Retrospective Multicenter Study on Outcome Measurement for Dyskinesia Improvement in Parkinson’s Disease Patients with Pallidal and Subthalamic Nucleus Deep Brain Stimulation
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Surgical Procedure
2.3. Assessments
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Patients
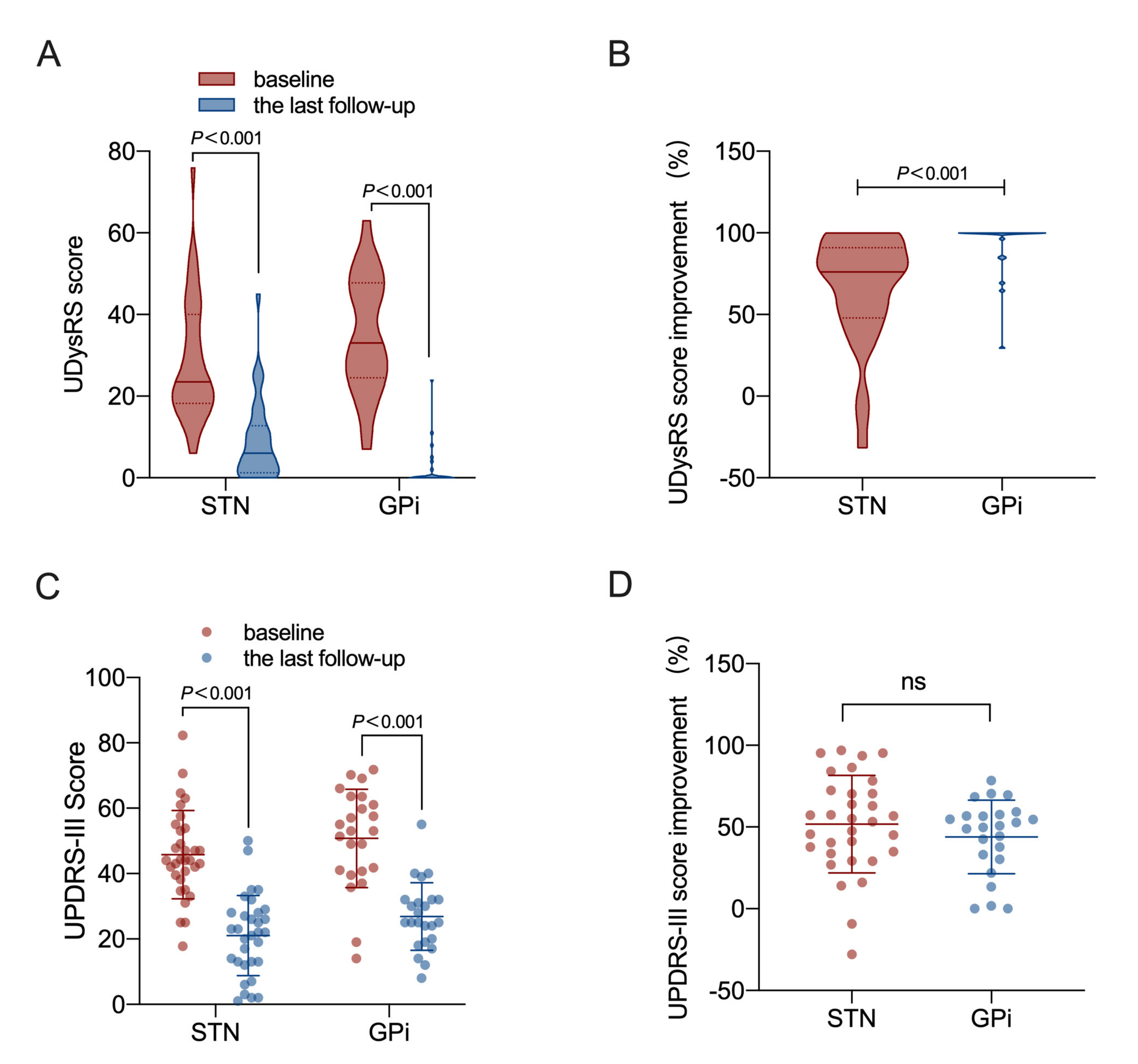
3.2. Outcome of UDysRS Scale
3.3. Outcome of the Med Off MDS-UPDRS-III
3.4. Outcome of the Levodopa Equivalent Daily Dose (LEDD)
3.5. A Grouping Approach for Dyskinesia Based on the UDysRS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Zesiewicz, T.A.; Sullivan, K.L.; Hauser, R.A. Levodopa-induced dyskinesia in Parkinson’s disease: Epidemiology, etiology, and treatment. Curr. Neurol. Neurosci. Rep. 2007, 7, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Morgante, F.; Merola, A.; Fasano, A.; Marsili, L.; Fox, S.H.; Bezard, E.; Picconi, B.; Calabresi, P.; Lang, A.E. Levodopa-induced dyskinesia in Parkinson disease: Current and evolving concepts. Ann. Neurol. 2018, 84, 797–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Sorbo, F.; Albanese, A. Levodopa-induced dyskinesias and their management. J. Neurol. 2008, 255 (Suppl. 4), 32–41. [Google Scholar] [CrossRef]
- Ahlskog, J.E.; Muenter, M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. Off. J. Mov. Disord. Soc. 2001, 16, 448–458. [Google Scholar] [CrossRef]
- Olanow, C.W.; Koller, W.C. An algorithm (decision tree) for the management of Parkinson’s disease: Treatment guidelines. American Academy of Neurology. Neurology 1998, 50, S1–S57. [Google Scholar] [CrossRef]
- Anderson, V.C.; Burchiel, K.J.; Hogarth, P.; Favre, J.; Hammerstad, J.P. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch. Neurol. 2005, 62, 554–560. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Jenner, P.; Antonini, A. Should there be less emphasis on levodopa-induced dyskinesia in Parkinson’s disease? Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 816–819. [Google Scholar] [CrossRef]
- Vitek, J.L.; Jain, R.; Chen, L.; Tröster, A.I.; Schrock, L.E.; House, P.A.; Giroux, M.L.; Hebb, A.O.; Farris, S.M.; Whiting, D.M.; et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson’s disease (INTREPID): A multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol. 2020, 19, 491–501. [Google Scholar] [CrossRef]
- Chiou, S.M.; Lin, Y.C.; Huang, H.M. One-year Outcome of Bilateral Subthalamic Stimulation in Parkinson Disease: An Eastern Experience. World Neurosurg. 2015, 84, 1294–1298. [Google Scholar] [CrossRef]
- Follett, K.A.; Weaver, F.M.; Stern, M.; Hur, K.; Harris, C.L.; Luo, P.; Marks, W.J., Jr.; Rothlind, J.; Sagher, O.; Moy, C.; et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2010, 362, 2077–2091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, F.M.; Follett, K.A.; Stern, M.; Luo, P.; Harris, C.L.; Hur, K.; Marks, W.J., Jr.; Rothlind, J.; Sagher, O.; Moy, C.; et al. Randomized trial of deep brain stimulation for Parkinson disease: Thirty-six-month outcomes. Neurology 2012, 79, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holewijn, R.A.; Verbaan, D.; van den Munckhof, P.M.; Bot, M.; Geurtsen, G.J.; Dijk, J.M.; Odekerken, V.J.; Beudel, M.; de Bie, R.M.A.; Schuurman, P.R. General Anesthesia vs Local Anesthesia in Microelectrode Recording-Guided Deep-Brain Stimulation for Parkinson Disease: The GALAXY Randomized Clinical Trial. JAMA Neurol. 2021, 78, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Oshima, H.; Kano, T.; Kobayashi, K.; Fukaya, C.; Yamamoto, T. Direct effect of subthalamic nucleus stimulation on levodopa-induced peak-dose dyskinesia in patients with Parkinson’s disease. Stereotact. Funct. Neurosurg. 2006, 84, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Follett, K.A. Comparison of pallidal and subthalamic deep brain stimulation for the treatment of levodopa-induced dyskinesias. Neurosurg. Focus 2004, 17, E3. [Google Scholar] [CrossRef]
- Fan, S.Y.; Wang, K.L.; Hu, W.; Eisinger, R.S.; Han, A.; Han, C.L.; Wang, Q.; Michitomo, S.; Zhang, J.G.; Wang, F.; et al. Pallidal versus subthalamic nucleus deep brain stimulation for levodopa-induced dyskinesia. Ann. Clin. Transl. Neurol. 2020, 7, 59–68. [Google Scholar] [CrossRef]
- Juhász, A.; Deli, G.; Aschermann, Z.; Janszky, J.; Harmat, M.; Makkos, A.; Kovács, M.; Komoly, S.; Balás, I.; Dóczi, T.; et al. How Efficient Is Subthalamic Deep Brain Stimulation in Reducing Dyskinesia in Parkinson’s Disease? Eur. Neurol. 2017, 77, 281–287. [Google Scholar] [CrossRef]
- Tinazzi, M.; Erro, R.; Mascia, M.M.; Esposito, M.; Ercoli, T.; Ferrazzano, G.; Di Biasio, F.; Pellicciari, R.; Eleopra, R.; Bono, F.; et al. Demographic and clinical determinants of neck pain in idiopathic cervical dystonia. J. Neural Transm. 2020, 127, 1435–1439. [Google Scholar] [CrossRef]
- Ali, K.; Morris, H.R. Parkinson’s disease: Chameleons and mimics. Pract. Neurol. 2015, 15, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Simonin, C.; Tir, M.; Devos, D.; Kreisler, A.; Dujardin, K.; Salleron, J.; Delval, A.; Blond, S.; Defebvre, L.; Destee, A.; et al. Reduced levodopa-induced complications after 5 years of subthalamic stimulation in Parkinson’s disease: A second honeymoon. J. Neurol. 2009, 256, 1736–1741. [Google Scholar] [CrossRef]
- Herrington, T.M.; Cheng, J.J.; Eskandar, E.N. Mechanisms of deep brain stimulation. J. Neurophysiol. 2016, 115, 19–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohodich, A.E.; Yalamanchili, H.; Raman, A.T.; Wan, Y.W.; Gundry, M.; Hao, S.; Jin, H.; Tang, J.; Liu, Z.; Zoghbi, H.Y. Forniceal deep brain stimulation induces gene expression and splicing changes that promote neurogenesis and plasticity. Elife 2018, 7, e34031. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Chen, J.; Zhou, H.; Tan, S. Electrical Stimulation Elicits Neural Stem Cells Activation: New Perspectives in CNS Repair. Front. Hum. Neurosci. 2015, 9, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunc-Ozcan, E.; Brooker, S.M.; Bonds, J.A.; Tsai, Y.H.; Rawat, R.; McGuire, T.L.; Peng, C.Y.; Kessler, J.A. Hippocampal BMP signaling as a common pathway for antidepressant action. Cell. Mol. Life Sci. 2021, 79, 31. [Google Scholar] [CrossRef]
- Gajera, C.R.; Emich, H.; Lioubinski, O.; Christ, A.; Beckervordersandforth-Bonk, R.; Yoshikawa, K.; Bachmann, S.; Christensen, E.I.; Götz, M.; Kempermann, G.; et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J. Cell Sci. 2010, 123, 1922–1930. [Google Scholar] [CrossRef] [Green Version]
- Malave, L.; Zuelke, D.R.; Uribe-Cano, S.; Starikov, L.; Rebholz, H.; Friedman, E.; Qin, C.; Li, Q.; Bezard, E.; Kottmann, A.H. Dopaminergic co-transmission with sonic hedgehog inhibits abnormal involuntary movements in models of Parkinson’s disease and L-Dopa induced dyskinesia. Commun. Biol. 2021, 4, 1071. [Google Scholar] [CrossRef]
- Kur, E.; Christa, A.; Veth, K.N.; Gajera, C.R.; Andrade-Navarro, M.A.; Zhang, J.; Willer, J.R.; Gregg, R.G.; Abdelilah-Seyfried, S.; Bachmann, S.; et al. Loss of Lrp2 in zebrafish disrupts pronephric tubular clearance but not forebrain development. Dev. Dyn. 2011, 240, 1567–1577. [Google Scholar] [CrossRef] [Green Version]
- Zhen, J.; Qian, Y.; Fu, J.; Su, R.; An, H.; Wang, W.; Zheng, Y.; Wang, X. Deep Brain Magnetic Stimulation Promotes Neurogenesis and Restores Cholinergic Activity in a Transgenic Mouse Model of Alzheimer’s Disease. Front. Neural Circuits 2017, 11, 48. [Google Scholar] [CrossRef]
- Mestre, T.A.; Beaulieu-Boire, I.; Aquino, C.C.; Phielipp, N.; Poon, Y.Y.; Lui, J.P.; So, J.; Fox, S.H. What is a clinically important change in the Unified Dyskinesia Rating Scale in Parkinson’s disease? Parkinsonism Relat. Disord. 2015, 21, 1349–1354. [Google Scholar] [CrossRef]
- Li, J.; Mei, S.; Jia, X.; Zhang, Y. Evaluation of the Direct Effect of Bilateral Deep Brain Stimulation of the Subthalamic Nucleus on Levodopa-Induced On-Dyskinesia in Parkinson’s Disease. Front. Neurol. 2021, 12, 595741. [Google Scholar] [CrossRef]
- Goetz, C.G.; Nutt, J.G.; Stebbins, G.T. The Unified Dyskinesia Rating Scale: Presentation and clinimetric profile. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
| Variable | STN | GPi | p Value |
|---|---|---|---|
| Number | 32 | 24 | |
| Gender (M/F) | 9/23 | 11/13 | 0.171 |
| Age of onset (year) | 47.8 ± 10.7 | 47.9 ± 9.5 | 0.769 |
| Duration of disease at DBS (year) | 9.5 ± 7.3 | 12.1 ± 3.7 | 0.142 |
| Age at DBS (year) | 59.3 ± 9.9 | 60.0 ± 8.5 | 0.268 |
| Levodopa equivalent daily dose (mg/day) | 871.6 ± 364.1 | 973.9 ± 418.5 | 0.393 |
| Dyskinesia score (UDysRS) | 23.5 (18.3–40.0) | 33.0 (24.5–47.8) | 0.056 |
| Med off MDS-UPDRS-III score | 45.8 ± 13.5 | 53.0 ± 15.0 | 0.118 |
| Hoehn–Yahr stage | |||
| 2 | 5 | 2 | |
| 2.5 | 7 | 6 | |
| 3 | 16 | 13 | |
| 4 | 4 | 3 | |
| Follow-up time (month) | 18 (6.0–34.5) | 24 (18.0–30.0) | 0.291 |
| Group | Improvement | STN-DBS, No. (%) | GPi-DBS, No. (%) |
|---|---|---|---|
| I IA IB | Dyskinesia almost disappears, 76–100% reduction in UDysRS scores No dyskinesia A little dyskinesia present but has no effect on patients | 16 (50%) 6 (18.8%) 10 (31.2%) | 21 (87.5%) 18 (75%) 3 (12.5%) |
| II | Dyskinesia improved significantly, 51–75% reduction in UDysRS scores | 8 (25%) | 2 (8.3%) |
| III | Dyskinesia improved partially, 26–50% reduction in UDysRS scores | 5 (15.6%) | 1 (4.2%) |
| IV IVA IVB | Dyskinesia improved slightly, 0–25% reduction in UDysRS scores Not improved at all A little change, but has no benefit to patients | 1 (3.1%) 1 (3.1%) 0 (0%) | 0 (0%) 0 (0%) 0 (0%) |
| V VA VB | Dyskinesia is aggravated, increase in UDysRS scores The original dyskinesia symptoms are aggravated New dyskinesia symptoms appear and do not alleviate | 2 (6.3%) 1 (3.1%) 1 (3.1%) | 0 (0%) 0 (0%) 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, F.; Cen, S.; Yi, Z.; Li, W.; Cai, G.; Wang, F.; Quintin, S.S.; Hey, G.E.; Hernandez, J.S.; Han, C.; et al. Retrospective Multicenter Study on Outcome Measurement for Dyskinesia Improvement in Parkinson’s Disease Patients with Pallidal and Subthalamic Nucleus Deep Brain Stimulation. Brain Sci. 2022, 12, 1054. https://doi.org/10.3390/brainsci12081054
Meng F, Cen S, Yi Z, Li W, Cai G, Wang F, Quintin SS, Hey GE, Hernandez JS, Han C, et al. Retrospective Multicenter Study on Outcome Measurement for Dyskinesia Improvement in Parkinson’s Disease Patients with Pallidal and Subthalamic Nucleus Deep Brain Stimulation. Brain Sciences. 2022; 12(8):1054. https://doi.org/10.3390/brainsci12081054
Chicago/Turabian StyleMeng, Fangang, Shanshan Cen, Zhiqiang Yi, Weiguo Li, Guoen Cai, Feng Wang, Stephan S. Quintin, Grace E. Hey, Jairo S. Hernandez, Chunlei Han, and et al. 2022. "Retrospective Multicenter Study on Outcome Measurement for Dyskinesia Improvement in Parkinson’s Disease Patients with Pallidal and Subthalamic Nucleus Deep Brain Stimulation" Brain Sciences 12, no. 8: 1054. https://doi.org/10.3390/brainsci12081054
APA StyleMeng, F., Cen, S., Yi, Z., Li, W., Cai, G., Wang, F., Quintin, S. S., Hey, G. E., Hernandez, J. S., Han, C., Fan, S., Gao, Y., Song, Z., Yi, J., Wang, K., Zhang, L., Ramirez-Zamora, A., & Zhang, J. (2022). Retrospective Multicenter Study on Outcome Measurement for Dyskinesia Improvement in Parkinson’s Disease Patients with Pallidal and Subthalamic Nucleus Deep Brain Stimulation. Brain Sciences, 12(8), 1054. https://doi.org/10.3390/brainsci12081054







