Distribution and Morphological Characteristics of Oligodendrocytes in Selected Areas of the Brain of Male and Female Red Kangaroos (Macropus rufus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sectioning and Histology
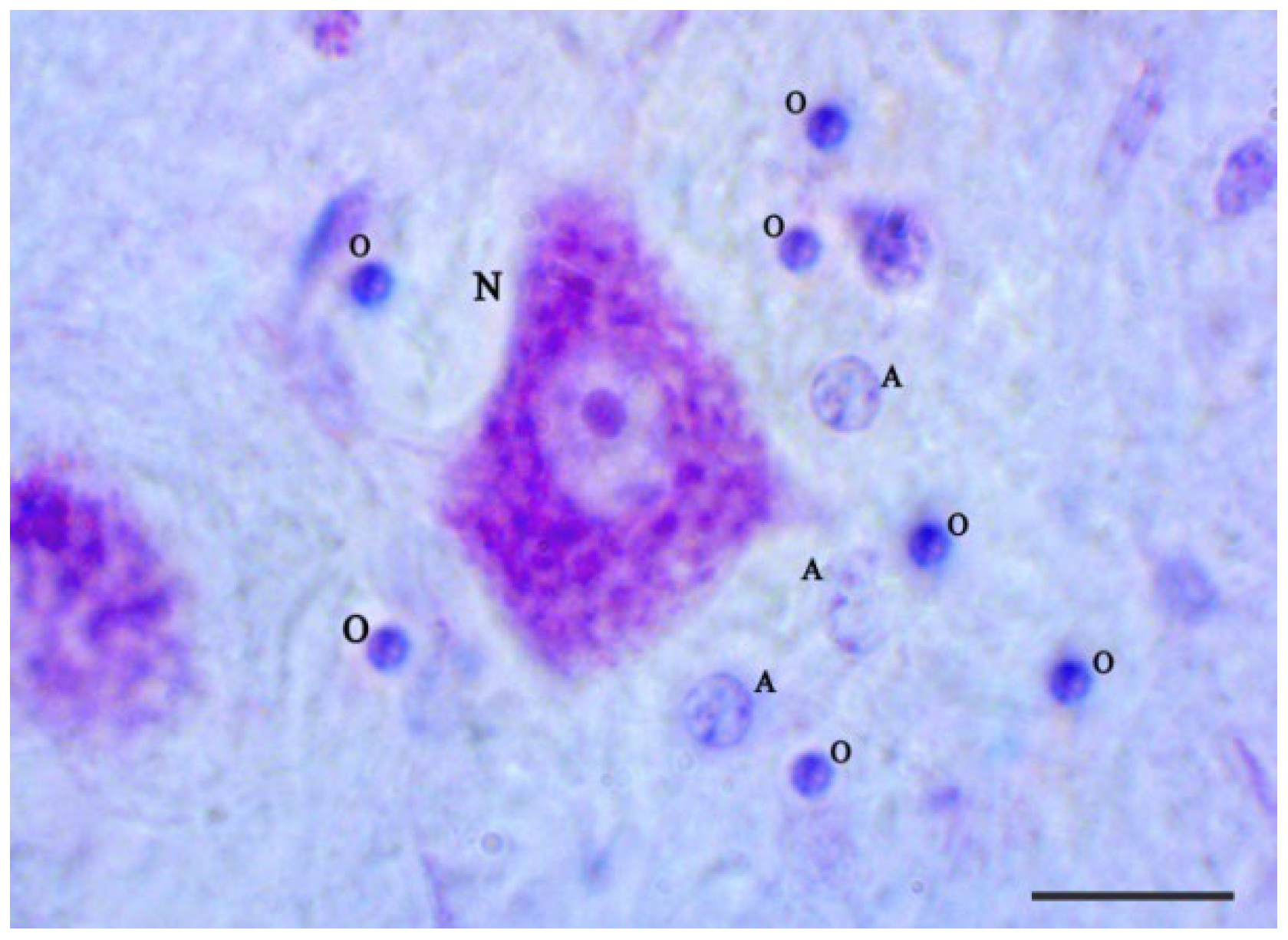
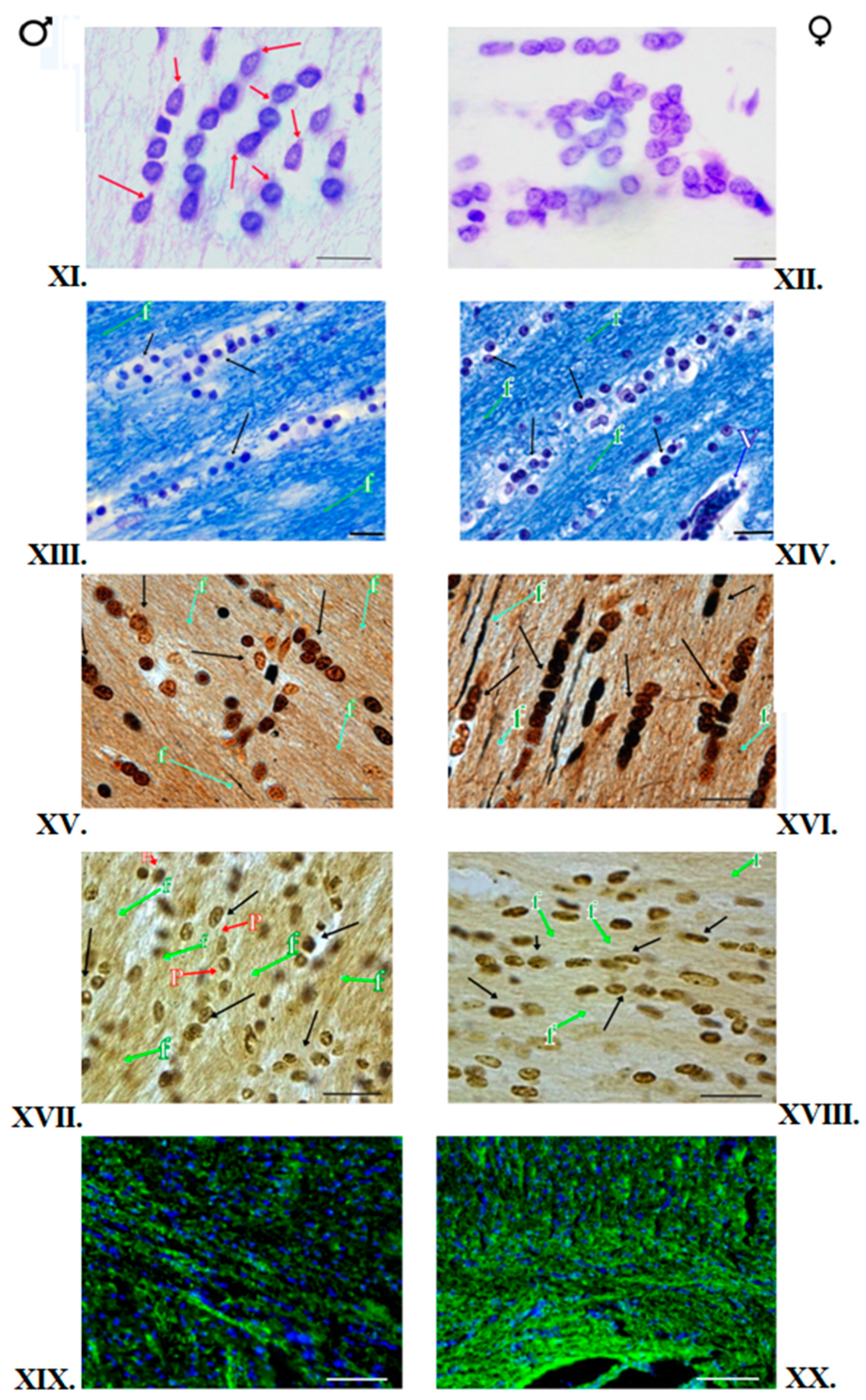
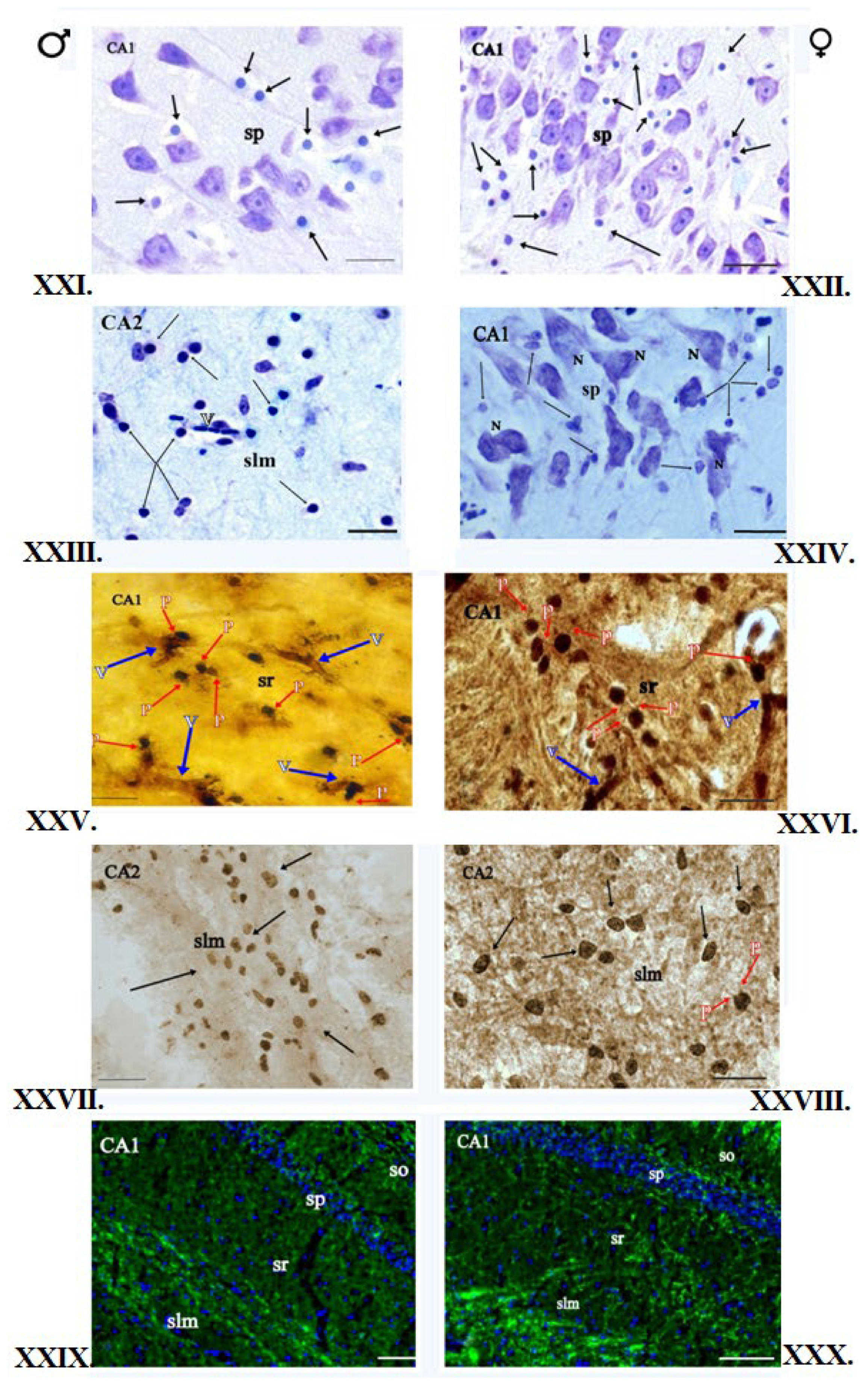
2.3. Nissl Staining of Oligodendrocytes
2.4. Morphological Criteria to Differentiate between Glial Cells and Neurons
2.5. Quantification
2.6. Klüver–Barrera Staining
2.7. Impregnation of Nervous Tissue with Silver Salts
2.8. Detecting the Presence of Iron Ions by Histochemical Method
2.9. Single Immunofluorescence Staining for Myelin Basic Protein
2.10. Photomicrography Production
2.11. Statistical Analysis
3. Results
3.1. Klüver–Barrera Staining
3.2. Nissl Staining
3.3. Impregnation with Silver Salts
3.4. Histochemical Method of Detection of Iron Presence in Oligodendrocytes
3.5. Immunofluorescent Staining
3.6. Determination of Oligodendrocyte Density in Some CNS Areas
4. Discussion
4.1. Current Knowledge about OLGs
4.2. Distribution and Role of Iron in Different Parts of CNS and Its Cells
4.3. Brain Cells Change Iron Requirements with Age
4.4. Metabolism Function of OLGs: Myelination
4.5. Sexual Dimorphism in the Hypothalamus and Number of Brain Cells
4.6. What This Paper Adds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kettenmann, H.; Verkhratsky, A. Neuroglia–Living Nerve Glue. Fortschr. Der Neurol.-Psychiatr. 2011, 79, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Leblond, C.P. Electron Microscopic Identification of Three Classes of Oligodendrocytes and a Preliminary Study of Their Proliferative Activity in the Corpus Callosum of Young Rats. J. Comp. Neurol. 1970, 139, 1–29. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of Oligodendrocyte and Myelin in the Mammalian Central Nervous System. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A. The Mystery and Magic of Glia: A Perspective on Their Roles in Health and Disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.R. Myelin Breakdown in Alzheimer’s Disease: A Commentary. Neurobiol. Aging 2004, 25, 45–47. [Google Scholar] [CrossRef]
- Todorich, B.; Pasquini, J.M.; Garcia, C.I.; Paez, P.M.; Connor, J.R. Oligodendrocytes and Myelination: The Role of Iron. Glia 2009, 57, 467–478. [Google Scholar] [CrossRef]
- McIver, S.R.; Muccigrosso, M.; Gonzales, E.R.; Lee, J.-M.; Roberts, M.S.; Sands, M.S.; Goldberg, M.P. Oligodendrocyte Degeneration and Recovery after Focal Cerebral Ischemia. Neuroscience 2010, 169, 1364–1375. [Google Scholar] [CrossRef]
- Van Dine, S.E.; Salem, E.; George, E.; Siu, N.Y.; Dotzler, T.; Ramos, R.L. Cellular and Axonal Diversity in Molecular Layer Heterotopia of the Rat Cerebellar Vermis. BioMed Res. Int. 2013, 2013, 805467. [Google Scholar] [CrossRef]
- Watakabe, A. Comparative Molecular Neuroanatomy of Mammalian Neocortex: What Can Gene Expression Tell Us about Areas and Layers? Dev. Growth Differ. 2009, 51, 343–354. [Google Scholar] [CrossRef]
- Yamamori, T.; Rockland, K.S. Neocortical Areas, Layers, Connections, and Gene Expression. Neurosci. Res. 2006, 55, 11–27. [Google Scholar] [CrossRef]
- Pakkenberg, B.; Gundersen, H.J.G. Neocortical Neuron Number in Humans: Effect of Sex and Age. J. Comp. Neurol. 1997, 384, 312–320. [Google Scholar] [CrossRef]
- Marner, L.; Nyengaard, J.R.; Tang, Y.; Pakkenberg, B. Marked Loss of Myelinated Nerve Fibers in the Human Brain with Age. J. Comp. Neurol. 2003, 462, 144–152. [Google Scholar] [CrossRef]
- Tang, W.; Fan, K.; Zhao, S.; Zhang, Y.; Li, Y.; Shao, S.; Wang, Z.; Ke, J. Correlations between Age, Biomedical Variables, and Cognition in Patients with Schizophrenia. Schizophr. Res. Cogn. 2020, 22, 100182. [Google Scholar] [CrossRef]
- Stevens, B.; Porta, S.; Haak, L.L.; Gallo, V.; Fields, R.D. Adenosine: A Neuron-Glial Transmitter Promoting Myelination in the CNS in Response to Action Potentials. Neuron 2002, 36, 855–868. [Google Scholar] [CrossRef]
- Fabri, M.; Pierpaoli, C.; Barbaresi, P.; Polonara, G. Functional Topography of the Corpus Callosum Investigated by DTI and FMRI. World J. Radiol. 2014, 6, 895. [Google Scholar] [CrossRef]
- Bercury, K.K.; Macklin, W.B. Dynamics and Mechanisms of CNS Myelination. Dev. Cell 2015, 32, 447–458. [Google Scholar] [CrossRef]
- Young, K.M.; Psachoulia, K.; Tripathi, R.B.; Dunn, S.-J.; Cossell, L.; Attwell, D.; Tohyama, K.; Richardson, W.D. Oligodendrocyte Dynamics in the Healthy Adult CNS: Evidence for Myelin Remodeling. Neuron 2013, 77, 873–885. [Google Scholar] [CrossRef]
- VanGuilder, H.D.; Freeman, W.M. The Hippocampal Neuroproteome with Aging and Cognitive Decline: Past Progress and Future Directions. Front. Aging Neurosci. 2011, 3, 8. [Google Scholar] [CrossRef]
- Lee, C.H.; Yoo, K.-Y.; Choi, J.H.; Park, O.K.; Hwang, I.K.; Kim, S.K.; Kang, I.-J.; Kim, Y.-M.; Won, M.-H. Neuronal Damage Is Much Delayed and Microgliosis Is More Severe in the Aged Hippocampus Induced by Transient Cerebral Ischemia Compared to the Adult Hippocampus. J. Neurol. Sci. 2010, 294, 1–6. [Google Scholar] [CrossRef]
- Alabarse, P.V.; Salomon, T.B.; Medeiros, T.M.; Hackenhaar, F.S.; Schüller, A.K.; Ehrenbrink, G.; Benfato, M.S. Oxidative Stress in the Kidney of Reproductive Male Rats during Aging. Exp. Gerontol. 2011, 46, 773–780. [Google Scholar] [CrossRef]
- McGEER, P.L.; McGEER, E.G. Inflammation and the Degenerative Diseases of Aging. Ann. N. Y. Acad. Sci. 2004, 1035, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Łuszczewska-Sierakowska, I.; Wawrzyniak-Gacek, A.; Guz, T.; Tatara, M.R.; Charuta, A. Morphometric Parameters of Pyramidal Cells in CA1-CA4 Fields in the Hippocampus of Arctic Fox (Vulpes lagopus). Folia Biol. (Kraków) 2015, 63, 263–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sweatt, J.D. Hippocampal Function in Cognition. Psychopharmacology 2004, 174, 99–110. [Google Scholar] [CrossRef]
- Rao, B.S.; Laxmi, T.R.; Meti, B.L.; Raju, T.R. Subicular Lesions Cause Dendritic Atrophy in CA1 and CA3 Pyramidal Neurons of the Rat Hippocampus. Neuroscience 2001, 102, 319–327. [Google Scholar]
- Miyashita, T.; Williams, C.L. Peripheral Arousal-Related Hormones Modulate Norepinephrine Release in the Hippocampus via Influences on Brainstem Nuclei. Behav. Brain Res. 2004, 153, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kesner, R.P.; Lee, I.; Gilbert, P. A Behavioral Assessment of Hippocampal Function Based on a Subregional Analysis. Rev. Neurosci. 2004, 15, 333–352. [Google Scholar] [CrossRef]
- Niewiadomska, G.; Baksalerska-Pazera, M.; Riedel, G. The Septo-Hippocampal System, Learning and Recovery of Function. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.A. How Hippocampus and Cortex Contribute to Recognition Memory: Revisiting the Complementary Learning Systems Model. Hippocampus 2010, 20, 1217–1227. [Google Scholar] [CrossRef]
- Langston, R.F.; Stevenson, C.H.; Wilson, C.L.; Saunders, I.; Wood, E.R. The Role of Hippocampal Subregions in Memory for Stimulus Associations. Behav. Brain Res. 2010, 215, 275–291. [Google Scholar] [CrossRef]
- West, M.J.; Slomianka, L.; Gundersen, H.J.G. Unbiased Stereological Estimation of the Total Number of Neurons in the Subdivisions of the Rat Hippocampus Using the Optical Fractionator. Anat. Rec. 1991, 231, 482–497. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Klüver, H.; Barrera, E. A Method for the Combined Staining of Cells and Fibers in the Nervous System. J. Neuropathol. Exp. Neurol. 1953, 12, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Okado, N.; Kojima, T. A New Technique of Silver Impregnation for Oligodendrocytes with Potassium Dicyanoargentate by Means of Perfusion-Fixation Method. Okajimas Folia Anat. Jpn. 1975, 52, 39–49. [Google Scholar] [CrossRef][Green Version]
- Wawrzyniak-Gacek, A. Distribution of Various Types of Oligodendrocytes and Cellular Localisation of Iron in the Frontal Cortex of the Adult Rat. Folia Morphol. 2002, 61, 115–121. [Google Scholar]
- Gundersen, H.J.G.; Jensen, E.B.V.; Kiêu, K.; Nielsen, J. The Efficiency of Systematic Sampling in Stereology—Reconsidered. J. Microsc. 1999, 193, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.W.; Peters, A. Neuroglial Cells in the Cerebral Cortex of Rats from Young Adulthood to Old Age: An Electron Microscope Study. J. Neurocytol. 1974, 3, 405–429. [Google Scholar] [CrossRef] [PubMed]
- LeVine, S.M.; Macklin, W.B. Iron-Enriched Oligodendrocytes: A Reexamination of Their Spatial Distribution. J. Neurosci. Res. 1990, 26, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.A.; Conceicão, L.E.; Rochai, E.; Marini-abreu, M.M. Age Changes in Cerebellar Oligodendrocytes: The Appearance of Nuclear Filaments and Increase in the Volume Density of the Nucleus and in the Number of Dark Cell Forms. Arch. Histol. Cytol. 1995, 58, 417–425. [Google Scholar] [CrossRef]
- Murtie, J.C.; Macklin, W.B.; Corfas, G. Morphometric Analysis of Oligodendrocytes in the Adult Mouse Frontal Cortex. J. Neurosci. Res. 2007, 85, 2080–2086. [Google Scholar] [CrossRef]
- Vinet, J.; Lemieux, P.; Tamburri, A.; Tiesinga, P.; Scafidi, J.; Gallo, V.; Sík, A. Subclasses of Oligodendrocytes Populate the Mouse Hippocampus. Eur. J. Neurosci. 2010, 31, 425–438. [Google Scholar] [CrossRef]
- Simpson, I.A.; Ponnuru, P.; Klinger, M.E.; Myers, R.L.; Devraj, K.; Coe, C.L.; Lubach, G.R.; Carruthers, A.; Connor, J.R. A Novel Model for Brain Iron Uptake: Introducing the Concept of Regulation. J. Cereb. Blood Flow Metab. 2015, 35, 48–57. [Google Scholar] [CrossRef]
- Del Zoppo, G.J.; Milner, R.; Mabuchi, T.; Hung, S.; Wang, X.; Koziol, J.A. Vascular Matrix Adhesion and the Blood–Brain Barrier. Biochem. Soc. Trans. 2006, 34, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, R.; Wenzel, J.; Schwartzkroin, P.A.; McKhann, G.M.; Janigro, D. Functional Specialization and Topographic Segregation of Hippocampal Astrocytes. J. Neurosci. 1998, 18, 4425–4438. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Fern, R.F.; Matute, C. Neurotransmitter Signaling in White Matter. Glia 2014, 62, 1762–1779. [Google Scholar] [CrossRef] [PubMed]
- Piñero, D.J.; Connor, J.R. Iron in the Brain: An Important Contributor in Normal and Diseased States. Neuroscientist 2000, 6, 435–453. [Google Scholar] [CrossRef]
- Francois, C.; Nguyen-Legros, J.; Percheron, G. Topographical and Cytological Localization of Iron in Rat and Monkey Brains. Brain Res. 1981, 215, 317–322. [Google Scholar] [CrossRef]
- Gilissen, E.P.; Ghosh, P.; Jacobs, R.E.; Allman, J.M. Topographical Localization of Iron in Brains of the Aged Fat-Tailed Dwarf Lemur (Cheirogaleus medius) and Gray Lesser Mouse Lemur (Microcebus murinus). Am. J. Primatol. 1998, 45, 291–299. [Google Scholar] [CrossRef]
- Schulz, K.; Vulpe, C.D.; Harris, L.Z.; David, S. Iron Efflux from Oligodendrocytes Is Differentially Regulated in Gray and White Matter. J. Neurosci. 2011, 31, 13301–13311. [Google Scholar] [CrossRef]
- Hill, J.M.; Switzer Iii, R.C. The Regional Distribution and Cellular Localization of Iron in the Rat Brain. Neuroscience 1984, 11, 595–603. [Google Scholar] [CrossRef]
- Erb, G.L.; Osterbur, D.L.; LeVine, S.M. The Distribution of Iron in the Brain: A Phylogenetic Analysis Using Iron Histochemistry. Dev. Brain Res. 1996, 93, 120–128. [Google Scholar] [CrossRef]
- Burdo, J.R.; Martin, J.; Menzies, S.L.; Dolan, K.G.; Romano, M.A.; Fletcher, R.J.; Garrick, M.D.; Garrick, L.M.; Connor, J.R. Cellular Distribution of Iron in the Brain of the Belgrade Rat. Neuroscience 1999, 93, 1189–1196. [Google Scholar] [CrossRef]
- Algarín, C.; Peirano, P.; Garrido, M.; Pizarro, F.; Lozoff, B. Iron Deficiency Anemia in Infancy: Long-Lasting Effects on Auditory and Visual System Functioning. Pediatric Res. 2003, 53, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, E.; Pasquini, J.M.; Thompson, K.; Felt, B.; Butkus, G.; Beard, J.; Connor, J.R. Effect of Manipulation of Iron Storage, Transport, or Availability on Myelin Composition and Brain Iron Content in Three Different Animal Models. J. Neurosci. Res. 2004, 77, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.F.; Connor, J.R. Functional Roles of Transferrin in the Brain. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Hulet, S.W.; Menzies, S.; Connor, J.R. Ferritin Binding in the Developing Mouse Brain Follows a Pattern Similar to Myelination and Is Unaffected by the Jimpy Mutation. Dev. Neurosci. 2002, 24, 208–213. [Google Scholar] [CrossRef]
- Beard, J. Recent Evidence from Human and Animal Studies Regarding Iron Status and Infant Development. J. Nutr. 2007, 137, 524S–530S. [Google Scholar] [CrossRef]
- Hulet, S.W.; Powers, S.; Connor, J.R. Distribution of Transferrin and Ferritin Binding in Normal and Multiple Sclerotic Human Brains. J. Neurol. Sci. 1999, 165, 48–55. [Google Scholar] [CrossRef]
- Peters, A. The Effects of Normal Aging on Myelinated Nerve Fibers in Monkey Central Nervous System. Front. Neuroanat. 2009, 3, 11. [Google Scholar] [CrossRef]
- Rasband, M.N.; Tayler, J.; Kaga, Y.; Yang, Y.; Lappe-Siefke, C.; Nave, K.-A.; Bansal, R. CNP Is Required for Maintenance of Axon–Glia Interactions at Nodes of Ranvier in the CNS. Glia 2005, 50, 86–90. [Google Scholar] [CrossRef]
- Fukunaga, M.; Li, T.-Q.; van Gelderen, P.; de Zwart, J.A.; Shmueli, K.; Yao, B.; Lee, J.; Maric, D.; Aronova, M.A.; Zhang, G. Layer-Specific Variation of Iron Content in Cerebral Cortex as a Source of MRI Contrast. Proc. Natl. Acad. Sci. USA 2010, 107, 3834–3839. [Google Scholar] [CrossRef]
- Nottebohm, F.; Arnold, A.P. Sexual Dimorphism in Vocal Control Areas of the Songbird Brain. Science 1976, 194, 211–213. [Google Scholar] [CrossRef]
- Gur, R.C.; Turetsky, B.I.; Matsui, M.; Yan, M.; Bilker, W.; Hughett, P.; Gur, R.E. Sex Differences in Brain Gray and White Matter in Healthy Young Adults: Correlations with Cognitive Performance. J. Neurosci. 1999, 19, 4065–4072. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Seidman, L.J.; Horton, N.J.; Makris, N.; Kennedy, D.N.; Caviness Jr, V.S.; Faraone, S.V.; Tsuang, M.T. Normal Sexual Dimorphism of the Adult Human Brain Assessed by in Vivo Magnetic Resonance Imaging. Cereb. Cortex 2001, 11, 490–497. [Google Scholar] [CrossRef]
- Cerghet, M.; Skoff, R.P.; Bessert, D.; Zhang, Z.; Mullins, C.; Ghandour, M.S. Proliferation and Death of Oligodendrocytes and Myelin Proteins Are Differentially Regulated in Male and Female Rodents. J. Neurosci. 2006, 26, 1439–1447. [Google Scholar] [CrossRef]
- Mong, J.A.; Glaser, E.; McCarthy, M.M. Gonadal Steroids Promote Glial Differentiation and Alter Neuronal Morphology in the Developing Hypothalamus in a Regionally Specific Manner. J. Neurosci. 1999, 19, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Barrera, Á.; Jiménez, L.; González, G.M.; Montiel, J.; Aboitiz, F. Dendritic Structure of Single Hippocampal Neurons According to Sex and Hemisphere of Origin in Middle-Aged and Elderly Human Subjects. Brain Res. 2001, 906, 31–37. [Google Scholar] [CrossRef]
- King, J.A.; Trinkler, I.; Hartley, T.; Vargha-Khadem, F.; Burgess, N. The Hippocampal Role in Spatial Memory and the Familiarity-Recollection Distinction: A Case Study. Neuropsychology 2004, 18, 405. [Google Scholar] [CrossRef]
- Nuñez, J.L.; Nelson, J.; Pych, J.C.; Kim, J.H.; Juraska, J.M. Myelination in the Splenium of the Corpus Callosum in Adult Male and Female Rats. Dev. Brain Res. 2000, 120, 87–90. [Google Scholar] [CrossRef]
- Jelsing, J.; Nielsen, R.; Olsen, A.K.; Grand, N.; Hemmingsen, R.; Pakkenberg, B. The Postnatal Development of Neocortical Neurons and Glial Cells in the Gottingen Minipig and the Domestic Pig Brain. J. Exp. Biol. 2006, 209, 1454–1462. [Google Scholar] [CrossRef]
- Mortensen, H.S.; Pakkenberg, B.; Dam, M.; Dietz, R.; Sonne, C.; Mikkelsen, B.; Eriksen, N. Quantitative Relationships in Delphinid Neocortex. Front. Neuroanat. 2014, 8, 132. [Google Scholar] [CrossRef]
- Hofman, M.A. Evolution of the Human Brain: When Bigger Is Better. Front. Neuroanat. 2014, 8, 15. [Google Scholar] [CrossRef]
- Keuker, J.I.; Luiten, P.G.; Fuchs, E. Preservation of Hippocampal Neuron Numbers in Aged Rhesus Monkeys. Neurobiol. Aging 2003, 24, 157–165. [Google Scholar] [CrossRef]
- Chen, L.; Lu, W.; Yang, Z.; Yang, S.; Li, C.; Shi, X.; Tang, Y. Age-Related Changes of the Oligodendrocytes in Rat Subcortical White Matter. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2011, 294, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; De Vos, R.A.; Donka, G.; Jansen Steur, E.N.; Mihaly, A.; Luiten, P.G. Age-Related Microvascular Degeneration in the Human Cerebral Periventricular White Matter. Acta Neuropathol. 2006, 111, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Tomimoto, H.; Ihara, M.; Wakita, H.; Ohtani, R.; Lin, J.-X.; Akiguchi, I.; Kinoshita, M.; Shibasaki, H. Chronic Cerebral Hypoperfusion Induces White Matter Lesions and Loss of Oligodendroglia with DNA Fragmentation in the Rat. Acta Neuropathol. 2003, 106, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Haro, D.; Mora-Loyola, E.; Soria-Ortiz, B.; García-Colunga, J. Regional Density of Glial Cells in the Rat Corpus Callosum. Biol. Res. 2013, 46, 27–32. [Google Scholar] [CrossRef]

| Brain Areas | NC | Hip | CC | |||
|---|---|---|---|---|---|---|
| ♂♀ | ♂♀ | ♂♀ | ||||
| MEAN± | 116,729.94 | 98,035.95 | 119,204.78 | 127,202,36 | 404,543.75 | 431,927.92 |
| MEAN ± SD | 24,456.56 | 7765.22 | 30,313.19 | 37,355.54 | 70,017.60 | 66,301.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyniak, A.; Balawender, K.; Lalak, R.; Golan, M.P.; Wróbel, K.; Boroń, D.; Staszkiewicz, R.; Grabarek, B.O. Distribution and Morphological Characteristics of Oligodendrocytes in Selected Areas of the Brain of Male and Female Red Kangaroos (Macropus rufus). Brain Sci. 2022, 12, 1035. https://doi.org/10.3390/brainsci12081035
Wawrzyniak A, Balawender K, Lalak R, Golan MP, Wróbel K, Boroń D, Staszkiewicz R, Grabarek BO. Distribution and Morphological Characteristics of Oligodendrocytes in Selected Areas of the Brain of Male and Female Red Kangaroos (Macropus rufus). Brain Sciences. 2022; 12(8):1035. https://doi.org/10.3390/brainsci12081035
Chicago/Turabian StyleWawrzyniak, Agata, Krzysztof Balawender, Roman Lalak, Maciej Przemysław Golan, Konrad Wróbel, Dariusz Boroń, Rafał Staszkiewicz, and Beniamin Oskar Grabarek. 2022. "Distribution and Morphological Characteristics of Oligodendrocytes in Selected Areas of the Brain of Male and Female Red Kangaroos (Macropus rufus)" Brain Sciences 12, no. 8: 1035. https://doi.org/10.3390/brainsci12081035
APA StyleWawrzyniak, A., Balawender, K., Lalak, R., Golan, M. P., Wróbel, K., Boroń, D., Staszkiewicz, R., & Grabarek, B. O. (2022). Distribution and Morphological Characteristics of Oligodendrocytes in Selected Areas of the Brain of Male and Female Red Kangaroos (Macropus rufus). Brain Sciences, 12(8), 1035. https://doi.org/10.3390/brainsci12081035






