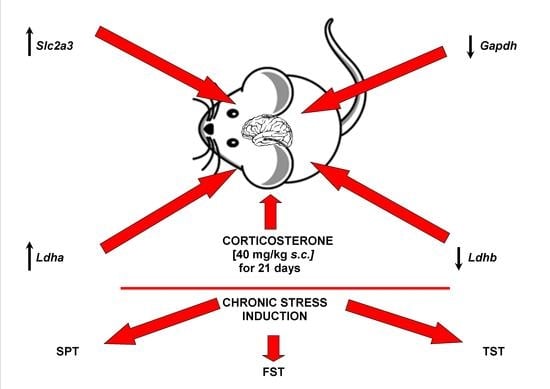
Alteration in the Expression of Genes Involved in Cerebral Glucose Metabolism as a Process of Adaptation to Stressful Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
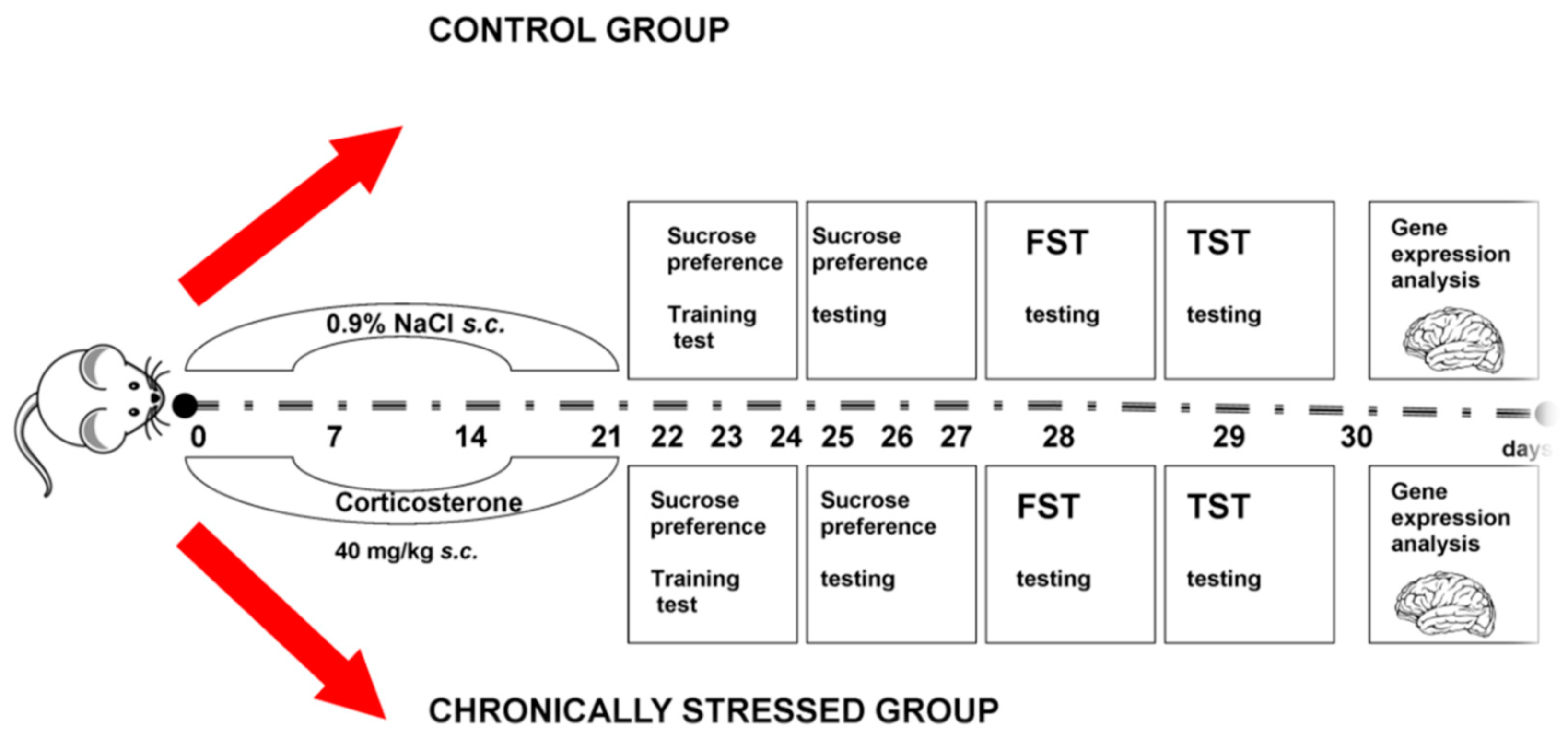
2.2. Experimental Project
2.3. Animal Behavioural Tests
2.3.1. Sucrose Preference Test (SPT)
2.3.2. Forced Swim Test (FST)
2.3.3. Tail Suspension Test (TST)
2.4. Collection of Tissues
2.5. The Quantitative Real-Time PCR Analysis (qRT-PCR)
2.5.1. RNA Isolation
2.5.2. cDNA Synthesis
2.5.3. Real-Time PCR
2.6. Statistical Analysis
3. Results
3.1. Body Weight of Mice
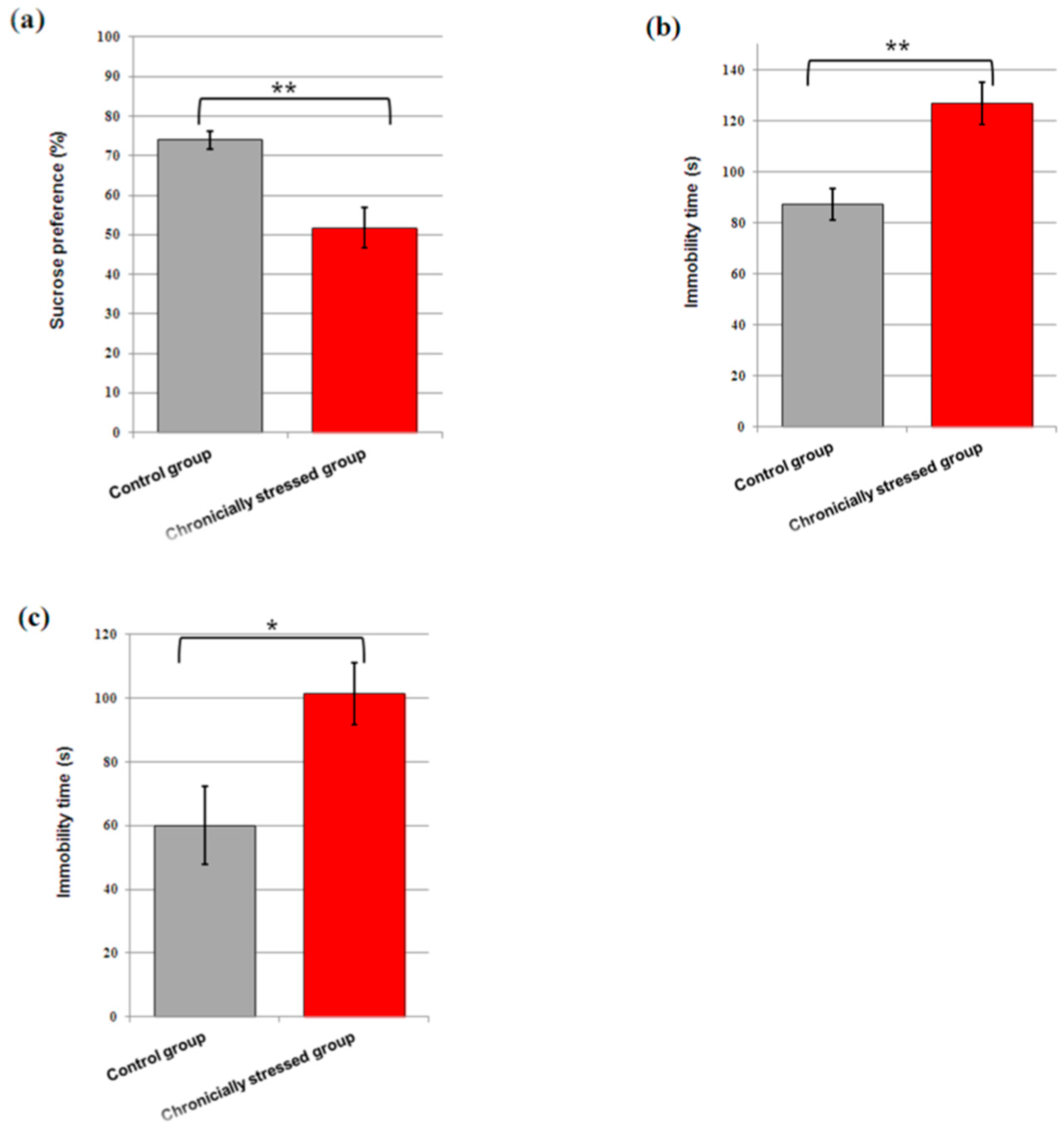
3.2. Consumption of Sucrose
3.3. Immobility Time in the FST and TST
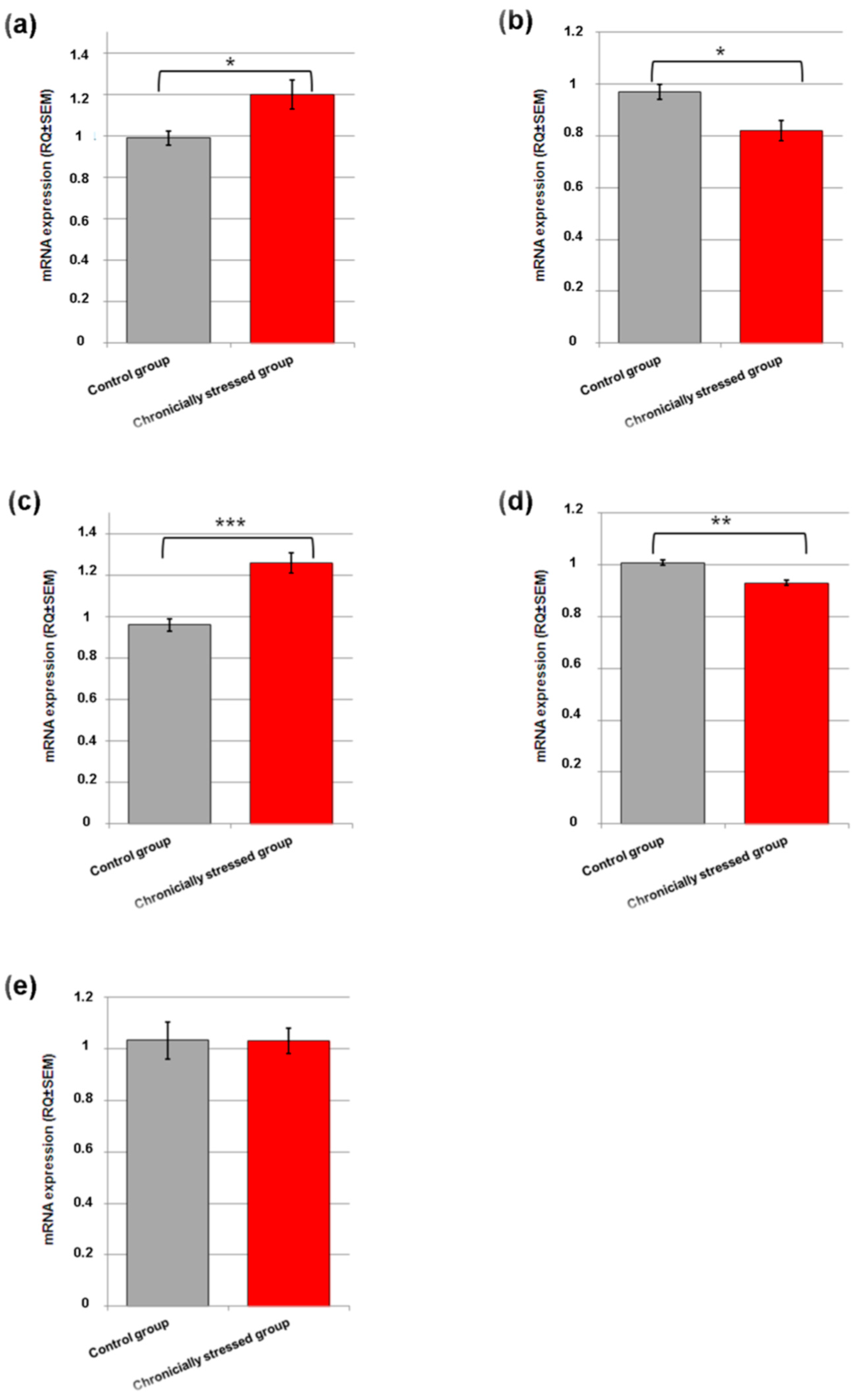
3.4. Gene Expression Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic Stress and Oxidative Stress as Common Factors of the Pathogenesis of Depression and Alzheimer’s Disease: The Role of Antioxidants in Prevention and Treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.Y.H.; Herbert, J. The Corticoid Environment: A Determining Factor for Neural Progenitors’ Survival in the Adult Hippocampus. Eur. J. Neurosci. 2004, 20, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Głombik, K.; Detka, J.; Kurek, A.; Budziszewska, B. Impaired Brain Energy Metabolism: Involvement in Depression and Hypothyroidism. Front. Neurosci. 2020, 14, 586939. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.A.; Zunszain, P.A. Neuroimmune and Neuroendocrine Abnormalities in Depression: Two Sides of the Same Coin. Ann. N. Y. Acad. Sci. 2015, 1351, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the Pathophysiology of Depression: From Monoamines to the Neurogenesis Hypothesis Model—Are We There Yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the Brain: The Role of Glucose in Physiological and Pathological Brain Function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Howarth, C.; Gleeson, P.; Attwell, D. Updated Energy Budgets for Neural Computation in the Neocortex and Cerebellum. J. Cereb. Blood Flow Metab. 2012, 32, 1222–1232. [Google Scholar] [CrossRef]
- Erbsloh, F.; Bernsmeier, A.; Hillesheim, H. The glucose consumption of the brain & its dependence on the liver. Arch. Psychiatr. Nervenkr. Z. Gesamte Neurol. Psychiatr. 1958, 196, 611–626. [Google Scholar] [CrossRef]
- Van Hall, G.; Strømstad, M.; Rasmussen, P.; Jans, O.; Zaar, M.; Gam, C.; Quistorff, B.; Secher, N.H.; Nielsen, H.B. Blood Lactate Is an Important Energy Source for the Human Brain. J. Cereb. Blood Flow Metab. 2009, 29, 1121–1129. [Google Scholar] [CrossRef]
- Lutas, A.; Yellen, G. The Ketogenic Diet: Metabolic Influences on Brain Excitability and Epilepsy. Trends Neurosci. 2013, 36, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Van der Kooij, M.A. The Impact of Chronic Stress on Energy Metabolism. Mol. Cell。 Neurosci. 2020, 107, 103525. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.M.; Pearson-Leary, J.; McNay, E.C. The Neuroenergetics of Stress Hormones in the Hippocampus and Implications for Memory. Front. Neurosci. 2015, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kloet, E.R.; Oitzl, M.S.; Joëls, M. Stress and Cognition: Are Corticosteroids Good or Bad Guys? Trends Neurosci. 1999, 22, 422–426. [Google Scholar] [CrossRef]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Nimgampalle, M.; Chakravarthy, H.; Devanathan, V. Chapter 8—Glucose Metabolism in the Brain: An Update. In Recent Developments in Applied Microbiology and Biochemistry; Viswanath, B., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 77–88. ISBN 978-0-12-821406-0. [Google Scholar]
- Herbet, M.; Izdebska, M.; Piątkowska-Chmiel, I.; Gawrońska-Grzywacz, M.; Natorska-Chomicka, D.; Pawłowski, K.; Sysa, M.; Ślaska, B.; Dudka, J. α-Tocopherol Ameliorates Redox Equilibrium and Reduces Inflammatory Response Caused by Chronic Variable Stress. BioMed Res. Int. 2018, 2018, 7210783. [Google Scholar] [CrossRef] [Green Version]
- King, A.; Gottlieb, E. Glucose Metabolism and Programmed Cell Death: An Evolutionary and Mechanistic Perspective. Curr. Opin. Cell Biol. 2009, 21, 885–893. [Google Scholar] [CrossRef]
- Deitmer, J.W.; Theparambil, S.M.; Ruminot, I.; Noor, S.I.; Becker, H.M. Energy Dynamics in the Brain: Contributions of Astrocytes to Metabolism and PH Homeostasis. Front. Neurosci. 2019, 13, 1301. [Google Scholar] [CrossRef]
- Gold, P.E. Regulation of Memory—From the Adrenal Medulla to Liver to Astrocytes to Neurons. Brain Res. Bull. 2014, 105, 25–35. [Google Scholar] [CrossRef] [Green Version]
- van der Kooij, M.A.; Jene, T.; Treccani, G.; Miederer, I.; Hasch, A.; Voelxen, N.; Walenta, S.; Müller, M.B. Chronic Social Stress-Induced Hyperglycemia in Mice Couples Individual Stress Susceptibility to Impaired Spatial Memory. Proc. Natl. Acad. Sci. USA 2018, 115, E10187–E10196. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ma, R.; Shen, J.; Su, H.; Xing, D.; Du, L. A Mouse Model of Depression Induced by Repeated Corticosterone Injections. Eur. J. Pharmacol. 2008, 581, 113–120. [Google Scholar] [CrossRef]
- Ždralević, M.; Brand, A.; Di Ianni, L.; Dettmer, K.; Reinders, J.; Singer, K.; Peter, K.; Schnell, A.; Bruss, C.; Decking, S.-M.; et al. Double Genetic Disruption of Lactate Dehydrogenases A and B Is Required to Ablate the “Warburg Effect” Restricting Tumor Growth to Oxidative Metabolism. J. Biol. Chem. 2018, 293, 15947–15961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Q.-Q.; Xian, Y.-F.; Ip, S.-P.; Tsai, S.-H.; Che, C.-T. Long-Term Treatment with Peony Glycosides Reverses Chronic Unpredictable Mild Stress-Induced Depressive-like Behavior via Increasing Expression of Neurotrophins in Rat Brain. Behav. Brain Res. 2010, 210, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. Behavioral Despair in Mice: A Primary Screening Test for Antidepressants. Arch. Int. Pharmacodyn. Ther. 1977, 229, 327–336. [Google Scholar] [PubMed]
- Porsolt, R.D. Behavioural Despair. Antidepressants Neurochem. Behavional Clin. Perspect. 1981, 121–139. [Google Scholar]
- Tillmann, S.; Pereira, V.S.; Liebenberg, N.; Christensen, A.K.; Wegener, G. ZL006, a Small Molecule Inhibitor of PSD-95/NNOS Interaction, Does Not Induce Antidepressant-like Effects in Two Genetically Predisposed Rat Models of Depression and Control Animals. PLoS ONE 2017, 12, e0182698. [Google Scholar] [CrossRef] [Green Version]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The Tail Suspension Test: A New Method for Screening Antidepressants in Mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Sakhaee, E.; Ostadhadi, S.; Khan, M.I.; Yousefi, F.; Norouzi-Javidan, A.; Akbarian, R.; Chamanara, M.; Zolfaghari, S.; Dehpour, A.-R. The Role of NMDA Receptor and Nitric Oxide/Cyclic Guanosine Monophosphate Pathway in the Antidepressant-like Effect of Dextromethorphan in Mice Forced Swimming Test and Tail Suspension Test. Biomed. Pharmacother. 2017, 85, 627–634. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Ali, S.H.; Madhana, R.M.; KV, A.; Kasala, E.R.; Bodduluru, L.N.; Pitta, S.; Mahareddy, J.R.; Lahkar, M. Resveratrol Ameliorates Depressive-like Behavior in Repeated Corticosterone-Induced Depression in Mice. Steroids 2015, 101, 37–42. [Google Scholar] [CrossRef]
- Camandola, S.; Mattson, M.P. Brain Metabolism in Health, Aging, and Neurodegeneration. EMBO J. 2017, 36, 1474–1492. [Google Scholar] [CrossRef]
- Herbet, M.; Korga, A.; Gawrońska-Grzywacz, M.; Izdebska, M.; Piątkowska-Chmiel, I.; Poleszak, E.; Wróbel, A.; Matysiak, W.; Jodłowska-Jędrych, B.; Dudka, J. Chronic Variable Stress Is Responsible for Lipid and DNA Oxidative Disorders and Activation of Oxidative Stress Response Genes in the Brain of Rats. Oxid. Med. Cell。 Longev. 2017, 2017, 7313090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Ju, P.; Liu, H.; Wu, X.; Niu, Z.; Zhu, Y.; Zhang, C.; Fang, Y. Ifenprodil Rapidly Ameliorates Depressive-like Behaviors, Activates MTOR Signaling and Modulates Proinflammatory Cytokines in the Hippocampus of CUMS Rats. Psychopharmacology 2020, 237, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, F.; Zhou, Y.; Ma, Y.; Huang, X.; Ning, Y.; Lang, X.; Luo, X.; Zhang, X. Association of Thyroid Dysfunction with Suicide Attempts in First-Episode and Drug Naïve Patients with Major Depressive Disorder. J. Affect. Disord. 2019, 259, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Jurcovicova, J. Glucose Transport in Brain—Effect of Inflammation. Endocr. Regul. 2014, 48, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Keyes, K.M.; Pratt, C.; Galea, S.; McLaughlin, K.A.; Koenen, K.C.; Shear, M.K. The Burden of Loss: Unexpected Death of a Loved One and Psychiatric Disorders across the Life Course in a National Study. Am. J. Psychiatry 2014, 171, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Fueling and Imaging Brain Activation. ASN Neuro 2012, 4, e00093. [Google Scholar] [CrossRef] [Green Version]
- Gałecki, P.; Talarowska, M. Inflammatory Theory of Depression. Psychiatr. Pol. 2018, 52, 437–447. [Google Scholar] [CrossRef]
- Xiong, Y.; Uys, J.D.; Tew, K.D.; Townsend, D.M. S-Glutathionylation: From Molecular Mechanisms to Health Outcomes. Antioxid. Redox Signal. 2011, 15, 233–270. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.-F.; Xiang, Y.; Wei, Y.-S. The Significance of Routine Biochemical Markers in Patients with Major Depressive Disorder. Sci. Rep. 2016, 6, 34402. [Google Scholar] [CrossRef] [Green Version]
- Ramachandraih, C.T.; Subramanyam, N.; Bar, K.J.; Baker, G.; Yeragani, V.K. Antidepressants: From MAOIs to SSRIs and More. Indian J. Psychiatry 2011, 53, 180–182. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Hardas, S.S.; Lange, M.L.B. Oxidatively Modified Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) and Alzheimer’s Disease: Many Pathways to Neurodegeneration. J. Alzheimers Dis. 2010, 20, 369–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Tang, B.L. Brain Activity-Induced Neuronal Glucose Uptake/Glycolysis: Is the Lactate Shuttle Not Required? Brain Res. Bull. 2018, 137, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Colell, A.; Ricci, J.-E.; Tait, S.; Milasta, S.; Maurer, U.; Bouchier-Hayes, L.; Fitzgerald, P.; Guio-Carrion, A.; Waterhouse, N.J.; Li, C.W.; et al. GAPDH and Autophagy Preserve Survival after Apoptotic Cytochrome c Release in the Absence of Caspase Activation. Cell 2007, 129, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishitani, R.; Chuang, D.M. Glyceraldehyde-3-Phosphate Dehydrogenase Antisense Oligodeoxynucleotides Protect against Cytosine Arabinonucleoside-Induced Apoptosis in Cultured Cerebellar Neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 9937–9941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LDHA Gene: MedlinePlus Genetics. Available online: https://medlineplus.gov/genetics/gene/ldha/ (accessed on 23 February 2022).
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of Pyruvate Metabolism and Human Disease. Cell. Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, M.A.; Beltrán, F.A.; Brauchi, S.; Concha, I.I. A Metabolic Switch in Brain: Glucose and Lactate Metabolism Modulation by Ascorbic Acid. J. Neurochem. 2009, 110, 423–440. [Google Scholar] [CrossRef]
- Feighner, J.P. Mechanism of Action of Antidepressant Medications. J. Clin. Psychiatry 1999, 60 (Suppl. S4), 4–13. [Google Scholar]
- Takanaga, H.; Mackenzie, B.; Peng, J.-B.; Hediger, M.A. Characterization of a Branched-Chain Amino-Acid Transporter SBAT1 (SLC6A15) That Is Expressed in Human Brain. Biochem. Biophys. Res. Commun. 2005, 337, 892–900. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Parraga-Alava, J.; Tu, S.-M. Testicular Germ Cell Tumors Type 2 Have High RNA Expression of LDHB, the Gene for Lactate Dehydrogenase Subunit B. Asian J. Androl. 2021, 23, 357–362. [Google Scholar] [CrossRef]
- Pearlstein, T.; Howard, M.; Salisbury, A.; Zlotnick, C. Postpartum Depression. Am. J. Obstet. Gynecol. 2009, 200, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Rodacka, A. Properties and functional diversity of glyceraldehyde-3-phosphate dehydrogenase. Postepy Hig. Med. Dosw. 2013, 67, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Tagliari, B.; Noschang, C.G.; Ferreira, A.G.K.; Ferrari, O.A.; Feksa, L.R.; Wannmacher, C.M.D.; Dalmaz, C.; Wyse, A.T.S. Chronic Variable Stress Impairs Energy Metabolism in Prefrontal Cortex and Hippocampus of Rats: Prevention by Chronic Antioxidant Treatment. Metab. Brain Dis. 2010, 25, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Herbet, M.; Natorska-Chomicka, D.; Korga, A.; Ostrowska, M.; Izdebska, M.; Gawrońska-Grzywacz, M.; Piątkowska-Chmiel, I.; Pawłowski, K.; Ślaska, B.; Poleszak, E.; et al. Altered Expression of Genes Involved in Brain Energy Metabolism as Adaptive Responses in Rats Exposed to Chronic Variable Stress; Changes in Cortical Level of Glucogenic and Neuroactive Amino Acids. Mol. Med. Rep. 2019, 19, 2386–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Symbol of the Gene | Name of the Gene | Ref Seq. | Assay ID | The Length of Amplicon |
|---|---|---|---|---|
| Slc2a3 | solute carrier family 2 (facilitated glucose transporter), member 3 | NM_011401 | Mm01184101_g1 | 80 |
| Gapdh | glyceraldehyde-3-phosphate dehydrogenase | NM_001289726.1 | Mm99999915_g1 | 107 |
| NM_008084.3 | ||||
| Ldha, | lactate dehydrogenase A | NM_001136069 | Mm01612132_g1 | 95 |
| NM_010699.2 | ||||
| Ldhb | lactate dehydrogenase B | AK084659.1 | Mm05733325_s1 | 90 |
| Pkfb3 | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 | NM_001177752.1 NM_001177753.1 NM_001177754.1 NM_001177755.1 NM_001177756.1 NM_001177757.1 NM_001177758.1 NM_133232.3 | Mm00504650_m1 | 98 |
| Hprt | Hypoxanthine guanine phosphoribosyl transferase | NM_013556.2 | Mm00446968_m1 | 65 |
| Tbp | TATA box-binding protein | NM_013684.3 | Mm00446974_m1 | 105 |
| Day of Experiment | 1. Control Group | 2. Chronicially Stressed Group |
|---|---|---|
| 1 | 29.21 ± 0.32 | 30.12 ± 0.89 |
| 7 | 32.23 ± 0.52 | 29.66 ± 0.62 ** |
| 14 | 34.05 ± 0.85 | 30.33 ± 0.74 ** |
| 21 | 34.48 ± 0.88 | 30.18 ± 0.66 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbet, M.; Piątkowska-Chmiel, I.; Motylska, M.; Gawrońska-Grzywacz, M.; Nieradko-Iwanicka, B.; Dudka, J. Alteration in the Expression of Genes Involved in Cerebral Glucose Metabolism as a Process of Adaptation to Stressful Conditions. Brain Sci. 2022, 12, 498. https://doi.org/10.3390/brainsci12040498
Herbet M, Piątkowska-Chmiel I, Motylska M, Gawrońska-Grzywacz M, Nieradko-Iwanicka B, Dudka J. Alteration in the Expression of Genes Involved in Cerebral Glucose Metabolism as a Process of Adaptation to Stressful Conditions. Brain Sciences. 2022; 12(4):498. https://doi.org/10.3390/brainsci12040498
Chicago/Turabian StyleHerbet, Mariola, Iwona Piątkowska-Chmiel, Monika Motylska, Monika Gawrońska-Grzywacz, Barbara Nieradko-Iwanicka, and Jarosław Dudka. 2022. "Alteration in the Expression of Genes Involved in Cerebral Glucose Metabolism as a Process of Adaptation to Stressful Conditions" Brain Sciences 12, no. 4: 498. https://doi.org/10.3390/brainsci12040498
APA StyleHerbet, M., Piątkowska-Chmiel, I., Motylska, M., Gawrońska-Grzywacz, M., Nieradko-Iwanicka, B., & Dudka, J. (2022). Alteration in the Expression of Genes Involved in Cerebral Glucose Metabolism as a Process of Adaptation to Stressful Conditions. Brain Sciences, 12(4), 498. https://doi.org/10.3390/brainsci12040498