. Engl. J. Med. 2007, 356, 2176–2183. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Krauss, R.M. American Heart Association call to action: Obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation 1998, 97, 2099–2100. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic complications of obesity. Endocrine 2000, 13, 155–165. [Google Scholar] [CrossRef]
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Lawrence, V.J.; Kopelman, P.G. Medical consequences of obesity. J. Clin. Endocrinol. Metab. 2004, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Kuwabara, R.; Niwa, K.; Hisatome, I.; Smits, G.; Roncal-Jimenez, C.A.; MacLean, P.S.; Yracheta, J.M.; Ohno, M.; Lanaspa, M.A.; et al. Different Risk for Hypertension, Diabetes, Dyslipidemia, and Hyperuricemia According to Level of Body Mass Index in Japanese and American Subjects. Nutrients 2018, 10, 1011. [Google Scholar] [CrossRef]
- Caulfield, L.E.; West, S.K.; Barron, Y.; Cid-Ruzafa, J. Anthropometric status and cataract: The Salisbury Eye Evaluation project. Am. J. Clin. Nutr. 1999, 69, 237–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bengtsson, B.; Heijl, A. A long-term prospective study of risk factors for glaucomatous visual field loss in patients with ocular hypertension. J. Glaucoma. 2005, 14, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Delcourt, C.; Michel, F.; Colvez, A.; Lacroux, A.; Delage, M.; Vernet, M.-H. Associations of cardiovascular disease and its risk factors with age-related macular degeneration: The POLA study. Ophthalmic Epidemiol. 2001, 8, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Bulpitt, C.J.; Hodes, C.; Everitt, M.G. Intraocular pressure and systemic blood pressure in the elderly. Br. J. Ophthalmol. 1975, 59, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.J.; Melton, L.J.; Dwyer, M.S.; Trautmann, J.C.; Chu, C.-P.; O’Fallon, W.M.; Palumbo, P.J. Risk factors for diabetic retinopathy: A population-based study in Rochester, Minnesota. Diabetes Care 1986, 9, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.D.; Donnenfeld, E.D. Dry eye diagnosis and management in 2004. Curr. Opin. Ophthalmol. 2004, 15, 299–304. [Google Scholar] [CrossRef]
- Krenzer, K.L.; Dana, M.R.; Ullman, M.D.; Cermak, J.M.; Tolls, D.B.; Evans, J.E.; Sullivan, D.A. Effect of androgen deficiency on the human meibomian gland and ocular surface. J. Clin. Endocrinol. Metab. 2000, 85, 4874–4882. [Google Scholar] [CrossRef]
- Jastaneiah, S.; Al-Rajhi, A.A. Association of aniridia and dry eyes. Ophthalmology 2005, 112, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.M.; Biser, S.A.; Perry, H.D.; Levinson, D.H.; Doshi, S.J.; Terraciano, A.; Donnenfeld, E.D. Conservative treatment of meibomian gland dysfunction. Eye Contact Lens 2004, 30, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Guliani, B.P.; Bhalla, A.; Naik, M.P. Association of the severity of meibomian gland dysfunction with dyslipidemia in Indian population. Indian J. Ophthalmol. 2018, 66, 1411–1416. [Google Scholar] [PubMed]
- Hu, J.Y.; Liao, Y.; Huang, C.H.; Wang, S.; Liu, Z.G. Association between meibomian gland dysfunction and body mass index in Chinese adults. Zhonghua Yi Xue Za Zhi 2021, 101, 2514–2518. [Google Scholar] [PubMed]
- Osae, E.A.; Steven, P.; Redfern, R.; Hanlon, S.; Smith, C.W.; Rumbaut, R.E.; Burns, A.R. Dyslipidemia and Meibomian Gland Dysfunction: Utility of Lipidomics and Experimental Prospects with a Diet-Induced Obesity Mouse Model. Int. J. Mol. Sci. 2019, 20, 3505. [Google Scholar] [CrossRef]
- Patel, P.S.; Buras, E.D.; Balasubramanyam, A. The role of the immune system in obesity and insulin resistance. J. Obes. 2013, 2013, 616193. [Google Scholar] [CrossRef]
- Bu, J.; Yu, J.; Wu, Y.; Cai, X.; Li, K.; Tang, L.; Jiang, N.; Jeyalatha, M.V.; Zhang, M.; Sun, H.; et al. Hyperlipidemia Affects Tight Junctions and Pump Function in the Corneal Endothelium. Am. J. Pathol. 2020, 190, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Wu, Y.; Cai, X.; Jiang, N.; Jeyalatha, M.V.; Yu, J.; He, X.; He, H.; Guo, Y.; Zhang, M.; et al. Hyperlipidemia induces meibomian gland dysfunction. Ocul. Surf. 2019, 17, 777–786. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Iozzo, P. Brain functional imaging in obese and diabetic patients. Acta. Diabetol. 2019, 56, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Laird, A.R.; Fox, P.M.; Eickhoff, S.B.; Turner, J.A.; Ray, K.L.; McKay, D.R.; Glahn, D.C.; Beckmann, C.F.; Smith, S.M.; Fox, P.T. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011, 23, 4022–4037. [Google Scholar] [CrossRef]
- Kamali, A.; Hasan, K.M.; Adapa, P.; Keser, A.R.A.; Lincoln, J.; Kramer, L.A. Distinguishing and quantification of the human visual pathways using high-spatial-resolution diffusion tensor tractography. Magn. Reson. Imaging 2014, 32, 796–803. [Google Scholar] [CrossRef]
- Fan, H.; Yang, X.; Zhang, J.; Chen, Y.; Li, T.; Ma, X. Analysis of voxel-mirrored homotopic connectivity in medication-free, current major depressive disorder. J. Affect. Disord. 2018, 240, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.N.; Kelly, C.; Di Martino, A.; Mennes, M.; Margulies, D.S.; Bangaru, S.; Grzadzinski, R.; Evans, A.C.; Zang, Y.-F.; Castellanos, F.X. Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. J. Neurosci. 2010, 30, 15034–15043. [Google Scholar] [CrossRef]
- Peng, J.; Yao, F.; Li, Q.; Ge, Q.; Shi, W.; Tang, L.; Pan, Y.; Liang, R.; Zhang, L.; Shao, Y. Alternations of interhemispheric functional connectivity in children with strabismus and amblyopia: A resting-state fMRI study. Sci. Rep. 2021, 11, 15059. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wei, R.; Huang, X.; Shi, W.-Q.; Yang, Q.-C.; Yuan, Q.; Zhu, P.-W.; Jiang, N.; Li, B.; Zhou, Q.; et al. Reduction in interhemispheric functional connectivity in the dorsal visual pathway in unilateral acute open globe injury patients: A resting-state fMRI study. Int. J. Ophthalmol. 2018, 11, 1056–1060. [Google Scholar]
- Shi, W.Q.; Liu, J.X.; Yuan, Q.; Ye, L.; Su, T.; Jiang, N.; Lin, Q.; Min, Y.-L.; Li, B.; Zhu, P.-W.; et al. Alternations of interhemispheric functional connectivity in corneal ulcer patients using voxel-mirrored homotopic connectivity: A resting state fMRI study. Acta Radiol. 2019, 60, 1159–1166. [Google Scholar] [CrossRef]
- Dong, Z.Z.; Zhu, F.Y.; Shi, W.Q.; Shu, Y.Q.; Chen, L.L.; Yuan, Q.; Lin, Q.; Zhu, P.W.; Liu, K.C.; Min, Y.L.; et al. Abnormalities of interhemispheric functional connectivity in individuals with acute eye pain: A resting-state fMRI study. Int. J. Ophthalmol. 2019, 12, 634–639. [Google Scholar] [PubMed]
- Mima, T.; Oluwatimilehin, T.; Hiraoka, T.; Hallett, M. Transient interhemispheric neuronal synchrony correlates with object recognition. J. Neurosci. 2001, 21, 3942–3948. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Bandt, S.K.; Werner, N.; Dines, J.; Rashid, S.; Eisenman, L.N.; Hogan, R.E.; Leuthardt, E.C.; Dowling, J. Trans-middle temporal gyrus selective amygdalohippocampectomy for medically intractable mesial temporal lobe epilepsy in adults: Seizure response rates, complications, and neuropsychological outcomes. Epilepsy. Behav. 2013, 28, 17–21. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Chen, W.; Tu, Y.; Hou, H.; Huang, X.; Chen, X.; Guo, Z.; Bai, G.; Cheng, W. The Abnormal Functional Connectivity between the Hypothalamus and the Temporal Gyrus Underlying Depression in Alzheimer’s Disease Patients. Front. Aging. Neurosci. 2018, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.J.; Gaffan, D.; Murray, E.A. Functional double dissociation between two inferior temporal cortical areas: Perirhinal cortex versus middle temporal gyrus. J. Neurophysiol. 1997, 77, 587–598. [Google Scholar] [CrossRef]
- Herath, P.; Kinomura, S.; Roland, P.E. Visual recognition: Evidence for two distinctive mechanisms from a PET study. Hum. Brain Mapp. 2001, 12, 110–119. [Google Scholar] [CrossRef]
- Convit, A.; de Asis, J.; de Leon, M.J.; Tarshish, C.Y.; Santi, S.D.; Rusinek, H. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer’s disease. Neurobiol. Aging 2000, 21, 19–26. [Google Scholar] [CrossRef]
- Tsapkini, K.; Frangakis, C.E.; Hillis, A.E. The function of the left anterior temporal pole: Evidence from acute stroke and infarct volume. Brain 2011, 134, 3094–3105. [Google Scholar] [CrossRef]
- Sato, W.; Kochiyama, T.; Uono, S.; Matsuda, K.; Usui, K.; Usui, N.; Inoue, Y.; Toichi, M. Motomi Toichi Gamma Oscillations in the Temporal Pole in Response to Eyes. PLoS ONE 2016, 11, e162039. [Google Scholar] [CrossRef] [PubMed]
- Wangbing, S.; Yuan, Y.; Chang, L.; Jing, L. The roles of the temporal lobe in creative insight: An integrated review. Think. Reason. 2017, 23, 321–375. [Google Scholar]
- Huang, X.; Cai, F.Q.; Hu, P.H.; Zhong, Y.L.; Zhang, Y.; Wei, R.; Pei, C.G.; Zhou, F.Q.; Shao, Y. Disturbed spontaneous brain-activity pattern in patients with optic neuritis using amplitude of low-frequency fluctuation: A functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 2015, 11, 3075–3083. [Google Scholar] [PubMed]
- Chhadva, P.; Goldhardt, R.; Galor, A. Meibomian Gland Disease: The Role of Gland Dysfunction in Dry Eye Disease. Ophthalmology 2017, 124, S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Tonkonogy, J.; Goodglass, H. Language function, foot of the third frontal gyrus, and rolandic operculum. Arch. Neurol. 1981, 38, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Indefrey, P.; Brown, C.M.; Hellwig, F.; Hagoort, P. A neural correlate of syntactic encoding during speech production. Proc. Natl. Acad. Sci. USA 2001, 98, 5933–5936. [Google Scholar] [CrossRef]
- Wu, C.; Zheng, Y.; Li, J.; Wu, H.; She, S.; Liu, S.; Ning, Y.; Li, L. Brain substrates underlying auditory speech priming in healthy listeners and listeners with schizophrenia. Psychol. Med. 2017, 47, 837–852. [Google Scholar] [CrossRef]
- Verma, G.; Woo, J.H.; Chawla, S.; Wang, S.; Sheriff, S.; Elman, L.B.; McCluskey, L.F.; Grossman, M.; Melhem, E.R.; Maudsley, A.A.; et al. Whole-brain analysis of amyotrophic lateral sclerosis by using echo-planar spectroscopic imaging. Radiology 2013, 267, 851–857. [Google Scholar] [CrossRef]
- Subira, M.; Alonso, P.; Segalas, C.; Real, E.; López-Solà, C.; Pujol, J.; Martínez-Zalacaín, L.; Harrison, B.J.; Menchón, J.M.; Cardoner, N.; et al. Brain structural alterations in obsessive-compulsive disorder patients with autogenous and reactive obsessions. PLoS ONE 2013, 8, e75273. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, O.A.; Veltman, D.J.; Groenewegen, H.J.; Cath, D.C.; van Balkomvan, A.J.L.M.; van Hartskamp, J.; Barkhof, F.; van Dyck, R. Frontal-striatal dysfunction during planning in obsessive-compulsive disorder. Arch. Gen. Psychiatry 2005, 62, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Huang, Y.R.; Liu, J.L.; Zhang, F.Q.; Zhang, B.Y.; Wu, J.C.; Ma, Y.; Xia, L.; Hao, Y.; Huo, J.W. Aberrant resting-state cerebral blood flow and its connectivity in primary dysmenorrhea on arterial spin labeling MRI. Magn. Reason. Imaging 2020, 73, 84–90. [Google Scholar] [CrossRef]
- Petro, N.M.; Gruss, L.F.; Yin, S.; Huang, H.; Miskovic, V.; Ding, M.; Kell, A. Multimodal Imaging Evidence for a Frontoparietal Modulation of Visual Cortex during the Selective Processing of Conditioned Threat. J. Cogn. Neurosci. 2017, 29, 953–967. [Google Scholar] [CrossRef]
- Ratan, D.B.; Sina, Z.M.; Hiroshi, O.; Carter, S.O.; James, T.R.; Elysa, W. Diffusion tensor tractography detection of functional pathway for the spread of epileptiform activity between temporal lobe and Rolandic region. Child’s Nerv. Syst. 2010, 26, 185–190. [Google Scholar]
- Lin, Y.C.; Shih, Y.C.; Tseng, W.Y.; Chu, Y.H.; Wu, M.T.; Chen, T.F.; Tang, P.F.; Chiu, M.J. Cingulum correlates of cognitive functions in patients with mild cognitive impairment and early Alzheimer’s disease: A diffusion spectrum imaging study. Brain Topogr. 2014, 27, 393–402. [Google Scholar] [CrossRef]
- Delano-Wood, L.; Stricker, N.H.; Sorg, S.F.; Nation, D.A.; Jak, A.J.; Woods, S.P.; Libon, D.J.; Delis, D.C.; Frank, L.R.; Lawrence, R.; et al. Posterior cingulum white matter disruption and its associations with verbal memory and stroke risk in mild cognitive impairment. J. Alzheimers Dis. 2012, 29, 589–603. [Google Scholar] [CrossRef]
- Takahashi, M.; Iwamoto, K.; Fukatsu, H.; Naganawa, S.; Iidaka, T.; Ozaki, N. White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: A diffusion tensor imaging study. Neurosci. Lett. 2010, 477, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Shinoura, N.; Yamada, R.; Tabei, Y.; Shiiode, T.; Itoi, C.; Saito, S.; Midorikawa, A. The right dorsal anterior cingulate cortex may play a role in anxiety disorder and visual function. Neurol. Res. 2013, 35, 65–70. [Google Scholar] [CrossRef]
- Tan, G.; Huang, X.; Ye, L.; Wu, A.; He, L.X.; Zhong, Y.L.; Jiang, N.; Zhou, F.Q.; Shao, Y. Altered spontaneous brain activity patterns in patients with unilateral acute open globe injury using amplitude of low-frequency fluctuation: A functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 2016, 12, 2015–2020. [Google Scholar] [PubMed]
- Shi, W.Q.; He, Y.; Li, Q.H.; Tang, L.Y.; Li, B.; Lin, Q.; Min, Y.L.; Yuan, Q.; Zhu, P.W.; Liang, R.B. Central network changes in patients with advanced monocular blindness: A voxel-based morphometric study. Brain Behav. 2019, 9, e1421. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, M.; Weed, E.; Ostergaard, L.; Mouridsen, K.; Roepstorff, A. Accessing the mental space-Spatial working memory processes for language and vision overlap in precuneus. Hum. Brain Mapp. 2008, 29, 524–532. [Google Scholar] [CrossRef]
- Horvath, A.; Kiss, M.; Szucs, A.; Kamondi, A. Precuneus-Dominant Degeneration of Parietal Lobe Is at Risk of Epilepsy in Mild Alzheimer’s Disease. Front. Neurol. 2019, 10, 878. [Google Scholar] [CrossRef]
- Xiao, J.X.; Xie, S.; Ye, J.T.; Liu, H.H.; Gan, X.L.; Gong, G.L.; Jiang, X.X. Detection of abnormal visual cortex in children with amblyopia by voxel-based morphometry. Am. J. Ophthalmol. 2007, 143, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gu, L.; Bao, D.; Hong, S.; He, W.; Tan, Y.; Zeng, X.; Gong, H.; Zhang, D.; Zhou, F. Altered homotopic connectivity in postherpetic neuralgia: A resting state fMRI study. J. Pain Res. 2016, 9, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, G.W.; Yu, F.X.; Liu, Y.; Li, M.Y.; Wang, Z.; Ding, H.Y.; Li, X.S.; Wang, H.; Jin, M.; et al. Abnormal Regional Neural Activity and Reorganized Neural Network in Obesity: Evidence from Resting-State fMRI. Obesity 2020, 28, 1283–1291. [Google Scholar] [CrossRef]
- Syan, S.K.; Mcintyre-Wood, C.; Minuzzi, L.; Hall, G.; McCabe, R.E.; MacKillop, J. Dysregulated resting state functional connectivity and obesity: A systematic review. Neurosci. Biobehav. Rev. 2021, 131, 270–292. [Google Scholar] [CrossRef] [PubMed]
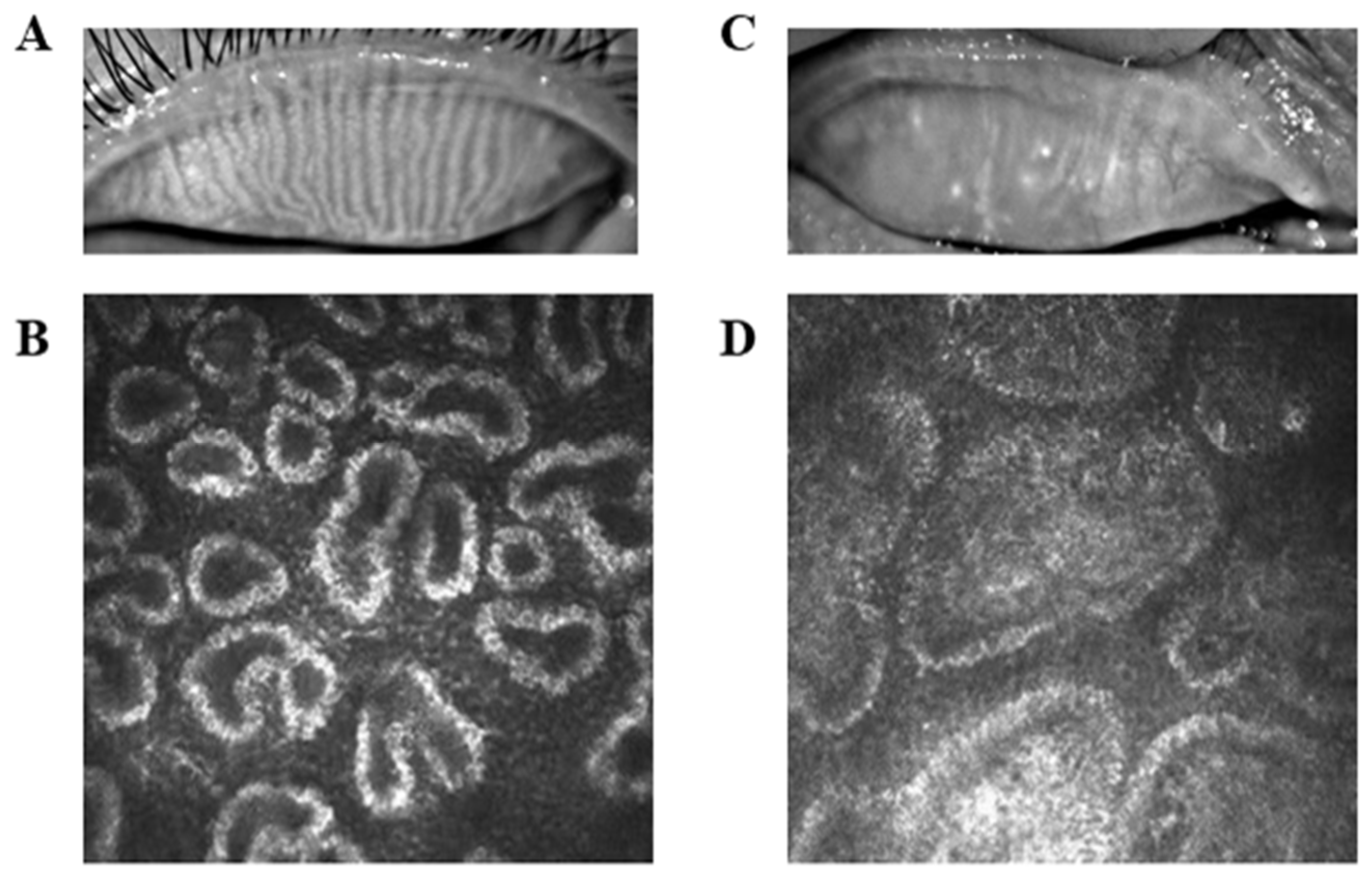
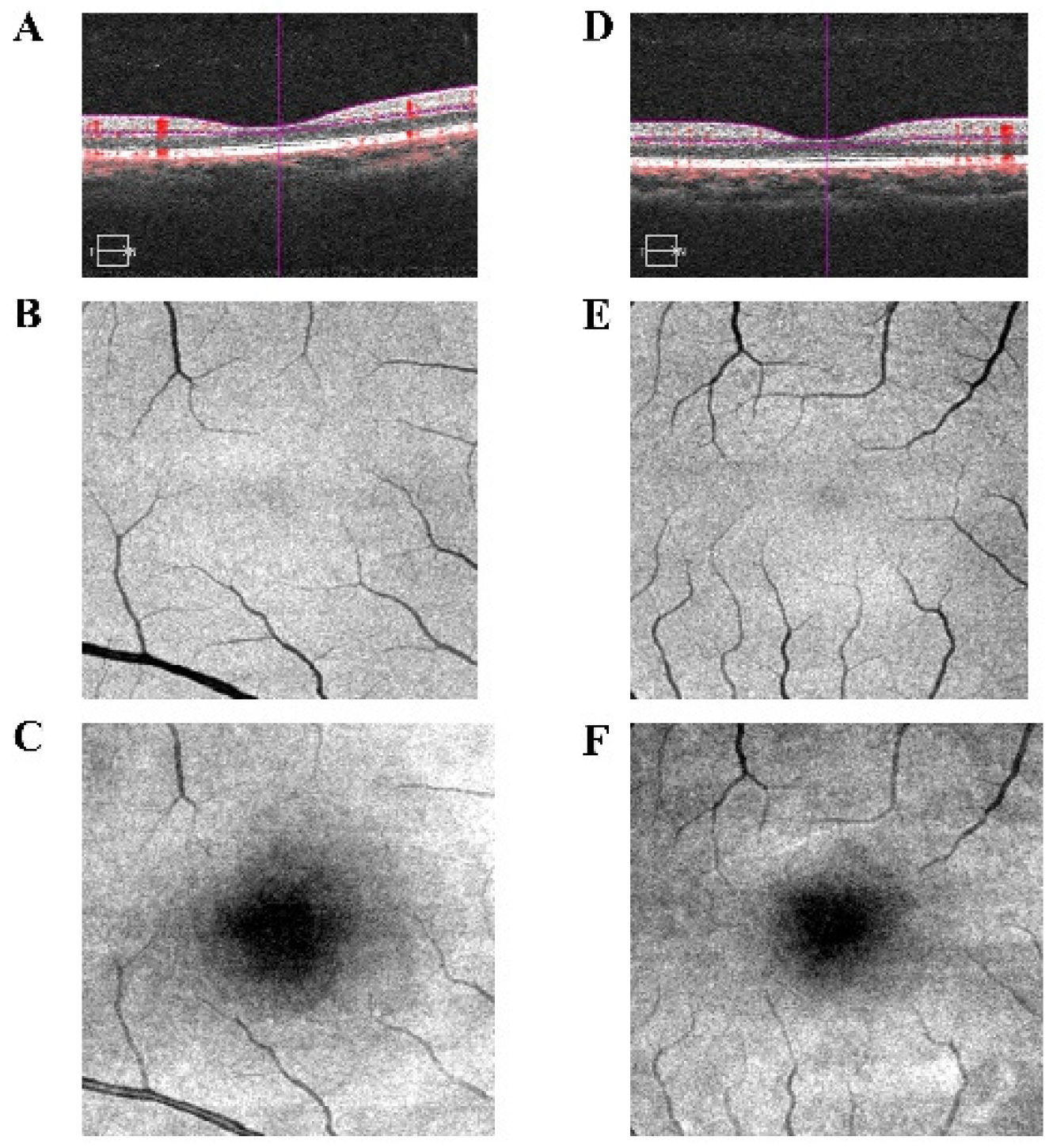




| Condition | PAT | HCs | t-Value | p-Value |
|---|---|---|---|---|
| Male/female | 4/8 | 6/6 | N/A | 0.680 |
| Age (years) | 34.25 ± 7.38 | 31.67 ± 6.24 | 0.925 | 0.365 |
| VA-L (log MAR) | 0.80 ± 0.17 | 0.23 ± 3.54 | 10.972 | <0.001 |
| VA-R (log MAR) | 0.83 ± 0.23 | 0.21 ± 0.08 | 8.867 | <0.001 |
| Blood pressure | ||||
| SP (mmHg) | 126.67 ± 11.85 | 130.50 ± 10.83 | −0.827 | 0.417 |
| DP(mmHg) | 82.41 ± 7.67 | 76.17 ± 10.57 | 1.658 | 0.112 |
| MMSE score | 21.42 ± 4.56 | 27.83 ± 2.52 | −4.266 | <0.001 |
| TG | 2.41 ± 1.20 | 1.62 ± 0.29 | 2.231 | 0.045 |
| Brain Areas | MNI Coordinates | BA | Peak Voxels | t-Value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| HCs > PAT | ||||||
| Temporal_Inf | −57 | −36 | −27 | 37 | 301 | 8.85 |
| Temporal_Pole_Mid | 57 | 3 | −36 | 21 | 153 | 6.59 |
| Rolandic_Oper | 45 | −18 | 15 | 13 | 477 | 6.55 |
| Temporal_Mid | −27 | −78 | 3 | 22 | 125 | 5.29 |
| HCs < PAT | ||||||
| Cingulum_Ant | 3 | 0 | 9 | 32 | 554 | −11.23 |
| Precuneus | −3 | −63 | 33 | 7 | 735 | −9.58 |
| Brain Region | Brain Function | Prospective Result |
|---|---|---|
| HCs > PAT | ||
| Temporal_Inf | Superior perceptual processing; Visual comprehensions; Visual object recognition. | Visual pathway damaged. |
| Temporal_Pole_Mid | Relationship between object naming and auditory comprehension; Later period of eye presence. | Abnormal eye movement. |
| Rolandic_Oper | Linguistic process; Utterance syntax encoding. | Possible impairment of speech function; Being influenced by temporal gyrus. |
| Temporal_Mid | Association processes; Construction of novel and useful information; Selective processing of conditioned threat in the visual cortex. | Impaired cognitive function. |
| HCs < PAT | ||
| Cingulum_Ant | Advanced activities (e.g., cognition, memory, and attention retention); Visual cognition. | Visual impairment. |
| Precuneus | Part of DMN; Visual network composition; Regulation of visually relevant spatial imaging and visual motion | Visual impairment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.-D.; Shu, H.-Y.; Liu, L.-Q.; Li, S.-Q.; Liao, X.-L.; Pan, Y.-C.; Su, T.; Zhang, L.-J.; Kang, M.; Ying, P.; et al. Voxel-Mirrored Homotopic Connectivity Is Altered in Meibomian Gland Dysfunction Patients That Are Morbidly Obese. Brain Sci. 2022, 12, 1078. https://doi.org/10.3390/brainsci12081078
Shi Y-D, Shu H-Y, Liu L-Q, Li S-Q, Liao X-L, Pan Y-C, Su T, Zhang L-J, Kang M, Ying P, et al. Voxel-Mirrored Homotopic Connectivity Is Altered in Meibomian Gland Dysfunction Patients That Are Morbidly Obese. Brain Sciences. 2022; 12(8):1078. https://doi.org/10.3390/brainsci12081078
Chicago/Turabian StyleShi, Yi-Dan, Hui-Ye Shu, Li-Qi Liu, Shi-Qi Li, Xu-Lin Liao, Yi-Cong Pan, Ting Su, Li-Juan Zhang, Min Kang, Ping Ying, and et al. 2022. "Voxel-Mirrored Homotopic Connectivity Is Altered in Meibomian Gland Dysfunction Patients That Are Morbidly Obese" Brain Sciences 12, no. 8: 1078. https://doi.org/10.3390/brainsci12081078
APA StyleShi, Y.-D., Shu, H.-Y., Liu, L.-Q., Li, S.-Q., Liao, X.-L., Pan, Y.-C., Su, T., Zhang, L.-J., Kang, M., Ying, P., & Shao, Y. (2022). Voxel-Mirrored Homotopic Connectivity Is Altered in Meibomian Gland Dysfunction Patients That Are Morbidly Obese. Brain Sciences, 12(8), 1078. https://doi.org/10.3390/brainsci12081078






