When Two Is Better Than One: A Pilot Study on Transcranial Magnetic Stimulation Plus Muscle Vibration in Treating Chronic Pelvic Pain in Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Setting
2.2. Outcome Measures
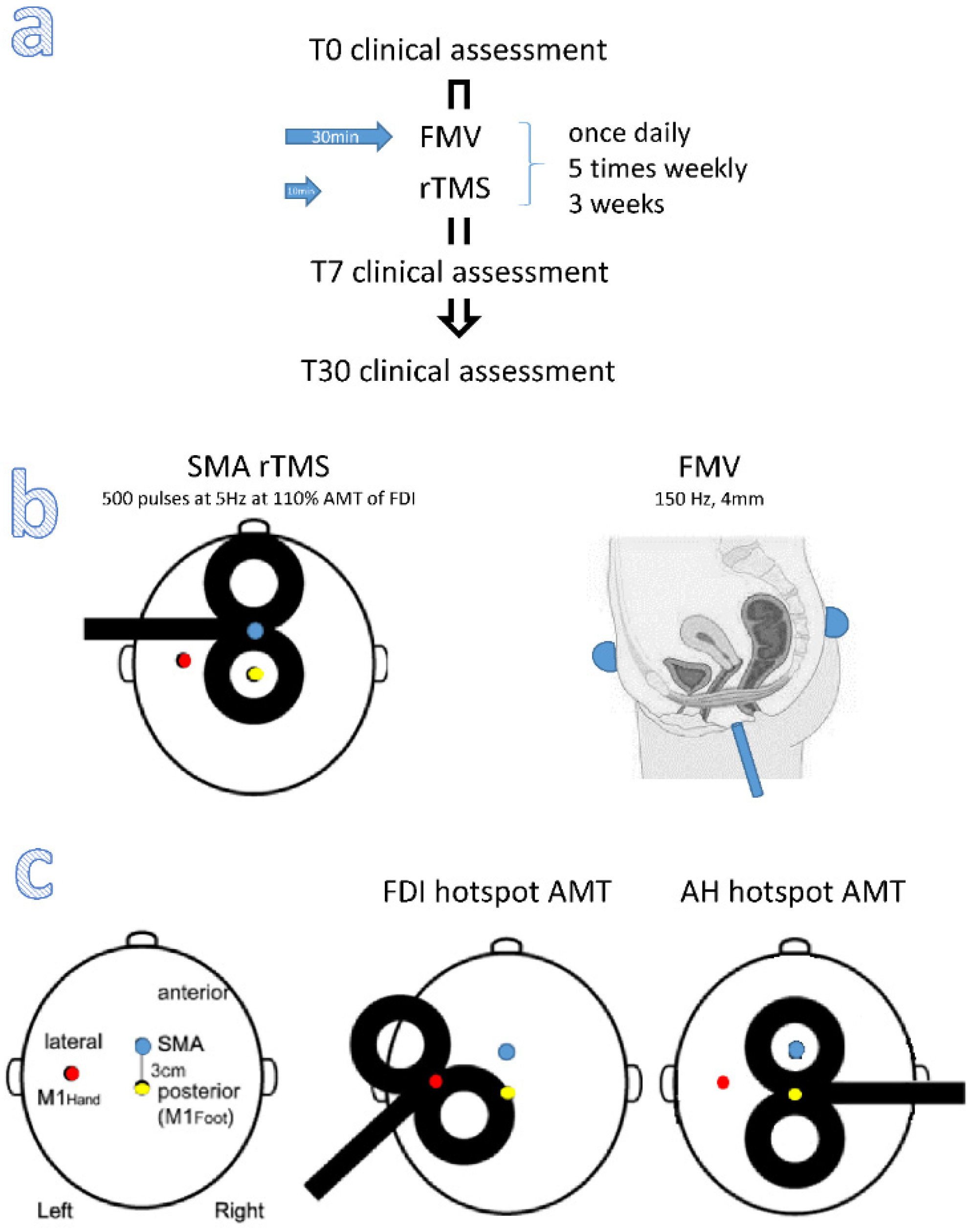
2.3. Stimulation Paradigm
2.4. Power Analysis
2.5. Statistical Analysis
3. Results
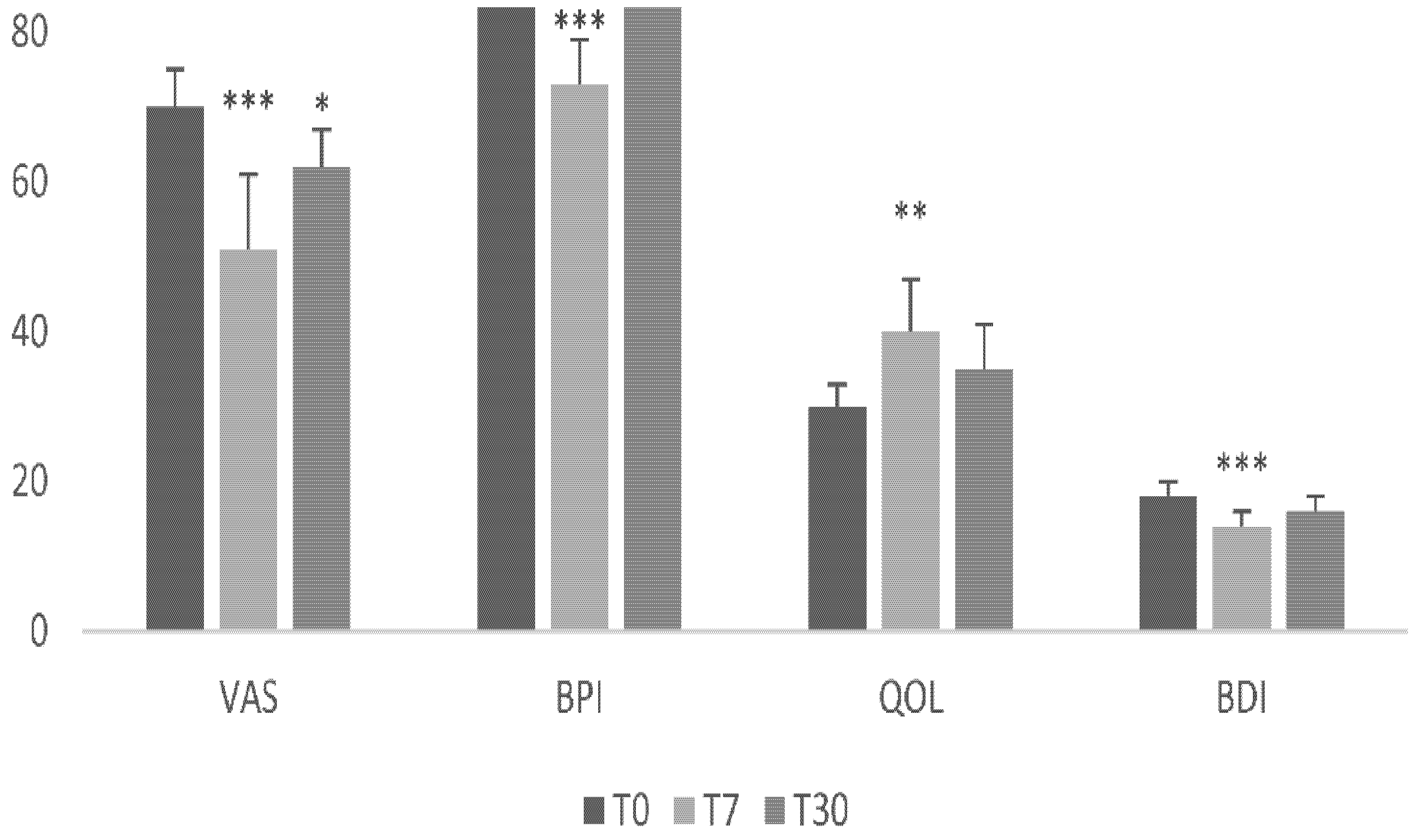
3.1. Primary Outcome Measure
3.2. Secondary Outcome Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dydyk, A.M.; Gupta, N. Chronic Pelvic Pain. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Sanses, T.V.; Chelimsky, G.; McCabe, N.P.; Zolnoun, D.; Janata, J.; Elston, R.; Buffington, C.A.; Simpson, P.; Zhang, L.; Chelimsky, T. The Pelvis and Beyond: Musculoskeletal Tender Points in Women with Chronic Pelvic Pain. Clin. J. Pain. 2016, 32, 659–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berghmans, B. Physiotherapy for pelvic pain and female sexual dysfunction: An untapped resource. Int. Urogynecol. J. 2018, 29, 631–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grinberg, K.; Weissman-Fogel, I.; Lowenstein, L.; Abramov, L.; Granot, M. How Does Myofascial Physical Therapy Attenuate Pain in Chronic Pelvic Pain Syndrome? Pain Res. Manag. 2019, 2019, 6091257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, D.; Kallewaard, J.W.; Rotte, A.; Jameson, J.; Caraway, D. Pain relief and improvement in quality of life with 10 kHz SCS therapy: Summary of clinicalevidence. CNS Neurosci. Ther. 2020, 26, 403–415. [Google Scholar] [CrossRef]
- Carrillo, J.; Witzeman, K.; Alappattu, M.; Lamvu, G. Musculoskeletal Considerations in Female Patients with Chronic Pelvic Pain. Semin. Reprod. Med. 2018, 36, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chang, M.C. Effect of Repetitive Transcranial Magnetic Stimulation on Pain Management: A Systematic Narrative Review. Front. Neurol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Yan, W.; Wan, R.; Lin, Y.; Zhu, X.; Song, G.; Zheng, K.; Wang, Y.; Wang, X. Effects of repetitive transcranial magnetic stimulation on neuropathic pain: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 132, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Passard, A.; Attal, N.; Benadhira, R.; Brasseur, L.; Saba, G.; Sichere, P.; Perrot, S.; Januel, D.; Bouhassira, D. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain 2007, 130, 2661–2670. [Google Scholar] [CrossRef]
- Boyer, L.; Dousset, A.; Roussel, P.; Dossetto, N.; Cammilleri, S.; Piano, V.; Khalfa, S.; Mundler, O.; Donnet, A.; Guedj, E. rTMS in fibromyalgia: A randomized trial evaluating QoL and its brain metabolic substrate. Neurology 2014, 82, 1231–1238. [Google Scholar] [CrossRef]
- Cruccu, G.; Garcia-Larrea, L.; Hansson, P.; Keindl, M.; Lefaucheur, J.; Paulus, W.; Taylor, R.; Tronnier, V.; Truini, A.; Attal, N. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur. J. Neurol. 2016, 23, 1489–1499. [Google Scholar] [CrossRef]
- Hou, W.-H.; Wang, T.-Y.; Kang, J.-H. The effects of add-on non-invasive brain stimulation in fibromyalgia: A meta-analysis and meta-regression of randomized controlled trials. Rheumatology 2016, 55, 1507–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinot-Monange, A.; Moisset, X.; Chauvet, P.; Gremeau, A.-S.; Comptour, A.; Canis, M.; Pereira, B.; Bourdel, N. Repetitive Transcranial Magnetic Stimulation Therapy (rTMS) for Endometriosis Patients with Refractory Pelvic Chronic Pain: A Pilot Study. J. Clin. Med. 2019, 8, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louppe, J.-M.; Nguyen, J.-P.; Robert, R.; Buffenoir, K.; de Chauvigny, E.; Riant, T.; Péréon, Y.; Labat, J.-J.; Nizard, J. Motor cortex stimulation in refractory pelvic and perineal pain: Report of two successful cases. Neurourol. Urodyn. 2013, 32, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.M.; Treister, R.; Raij, T.; Pascual-Leone, A.; Park, L.; Nurmikko, T.; Lenz, F.; Lefaucheur, J.-P.; Lang, M.; Hallett, M.; et al. Transcranial magnetic stimulation of the brain: Guidelines for pain treatment research. Pain 2015, 156, 1601. [Google Scholar] [CrossRef] [Green Version]
- Cervigni, M.; Onesti, E.; Ceccanti, M.; Gori, M.C.; Tartaglia, G.; Campagna, G.; Panico, G.; Vacca, L.; Cambieri, C.; Libonati, L.; et al. Repetitive transcranial magnetic stimulation for chronic neuropathic pain in patients with bladder pain syndrome/interstitial cystitis. Neurourol. Urodyn. 2018, 37, 2678–2687. [Google Scholar] [CrossRef] [PubMed]
- Kairys, A.E.; Schmidt-Wilcke, T.; Puiu, T.; Ichesco, E.; Labus, J.S.; Martucci, K.; Farmer, M.; Ness, T.; Deutsch, G.; Mayer, E.A.; et al. Increased Brain Gray Matter in the Primary Somatosensory Cortex is Associated with Increased Pain and Mood Disturbance in Patients with Interstitial Cystitis/Painful Bladder Syndrome. J. Urol. 2015, 193, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, L.A.; Kutch, J.J.; Tillisch, K.; Naliboff, B.D.; Labus, J.S.; Jiang, Z.; Farmer, M.; Apkarian, A.V.; Mackey, S.; Martucci, K.; et al. Alterations in Resting State Oscillations and Connectivity in Sensory and Motor Networks in Women with Interstitial Cystitis/Painful Bladder Syndrome. J. Urol. 2014, 192, 947–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutch, J.J.; Yani, M.S.; Asavasopon, S.; Kirages, D.J.; Rana, M.; Cosand, L.; Labus, J.S.; Kilpatrick, L.A.; Ashe-McNalley, C.; Farmer, M.A.; et al. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP: Research Network Neuroimaging Study. NeuroImage Clin. 2015, 8, 493–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodworth, D.; Mayer, E.; Leu, K.; Ashe-McNalley, C.; Naliboff, B.D.; Labus, J.S.; Tillisch, K.; Kutch, J.J.; Farmer, M.A.; Apkarian, A.V.; et al. Unique Microstructural Changes in the Brain Associated with Urological Chronic Pelvic Pain Syndrome (UCPPS) Revealed by Diffusion Tensor MRI, Super-Resolution Track Density Imaging, and Statistical Parameter Mapping: A MAPP Network Neuroimaging Study. PLoS ONE 2015, 10, e0140250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrum, A.; Wolff, S.; Van Der Horst, C.; Kuhtz-Buschbeck, J. Motor Cortical Representation of the Pelvic Floor Muscles. J. Urol. 2011, 186, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Yani, M.S.; Wondolowski, J.H.; Eckel, S.P.; Kulig, K.; Fisher, B.E.; Gordon, J.E.; Kutch, J.J. Distributed representation of pelvic floor muscles in human motor cortex. Sci. Rep. 2018, 8, 7213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, M.; Yani, M.S.; Asavasopon, S.; Fisher, B.E.; Kutch, J.J. Brain Connectivity Associated with Muscle Synergies in Humans. J. Neurosci. 2015, 35, 14708–14716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guedes-Aguiar, E.D.O.; Sá-Caputo, D.D.C.D.; Moreira-Marconi, E.; de Macêdo Uchôa, S.M.; De Barros, P.Z.; Valentin, E.K.; Bergmann, A.; Taiar, R.; Bernardo-Filho, M. Effect of whole-body vibration exercise in the pelvic floor muscles of healthy and unhealthy individuals: A narrative review. Transl. Androl. Urol. 2019, 8, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.P.; Paiva, L.L.; Ramos, J.G.L.; Ferla, L. Vibratory perineal stimulation for the treatment of female stress urinary incontinence: A systematic review. Int. Urogynecol. J. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Paolucci, T.; Bellomo, R.G.; Pezzi, L.; Frondaroli, F.; Frondaroli, S.; Santarelli, A.; Barbato, C.; Porreca, A.; Saggini, R. A Novel Rehabilitative Protocol in the Treatment of Mixed Urinary Incontinence in Women: The Effects of Focused Mechano-Acoustic Vibration. BioRes. Open Access 2019, 8, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, I.; Rebolledo, G.; Acharya, G.; Leivseth, G. Mechanical oscillations superimposed on the pelvic floor muscles during Kegel exercises reduce urine leakage in women suffering from stress urinary incontinence: A prospective cohort study with a 2-year follow up. Acta Obstet. Gynecol. Scand. 2018, 97, 1185–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barassi, G.; Bellomo, R.G.; Frondaroli, F.; Frondaroli, S.; Santarelli, A.; Di Felice, P.A.; Supplizi, M.; Palermo, T.; Saggini, R. Integrated Rehabilitation Approach with Manual and Mechanic-Acoustic Vibration Therapies for Urinary Incontinence. In Advancements and Innovations in Health Sciences. Advances in Experimental Medicine and Biology; Pokorski, M., Ed.; Springer: Cham, Switzerland, 2019; Volume 1211. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Pullia, M.; Porcari, B.; Torrisi, M.; La Rosa, G.; Manuli, A.; Billeri, L.; Bramanti, P.; Quattrini, F. Improving Sexual Function by Using Focal Vibrations in Men with Spinal Cord Injury: Encouraging Findings from a Feasibility Study. J. Clin. Med. 2019, 8, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iodice, P.; Ripari, P.; Pezzulo, G. Local high-frequency vibration therapy following eccentric exercises reduces muscle soreness perception and posture alterations in elite athletes. Eur. J. Appl. Physiol. 2019, 119, 539–549. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Milardi, D.; Leo, A.; Filoni, S.; Trinchera, A.; Bramanti, P. Is two better than one? Muscle vibration plus robotic rehabilitation to improve upper limb spasticity and function: A pilot randomized controlled trial. PLoS ONE 2017, 12, e0185936. [Google Scholar] [CrossRef]
- Fall, M.; Baranowski, A.P.; Elneil, S.; Engeler, D.; Hughes, J.; Messelink, E.J.; Oberpenningg, F.; de, C. Williamshet, A.C. EAU guidelines on chronic pelvic pain. Eur. Urol. 2010, 57, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. 1994, 23, 129–138. [Google Scholar]
- Hurst, H.; Bolton, J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J. Manip. Physiol. Ther. 2004, 27, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.; Brown, G. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Burckhardt, C.S.; Anderson, K.L. The Quality of Life Scale (QOLS): Reliability, Validity, and Utilization. Health Qual. Life Outcomes 2003, 1, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauvet, P.; Auclair, C.; Mourgues, C.; Canis, M.; Gerbaud, L.; Bourdel, N. Psychometric properties of the French version of the Endometriosis Health Profile-30, a health-related quality of life instrument. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Nikkola, J.; Holm, A.; Seppänen, M.; Joutsi, T.; Rauhala, E.; Kaipia, A. Repetitive Transcranial Magnetic Stimulation for Chronic Prostatitis/Chronic Pelvic Pain Syndrome: A Prospective Pilot Study. Int. Neurourol. J. 2020, 24, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Brasil-Neto, J.P.; Cohen, L.G.; Panizza, M.; Nilsson, J.; Roth, B.J.; Hallett, M. Optimal focal transcranial magnetic activation of the human motor cortex: Effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J. Clin. Neurophysiol. 1992, 9, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Maruyama, A.; Fujiwara, T.; Nakanishi, R.; Tsuji, S.; Rothwell, J.C. Increased corticospinal excitability after 5 Hz rTMS over the human supplementary motor area. J. Physiol. 2005, 562, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yani, M.S.; Fenske, S.J.; Rodriguez, L.V.; Kutch, J.J. Motor cortical neuromodulation of pelvic floor muscle tone: Potential implications for the treatment of urologic conditions. Neurourol. Urodyn. 2019, 38, 1517–1523. [Google Scholar] [CrossRef]
- Naro, A.; Milardi, D.; Russo, M.; Terranova, C.; Rizzo, V.; Cacciola, A.; Marino, S.; Calabro, R.S.; Quartarone, A. Non-invasive Brain Stimulation, a Tool to Revert Maladaptive Plasticity in Neuropathic Pain. Front. Hum. Neurosci. 2016, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Cocks, K.; Torgerson, D. Sample size calculations for pilot randomized trials: A confidence interval approach. J. Clin. Epidemiol. 2013, 66, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Morley, S.; Dowzer, C.N. Manual for the Leeds Reliable Change Indicator: Simple Excel(tm) Applications for the Analysis of Individual Patient and Group Data; University of Leeds: Leeds, UK, 2014. [Google Scholar]
- Glass, G.V. Primary, Secondary, and Meta-Analysis of Research. Educ. Res. 1976, 5, 3–8. [Google Scholar] [CrossRef]
- Durlak, J.A. How to Select, Calculate, and Interpret Effect Sizes. J. Pediatr. Psychol. 2009, 34, 917–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mease, P.J.; Spaeth, M.; Clauw, D.J.; Arnold, L.M.; Bradley, L.; Russell, I.J.; Kajdasz, D.K.; Walker, D.J.; Chappell, A.S. Estimation of minimum clinically important difference for pain in fibromyalgia. Arthritis Care Res. 2011, 63, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Zeng, L.; Zhang, L.; Bedard, G.; Wong, E.; Tsao, M.; Barnes, E.; Danjoux, C.; Sahgal, A.; Holden, L.; et al. Minimal clinically important di_erences in the brief pain inventory in patients with bone metastases. Support. Care Cancer 2013, 21, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Ding, K.; Chow, E.; Meyer, R.M.; van der Linden, Y.M.; Roos, D.; Hartsell, W.F.; Hoskin, P.; Wu, J.S.Y.; Nabid, A.; et al. Minimal clinically important di_erences in the EORTC QLQ-C30 and brief pain inventory in patients undergoing re-irradiation for painful bone metastases. Qual. Life Res. 2018, 27, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Nizard, J.; Esnault, J.; Bouche, B.; Moreno, A.S.; Lefaucheur, J.-P.; Nguyen, J.-P. Long-Term Relief of Painful Bladder Syndrome by High-Intensity, Low-Frequency Repetitive Transcranial Magnetic Stimulation of the Right and Left Dorsolateral Prefrontal Cortices. Front. Neurosci. 2018, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- De Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 5, 327. [Google Scholar]
- Griffiths, D. Neural control of micturition in humans: A working model. Nat. Rev. Urol. 2015, 12, 695. [Google Scholar] [CrossRef]
- Griffiths, D.; Clarkson, B.; Tadic, S.D.; Resnick, N.M. Brain Mechanisms Underlying Urge Incontinence and its Response to Pelvic Floor Muscle Training. J. Urol. 2015, 194, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Lamusuo, S.; Hirvonen, J.; Lindholm, P.; Martikainen, I.K.; Hagelberg, N.; Parkkola, R.; Taiminen, T.; Hietala, J.; Helin, S.; Virtanen, A.; et al. Neurotransmitters behind pain relief with transcranial magnetic stimulation—Positron emission tomography evidence for release of endogenous opioids. Eur. J. Pain 2017, 21, 1505–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poenaru, D.; Cinteza, D.; Petrusca, I.; Cioc, L.; Dumitrascu, D. Local Application of Vibration in Motor Rehabilitation—Scientific and Practical Considerations. Maedica 2016, 11, 227–231. [Google Scholar] [PubMed]
- Chang, E.; Ghosh, N.; Yanni, D.; Lee, S.; Alexandru, D.; Mozaffar, T. A Review of Spasticity Treatments: Pharmacological and Interventional Approaches. Crit. Rev. Phys. Rehabil. Med. 2013, 25, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, S.; Lin, W.; Li, X.; Andersen, L.L.; Wang, Y. Efficacy of whole body vibration therapy on pain and functional ability in people with non-specific low back pain: A systematic review. BMC Complement. Med. Ther. 2020, 20, 158. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.F.; Calabrò, R.S.; Sale, P.; Vergura, F.; De Cola, M.C.; Militi, A.; Bramanti, P.; Portaro, S.; Filoni, S. Can muscle vibration be the future in the treatment of cerebral palsy-related drooling? A feasibility study. Int. J. Med. Sci. 2019, 16, 1447–1452. [Google Scholar] [CrossRef] [Green Version]
- Chandrashekhar, R.; Wang, H.; Dionne, C.; James, S.; Burzycki, J. Wearable Focal Muscle Vibration on Pain, Balance, Mobility, and Sensation in Individuals with Diabetic Peripheral Neuropathy: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 2415. [Google Scholar] [CrossRef] [PubMed]
- Lauper, M.; Kuhn, A.; Gerber, R.; Luginbühl, H.; Radlinger, L. Pelvic Floor Stimulation: What are the good vibrations? Neurourol. Urodyn. 2008, 28, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Farzinmehr, A.; Moezy, A.; Koohpayehzadeh, J.; Kashanian, M. A Comparative Study of Whole Body Vibration Training and Pelvic Floor Muscle Training on Women’s Stress Urinary Incontinence: Three-Month Follow-Up. J. Fam. Reprod. Health 2015, 9, 147–154. [Google Scholar]
- Marconi, B.; Filippi, G.M.; Koch, G.; Giacobbe, V.; Pecchioli, C.; Versace, V.; Camerota, F.; Saraceni, V.M.; Caltagirone, C. Long-Term Effects on Cortical Excitability and Motor Recovery Induced by Repeated Muscle Vibration in Chronic Stroke Patients. Neurorehabilit. Neural Repair 2010, 25, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Maloney-Hinds, C.; Petrofsky, J.S.; Zimmerman, G.; Hessinger, D.A. The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol. Ther. 2009, 11, 39–43. [Google Scholar] [CrossRef]
- Maloney-Hinds, C.; Petrofsky, J.S.; Zimmerman, G. The effect of 30 Hz vs. 50 Hz passive vibration and duration of vibration on skin blood flow in the arm. Med. Sci. Monit. 2008, 14, CR112–CR116. [Google Scholar] [PubMed]
- Yu, C.O.-L.; Leung, K.-S.; Jiang, J.L.; Wang, T.B.-Y.; Chow, S.K.-H.; Cheung, W.-H. Low-magnitude high-frequency vibration accelerated the foot wound healing of n5-streptozotocin-induced diabetic rats by enhancing glucose transporter 4 and blood microcirculation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khedr, E.M.; Kotb, H.; Kamel, N.F.; AAhmed, M.; Sadek, R.; Rothwell, J.C. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J. Neurol. Neurosurg. Psychiatry 2005, 76, 833–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onesti, E.; Gabriele, M.; Cambieri, C.; Ceccanti, M.; Raccah, R.; Di Stefano, G.; Biasiotta, A.; Truini, A.; Zangen, A.; Inghilleri, M. H-coil repetitive transcranial magnetic stimulation for pain relief in patients with diabetic neuropathy. Eur. J. Pain 2013, 17, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Hirayama, A.; Kishima, H.; Shimokawa, T.; Oshino, S.; Hirata, M.; Tani, N.; Kato, A.; Yoshimine, T. Reduction of intractable deafferentation pain due to spinal cord or peripheral lesion by high-frequency repetitive transcranial magnetic stimulation of the primary motor cortex. J. Neurosurg. 2007, 107, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.; Park, H.; Kim, S. Neural Plasticity in the Brain during Neuropathic Pain. Biomedicines 2021, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Truini, A. A review of Neuropathic Pain: From Guidelines to Clinical Practice. Pain Ther. 2017, 6 (Suppl. 1), 35–42. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20 (Suppl. 1), S2–S12. [Google Scholar] [CrossRef] [Green Version]
- Murer, S.; Polidori, G.; Beaumont, F.; Bogard, F.; Polidori, É.; Kinne, M. Advances in the therapeutic approach of pudendal neuralgia: A systematic review. J. Osteopat. Med. 2021, 122, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.D.; Bennett, W.; Bindoff, A.D.; Bolland, S.; Collins, J.; Langley, R.C.; Garry, M.I.; Summers, J.J.; Hinder, M.R.; Rodger, J.; et al. Subthreshold repetitive transcranial magnetic stimulation drives structural synaptic plasticity in the young and aged motor cortex. Brain Stimul. 2021, 14, 1498–1507. [Google Scholar] [CrossRef]
- Murnion, B.P. Neuropathic pain: Current definition and review of drug treatment. Aust. Prescr. 2018, 41, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Hodaj, H.; Payen, J.-F.; Hodaj, E.; Dumolard, A.; Maindet, C.; Cracowski, J.-L.; Delon-Martin, C.; Lefaucheur, J.-P. Long-term treatment of chronic orofacial, pudendal, and central neuropathic limb pain with repetitive transcranial magnetic stimulation of the motor cortex. Clin. Neurophysiol. 2020, 131, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
| Age (Years) | Disease Duration (Years) | Pain Treatment |
|---|---|---|
| 35 | 8 | no |
| 42 | 5 | tramadol |
| 56 | 13 | NSAID pregabalin |
| 37 | 8 | no |
| 55 | 12 | amitriptyline |
| 32 | 11 | NSAID pregabalin |
| 34 | 9 | no |
| 42 ± 10 | 9 ± 3 | 4 treated 3 not treated |
| T0 | T7 | T30 | ||||||
|---|---|---|---|---|---|---|---|---|
| PGIC | PGIC | |||||||
| 68 | 39 | −42% | 6.4 * | much improved | 64 | −6% | 0.9 | no change |
| 73 | 53 | −27% | 4.4 * | minimally improved | 70 | −4% | 0.6 | no change |
| 60 | 39 | −35% | 4.7 * | improved | 56 | −7% | 0.9 | no change |
| 70 | 49 | −30% | 4.7 * | improved | 59 | −17% | 2.6 * | minimally improved |
| 75 | 59 | −22% | 3.8 * | minimally improved | 62 | −17% | 2.9 * | minimally improved |
| 72 | 65 | −9% | 1.5 | no change | 68 | −5% | 0.9 | no change |
| 70 | 55 | −22% | 3.5 * | minimally improved | 59 | −17% | 2.6 * | minimally improved |
| 70 ± 5 | 51 ± 10 | −27 ± 11% | 3 improved 3 partially improved 1 no improvement | 62 ± 5 | −10 ± 6% | 3 partially improved 4 no improvement | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabrò, R.S.; Billeri, L.; Porcari, B.; Pignolo, L.; Naro, A. When Two Is Better Than One: A Pilot Study on Transcranial Magnetic Stimulation Plus Muscle Vibration in Treating Chronic Pelvic Pain in Women. Brain Sci. 2022, 12, 396. https://doi.org/10.3390/brainsci12030396
Calabrò RS, Billeri L, Porcari B, Pignolo L, Naro A. When Two Is Better Than One: A Pilot Study on Transcranial Magnetic Stimulation Plus Muscle Vibration in Treating Chronic Pelvic Pain in Women. Brain Sciences. 2022; 12(3):396. https://doi.org/10.3390/brainsci12030396
Chicago/Turabian StyleCalabrò, Rocco Salvatore, Luana Billeri, Bruno Porcari, Loris Pignolo, and Antonino Naro. 2022. "When Two Is Better Than One: A Pilot Study on Transcranial Magnetic Stimulation Plus Muscle Vibration in Treating Chronic Pelvic Pain in Women" Brain Sciences 12, no. 3: 396. https://doi.org/10.3390/brainsci12030396
APA StyleCalabrò, R. S., Billeri, L., Porcari, B., Pignolo, L., & Naro, A. (2022). When Two Is Better Than One: A Pilot Study on Transcranial Magnetic Stimulation Plus Muscle Vibration in Treating Chronic Pelvic Pain in Women. Brain Sciences, 12(3), 396. https://doi.org/10.3390/brainsci12030396







