New Insight in Hyperinsulinism/Hyperammonemia Syndrome by Magnetic Resonance Imaging and Spectroscopy
Abstract
1. Introduction
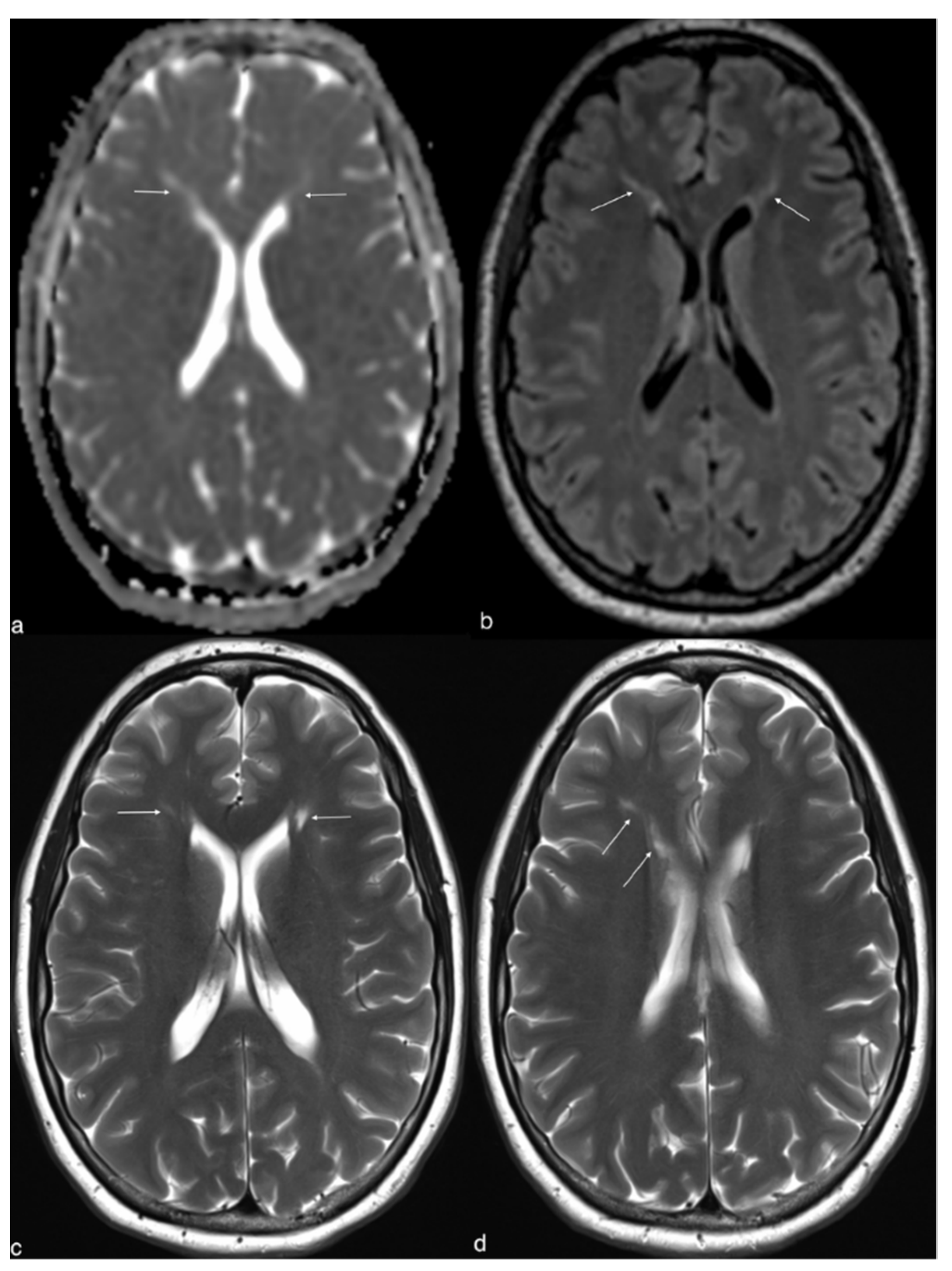
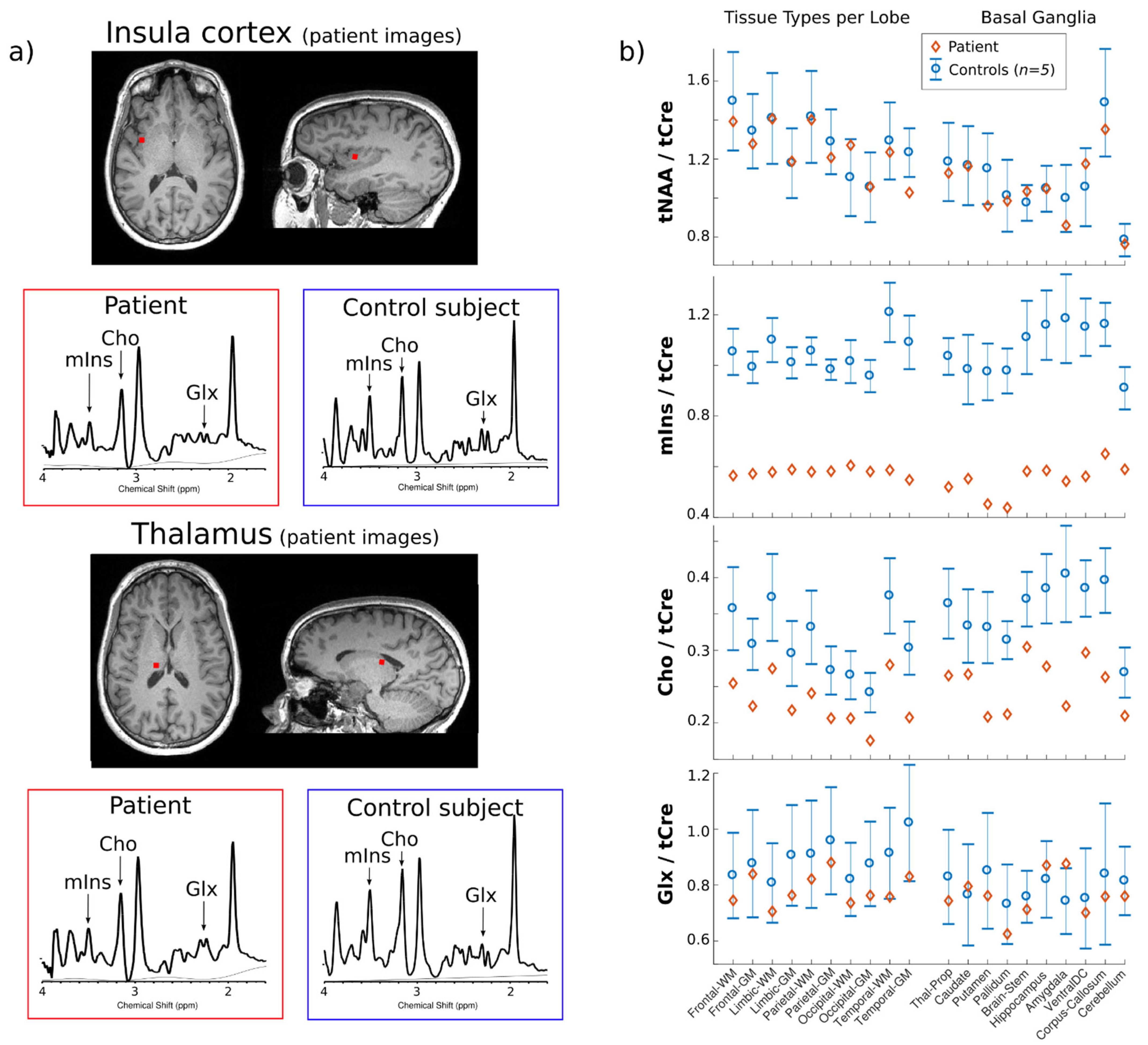
2. Case Report
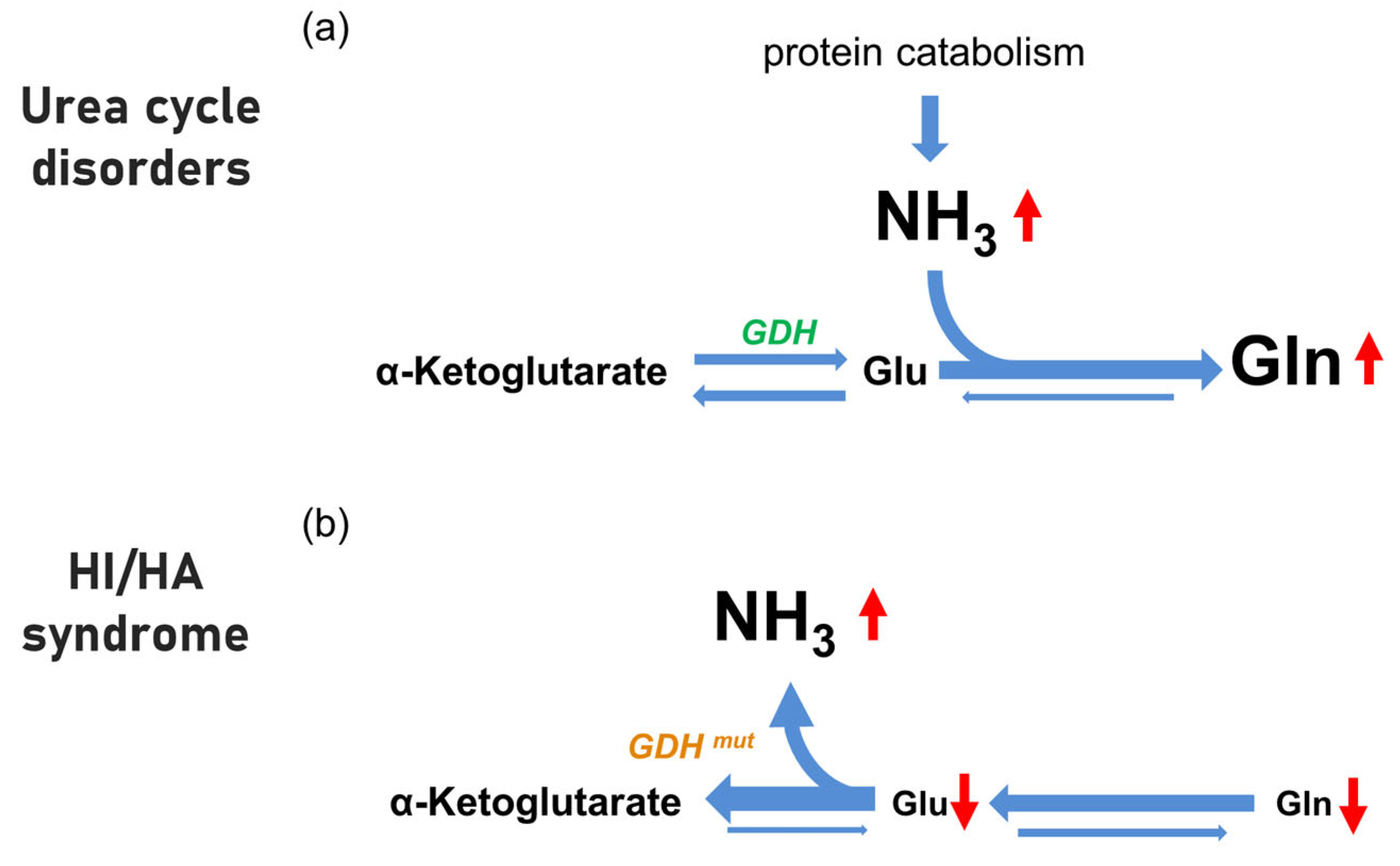
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cochrane, W.A.; Payne, W.W.; Simpkiss, M.J.; Woolf, L.I. Familial hypoglycemia precipitated by amino acids. J. Clin. Investig. 1956, 35, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.A.; Lieu, Y.K.; Hsu, B.Y.; Burlina, A.B.; Greenberg, C.R.; Hopwood, N.J.; Perlman, K.; Rich, B.H.; Zammarchi, E.; Poncz, M. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N. Engl. J. Med. 1998, 338, 1352–1357. [Google Scholar] [CrossRef]
- Stanley, C.A. Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children. Am. J. Clin. Nutr. 2009, 90, 862S–866S. [Google Scholar] [CrossRef] [PubMed]
- Bahi-Buisson, N.; Roze, E.; Dionisi, C.; Escande, F.; Valayannopoulos, V.; Feillet, F.; Heinrichs, C.; Chadefaux-Vekemans, B.; Dan, B.; de Lonlay, P. Neurological aspects of hyperinsulinism-hyperammonaemia syndrome. Dev. Med. Child Neurol. 2008, 50, 945–949. [Google Scholar] [CrossRef]
- Treberg, J.R.; Brosnan, M.E.; Watford, M.; Brosnan, J.T. On the reversibility of glutamate dehydrogenase and the source of hyperammonemia in the hyperinsulinism/hyperammonemia syndrome. Adv. Enzyme Regul. 2010, 50, 34–43. [Google Scholar] [CrossRef] [PubMed]
- De Lonlay, P.; Benelli, C.; Fouque, F.; Ganguly, A.; Aral, B.; Dionisi-Vici, C.; Touati, G.; Heinrichs, C.; Rabier, D.; Kamoun, P.; et al. Hyperinsulinism and hyperammonemia syndrome: Report of twelve unrelated patients. Pediatric Res. 2001, 50, 353–357. [Google Scholar] [CrossRef]
- Adeva, M.M.; Souto, G.; Blanco, N.; Donapetry, C. Ammonium metabolism in humans. Metab. Clin. Exp. 2012, 61, 1495–1511. [Google Scholar] [CrossRef]
- Haberle, J. Clinical and biochemical aspects of primary and secondary hyperammonemic disorders. Arch. Biochem. Biophys. 2013, 536, 101–108. [Google Scholar] [CrossRef]
- Gonzalez Melo, M.; Remacle, N.; Cudre-Cung, H.P.; Roux, C.; Poms, M.; Cudalbu, C.; Barroso, M.; Gersting, S.W.; Feichtinger, R.G.; Mayr, J.A.; et al. The first knock-in rat model for glutaric aciduria type I allows further insights into pathophysiology in brain and periphery. Mol. Genet. Metab. 2021, 133, 157–181. [Google Scholar] [CrossRef]
- Gonzalez Melo, M.; Fontana, A.O.; Viertl, D.; Allenbach, G.; Prior, J.O.; Rotman, S.; Feichtinger, R.G.; Mayr, J.A.; Costanzo, M.; Caterino, M.; et al. A knock-in rat model unravels acute and chronic renal toxicity in glutaric aciduria type I. Mol. Genet. Metab. 2021, 134, 287–300. [Google Scholar] [CrossRef]
- Oz, G.; Alger, J.R.; Barker, P.B.; Bartha, R.; Bizzi, A.; Boesch, C.; Bolan, P.J.; Brindle, K.M.; Cudalbu, C.; Dincer, A.; et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014, 270, 658–679. [Google Scholar] [CrossRef] [PubMed]
- Rudkin, T.M.; Arnold, D.L. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch. Neurol. 1999, 56, 919–926. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gropman, A.L.; Sailasuta, N.; Harris, K.C.; Abulseoud, O.; Ross, B.D. Ornithine transcarbamylase deficiency with persistent abnormality in cerebral glutamate metabolism in adults. Radiology 2009, 252, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Colon, I.; Fricke, S.; VanMeter, J.; Gropman, A.L. Advances in urea cycle neuroimaging: Proceedings from the 4th International Symposium on urea cycle disorders, Barcelona, Spain, September 2013. Mol. Genet. Metab. 2014, 113, 118–126. [Google Scholar] [CrossRef]
- Haberle, J.; Burlina, A.; Chakrapani, A.; Dixon, M.; Karall, D.; Lindner, M.; Mandel, H.; Martinelli, D.; Pintos-Morell, G.; Santer, R.; et al. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J. Inherit. Metab. Dis. 2019, 42, 1192–1230. [Google Scholar] [CrossRef]
- Luczkowska, K.; Stekelenburg, C.; Sloan-Bena, F.; Ranza, E.; Gastaldi, G.; Schwitzgebel, V.; Maechler, P. Hyperinsulinism associated with GLUD1 mutation: Allosteric regulation and functional characterization of p.G446V glutamate dehydrogenase. Hum. Genom. 2020, 14, 9. [Google Scholar] [CrossRef]
- Klauser, A.; Klauser, P.; Grouiller, F.; Courvoisier, S.; Lazeyras, F. Whole-brain high-resolution metabolite mapping with 3D compressed-sensing SENSE low-rank (1) H FID-MRSI. NMR Biomed. 2022, 35, e4615. [Google Scholar] [CrossRef]
- U-King-Im , J.M.; Yu, E.; Bartlett, E.; Soobrah, R.; Kucharczyk, W. Acute hyperammonemic encephalopathy in adults: Imaging findings. Am. J. Neuroradiol. 2011, 32, 413–418. [Google Scholar] [CrossRef]
- Hershman, M.; Carmody, R.; Udayasankar, U.K. Case 252: Acute Hyperammonemic Encephalopathy Resulting from Late-Onset Ornithine Transcarbamylase Deficiency. Radiology 2018, 287, 353–359. [Google Scholar] [CrossRef]
- Upadhyay, R.; Bleck, T.P.; Busl, K.M. Hyperammonemia: What Urea-lly Need to Know: Case Report of Severe Noncirrhotic Hyperammonemic Encephalopathy and Review of the Literature. Case Rep. Med. 2016, 2016, 8512721. [Google Scholar] [CrossRef]
- Brusilow, W.S.A. Saul Brusilow: Understanding and treating diseases of ammonia toxicity. Anal. Biochem. 2022, 636, 114478. [Google Scholar] [CrossRef] [PubMed]
- Haussinger, D.; Butz, M.; Schnitzler, A.; Gorg, B. Pathomechanisms in hepatic encephalopathy. Biol. Chem. 2021, 402, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Lichter-Konecki, U. Profiling of astrocyte properties in the hyperammonaemic brain: Shedding new light on the pathophysiology of the brain damage in hyperammonaemia. J. Inherit. Metab. Dis. 2008, 31, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Rackayova, V.; Pierzchala, K.; Grosse, J.; McLin, V.A.; Cudalbu, C. Longitudinal neurometabolic changes in the hippocampus of a rat model of chronic hepatic encephalopathy. J. Hepatol. 2019, 71, 505–515. [Google Scholar] [CrossRef]
- Rackayova, V.; Braissant, O.; McLin, V.A.; Berset, C.; Lanz, B.; Cudalbu, C. 1H and 31P magnetic resonance spectroscopy in a rat model of chronic hepatic encephalopathy: In vivo longitudinal measurements of brain energy metabolism. Metab. Brain Dis. 2016, 31, 1303–1314. [Google Scholar] [CrossRef]
- Wong, Y.C.; Au, W.L.; Xu, M.; Ye, J.; Lim, C.C. Magnetic resonance spectroscopy in adult-onset citrullinemia: Elevated glutamine levels in comatose patients. Arch. Neurol. 2007, 64, 1034–1037. [Google Scholar] [CrossRef][Green Version]
- Balata, S.; Olde Damink, S.W.; Ferguson, K.; Marshall, I.; Hayes, P.C.; Deutz, N.E.; Williams, R.; Wardlaw, J.; Jalan, R. Induced hyperammonemia alters neuropsychology, brain MR spectroscopy and magnetization transfer in cirrhosis. Hepatology 2003, 37, 931–939. [Google Scholar] [CrossRef]
- Shawcross, D.L.; Balata, S.; Olde Damink, S.W.; Hayes, P.C.; Wardlaw, J.; Marshall, I.; Deutz, N.E.; Williams, R.; Jalan, R. Low myo-inositol and high glutamine levels in brain are associated with neuropsychological deterioration after induced hyperammonemia. Am. J. Physiol. Gastrointest Liver Physiol. 2004, 287, G503–G509. [Google Scholar] [CrossRef][Green Version]
- Sen, K.; Whitehead, M.T.; Gropman, A.L. Multimodal imaging in urea cycle-related neurological disease—What can imaging after hyperammonemia teach us? Transl. Sci. Rare Dis. 2020, 5, 87–95. [Google Scholar] [CrossRef]
- Sen, K.; Anderson, A.A.; Whitehead, M.T.; Gropman, A.L. Review of Multi-Modal Imaging in Urea Cycle Disorders: The Old, the New, the Borrowed, and the Blue. Front. Neurol. 2021, 12, 632307. [Google Scholar] [CrossRef]
- Roze, E.; Azuar, C.; Menuel, C.; Haberle, J.; Guillevin, R. Usefulness of magnetic resonance spectroscopy in urea cycle disorders. Pediatric Neurol. 2007, 37, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.G.; Yoo, H.W. Localized proton MR spectroscopy in infants with urea cycle defect. Am. J. Neuroradiol. 2001, 22, 834–837. [Google Scholar] [PubMed]
- Chavarria, L.; Alonso, J.; Garcia-Martinez, R.; Simon-Talero, M.; Ventura-Cots, M.; Ramirez, C.; Torrens, M.; Vargas, V.; Rovira, A.; Cordoba, J. Brain magnetic resonance spectroscopy in episodic hepatic encephalopathy. J. Cereb. Blood Flow Metab. 2013, 33, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Spahr, L.; Vingerhoets, F.; Lazeyras, F.; Delavelle, J.; DuPasquier, R.; Giostra, E.; Mentha, G.; Terrier, F.; Hadengue, A. Magnetic resonance imaging and proton spectroscopic alterations correlate with parkinsonian signs in patients with cirrhosis. Gastroenterology 2000, 119, 774–781. [Google Scholar] [CrossRef]
- Ziyeh, S.; Thiel, T.; Spreer, J.; Klisch, J.; Schumacher, M. Valproate-induced encephalopathy: Assessment with MR imaging and 1H MR spectroscopy. Epilepsia 2002, 43, 1101–1105. [Google Scholar] [CrossRef]
- Gropman, A.L.; Prust, M.; Breeden, A.; Fricke, S.; VanMeter, J. Urea cycle defects and hyperammonemia: Effects on functional imaging. Metab. Brain Dis. 2013, 28, 269–275. [Google Scholar] [CrossRef]
- Gropman, A.L.; Seltzer, R.R.; Yudkoff, M.; Sawyer, A.; VanMeter, J.; Fricke, S.T. 1H MRS allows brain phenotype differentiation in sisters with late onset ornithine transcarbamylase deficiency (OTCD) and discordant clinical presentations. Mol. Genet. Metab. 2008, 94, 52–60. [Google Scholar] [CrossRef][Green Version]
- Karaca, M.; Martin-Levilain, J.; Grimaldi, M.; Li, L.; Dizin, E.; Emre, Y.; Maechler, P. Liver Glutamate Dehydrogenase Controls Whole-Body Energy Partitioning Through Amino Acid-Derived Gluconeogenesis and Ammonia Homeostasis. Diabetes 2018, 67, 1949–1961. [Google Scholar] [CrossRef]
- Stanley, C.A. Hyperinsulinism/hyperammonemia syndrome: Insights into the regulatory role of glutamate dehydrogenase in ammonia metabolism. Mol. Genet. Metab. 2004, 81 (Suppl. 1), S45–S51. [Google Scholar] [CrossRef]
- Palladino, A.A.; Stanley, C.A. The hyperinsulinism/hyperammonemia syndrome. Rev. Endocr. Metab. Disord. 2010, 11, 171–178. [Google Scholar] [CrossRef]
- El-Gharbawy, A.H. Hyperinsulinism/Hyperammonemia Syndrome: A synopsis. Mol. Genet. Metab. 2005, 84, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Jeitner, T.M. Central Role of Glutamate Metabolism in the Maintenance of Nitrogen Homeostasis in Normal and Hyperammonemic Brain. Biomolecules 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Brusilow, S.W.; Koehler, R.C.; Traystman, R.J.; Cooper, A.J. Astrocyte glutamine synthetase: Importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics 2010, 7, 452–470. [Google Scholar] [CrossRef] [PubMed]
| Disease | MRI Findings | MRS Findings | Ref. |
|---|---|---|---|
| OTC | NA | Elevated glutamine in posterior cingulate gray matter, parietal and frontal WM. Reduction in myoinositol and choline in parietal and frontal white matter, thalamus and posterior cingulate gray matter | [36] |
| OTC | Increased signal on T2-weighted and diffusion-weighted images in the basal ganglia, claustrum, frontoparietal WM, pontine tegmentum, and left brachium pontis | Elevated glutamine. Reduction in myoinositol and choline | [29] |
| OTC | No structural abnormalities in gray or white matter | Elevated glutamine and glutamate. Reduction in myoinositol and choline | [37] |
| Type II citrullinemia | Bilateral, non-enhancing abnormalities of the globus pallidus, insular cortex, and cingulate gyrus on T2-weighted and diffusion-weighted MRI | Elevated glutamine and glutamate. Reduction in myoinositol and choline | [26] |
| Hepatic encephalopathy | Elevated apparent diffusion coefficient values in the corticospinal tract and parietal white matter | Elevated glutamine, reduced myoinositol and choline and non-significant difference in glutamate and N-acetylaspartate | [33] |
| Hepatic encephalopathy | NA | Elevated glutamine and glutamate. Reduction in myoinositol and choline | [27] |
| Hepatic encephalopathy | Occipital white matter and basal ganglia had significant hyperintensity | Elevated glutamine and glutamate. Reduction in myoinositol and choline | [34] |
| Valproate-induced encephalopathy | Metabolic-toxic lesion pattern with bilateral T2-hyperintense lesion in the cerebellar white matter and in the globus pallidus | Elevated glutamine and glutamate. Reduction in myoinositol and choline | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gariani, K.; Klauser, A.; Vargas, M.I.; Lazeyras, F.; Tran, C. New Insight in Hyperinsulinism/Hyperammonemia Syndrome by Magnetic Resonance Imaging and Spectroscopy. Brain Sci. 2022, 12, 389. https://doi.org/10.3390/brainsci12030389
Gariani K, Klauser A, Vargas MI, Lazeyras F, Tran C. New Insight in Hyperinsulinism/Hyperammonemia Syndrome by Magnetic Resonance Imaging and Spectroscopy. Brain Sciences. 2022; 12(3):389. https://doi.org/10.3390/brainsci12030389
Chicago/Turabian StyleGariani, Karim, Antoine Klauser, Maria Isabel Vargas, François Lazeyras, and Christel Tran. 2022. "New Insight in Hyperinsulinism/Hyperammonemia Syndrome by Magnetic Resonance Imaging and Spectroscopy" Brain Sciences 12, no. 3: 389. https://doi.org/10.3390/brainsci12030389
APA StyleGariani, K., Klauser, A., Vargas, M. I., Lazeyras, F., & Tran, C. (2022). New Insight in Hyperinsulinism/Hyperammonemia Syndrome by Magnetic Resonance Imaging and Spectroscopy. Brain Sciences, 12(3), 389. https://doi.org/10.3390/brainsci12030389






