Hippocampal Subfields and White Matter Connectivity in Patients with Subclinical Geriatric Depression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Neuropsychological Tests
2.2. Structural MRI Acquisition
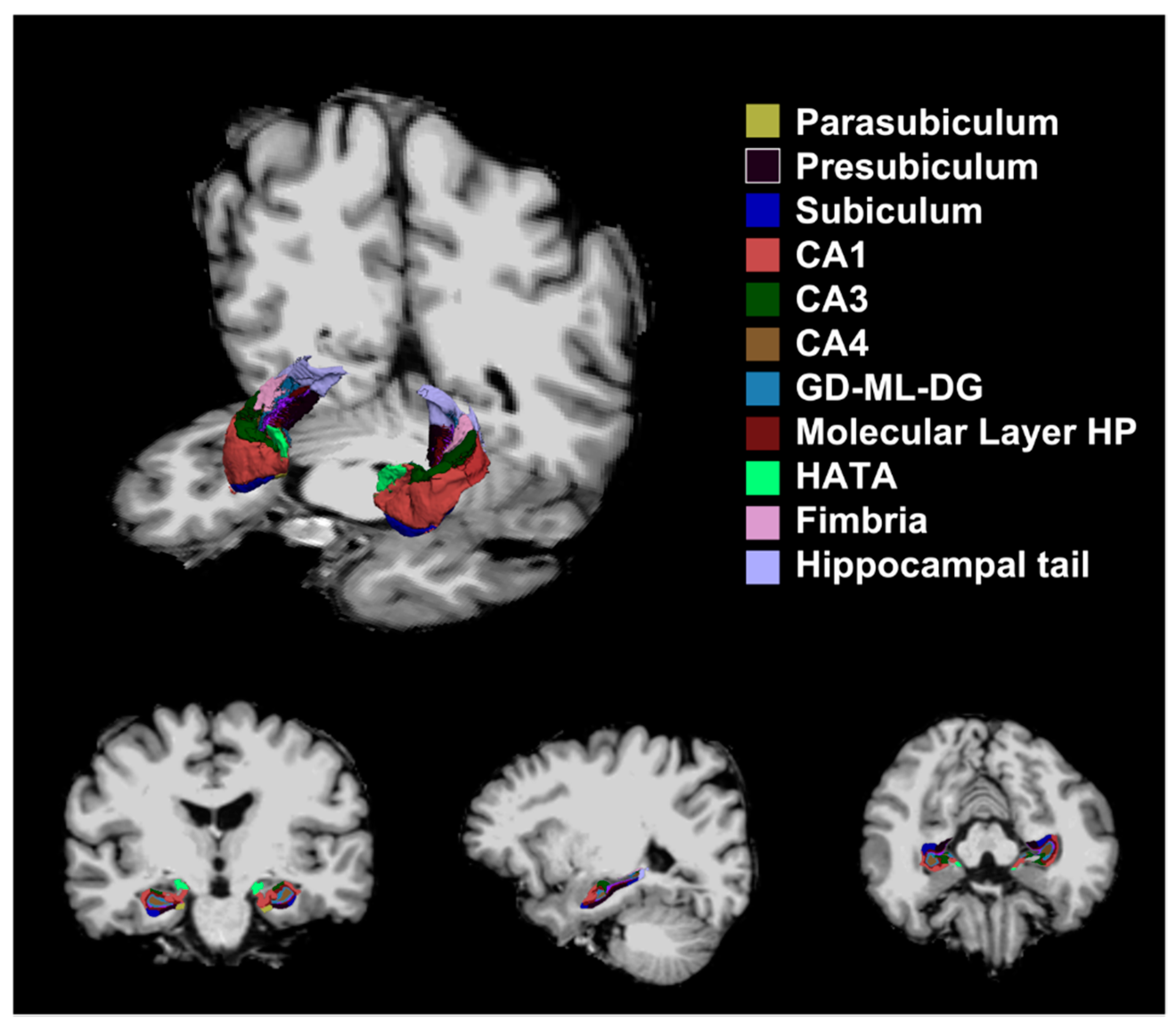
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics and Neuropsychological Functions in Each Group
3.2. Hippocampal Microstructure
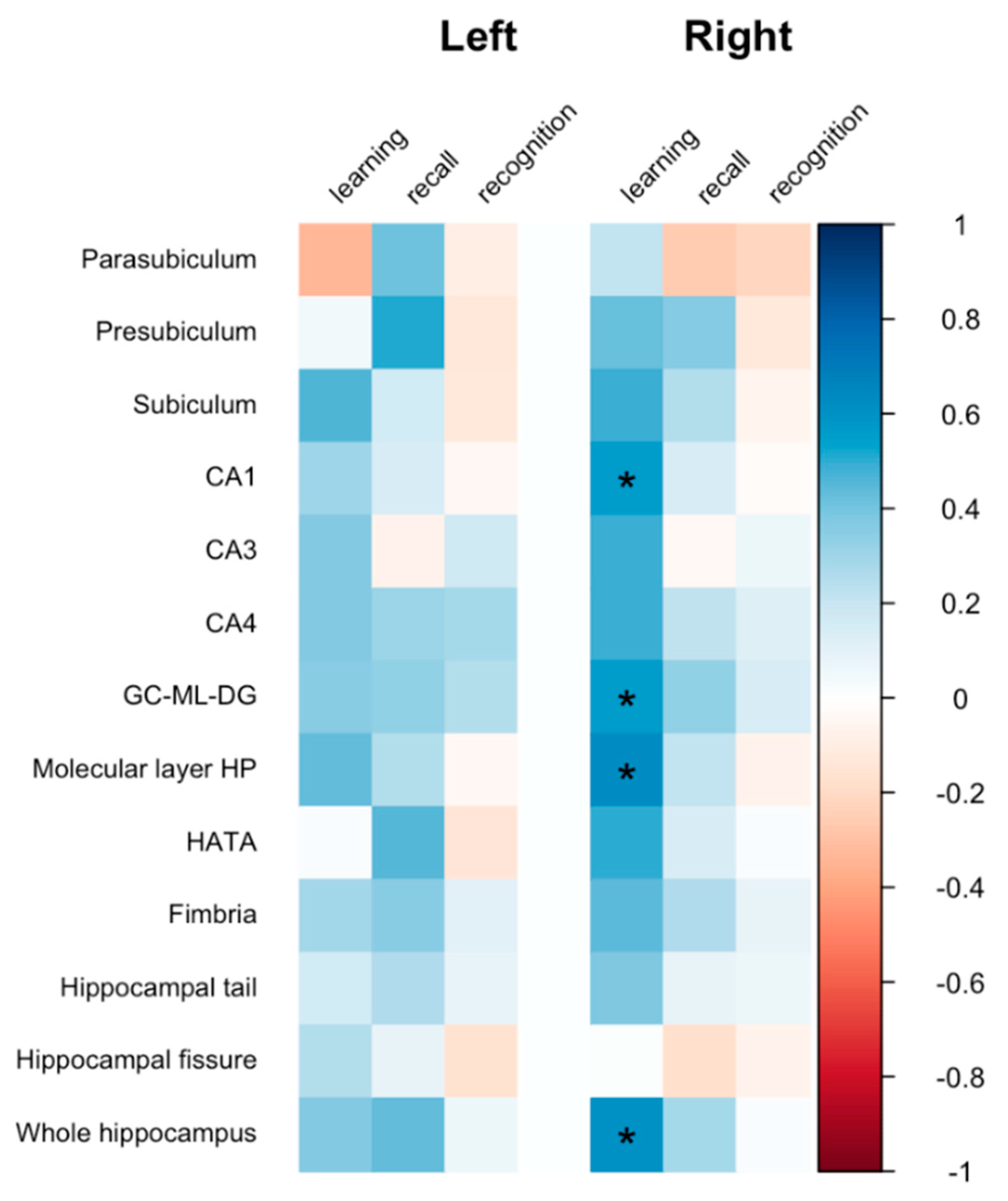
3.3. Association between Hippocampal Microstructure and Verbal Memory
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korten, N.C.M.; Penninx, B.W.J.H.; Kok, R.M.; Stek, M.L.; Voshaar, R.C.O.; Deeg, D.J.H.; Comijs, H.C. Heterogeneity of Late-Life Depression: Relationship with Cognitive Functioning. Int. Psychogeriatr. 2014, 26, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, H.G.; Blazer, D.G. Epidemiology of Geriatric Affective Disorders. Clin. Geriatr. Med. 1992, 8, 235–251. [Google Scholar] [CrossRef]
- Kahn, R.L.; Zarit, S.H.; Hilbert, N.M.; Niederehe, G. Memory Complaint and Impairment in the Aged: The Effect of Depression and Altered Brain Function. Arch. Gen. Psychiatry 1975, 32, 1569–1573. [Google Scholar] [CrossRef]
- Potter, G.G.; Steffens, D.C. Contribution of Depression to Cognitive Impairment and Dementia in Older Adults. Neurologist 2007, 13, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Jorm, A.F. Is Depression a Risk Factor for Dementia or Cognitive Decline? Neurobiol. Aging 2000, 21, 215. [Google Scholar] [CrossRef]
- Aisen, P.S.; Petersen, R.C.; Donohue, M.C.; Gamst, A.; Raman, R.; Thomas, R.G.; Walter, S.; Trojanowski, J.Q.; Shaw, L.M.; Beckett, L.A.; et al. Clinical Core of the Alzheimer’s Disease Neuroimaging Initiative: Progress and Plans. Alzheimer’s Dement. 2010, 6, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosconi, L.; Tsui, W.-H.; Santi, S.D.; Li, J.; Rusinek, H.; Convit, A.; Li, Y.; Boppana, M.; de Leon, M.J. Reduced Hippocampal Metabolism in MCI and AD. Neurology 2005, 64, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Videbech, P.; Ravnkilde, B. Hippocampal Volume and Depression: A Meta-Analysis of MRI Studies. Am. J. Psychiat. 2004, 161, 1957–1966. [Google Scholar] [CrossRef]
- Dotson, V.M.; Davatzikos, C.; Kraut, M.A.; Resnick, S.M. Depressive Symptoms and Brain Volumes in Older Adults: A Longitudinal Magnetic Resonance Imaging Study. J. Psychiatry Neurosci. Jpn. 2009, 34, 367–375. [Google Scholar]
- Salloway, S.; Malloy, P.; Kohn, R.; Gillard, E.; Duffy, J.; Rogg, J.; Tung, G.; Richardson, E.; Thomas, C.; Westlake, R. MRI and Neuropsychological Differences in Early- and Late-Life-Onset Geriatric Depression. Neurology 1996, 46, 1567–1574. [Google Scholar] [CrossRef]
- Geerlings, M.I.; Brickman, A.M.; Schupf, N.; Devanand, D.P.; Luchsinger, J.A.; Mayeux, R.; Small, S.A. Depressive Symptoms, Antidepressant Use, and Brain Volumes on MRI in a Population-Based Cohort of Old Persons without Dementia. J. Alzheimer’s Dis. 2012, 30, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Negreira, A.; LaViolette, P.; Bakkour, A.; Sperling, R.A.; Dickerson, B.C. Intrinsic Interhemispheric Hippocampal Functional Connectivity Predicts Individual Differences in Memory Performance Ability. Hippocampus 2010, 20, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postans, M.; Parker, G.D.; Lundell, H.; Ptito, M.; Hamandi, K.; Gray, W.P.; Aggleton, J.P.; Dyrby, T.B.; Jones, D.K.; Winter, M. Uncovering a Role for the Dorsal Hippocampal Commissure in Recognition Memory. Cereb. Cortex 2019, 30, 1001–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.N.; Cho, M.J. Development of the Korean Version of the Geriatric Depression Scale and Its Short Form among Elderly Psychiatric Patients. J. Psychosom. Res. 2004, 57, 297–305. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.U.; Lee, D.Y.; Kim, K.W.; Jhoo, J.H.; Kim, J.H.; Lee, K.H.; Kim, S.Y.; Han, S.H.; Woo, J.I. Development of the Korean Version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): Clinical and Neuropsychological Assessment Batteries. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2002, 57, P47–P53. [Google Scholar] [CrossRef] [Green Version]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Buckner, R.L.; Head, D.; Parker, J.; Fotenos, A.F.; Marcus, D.; Morris, J.C.; Snyder, A.Z. A Unified Approach for Morphometric and Functional Data Analysis in Young, Old, and Demented Adults Using Automated Atlas-Based Head Size Normalization: Reliability and Validation against Manual Measurement of Total Intracranial Volume. Neuroimage 2004, 23, 724–738. [Google Scholar] [CrossRef]
- Lee, J.; Im, S.-J.; Lee, S.-G.; Stadlin, A.; Son, J.-W.; Shin, C.-J.; Ju, G.; Lee, S.-I.; Kim, S. Volume of Hippocampal Subfields in Patients with Alcohol Dependence. Psychiatry Res. Neuroimaging 2016, 258, 16–22. [Google Scholar] [CrossRef]
- Sämann, P.G.; Iglesias, J.E.; Gutman, B.; Grotegerd, D.; Leenings, R.; Flint, C.; Dannlowski, U.; Clarke-Rubright, E.K.; Morey, R.A.; van Erp, T.G.M.; et al. FreeSurfer-based Segmentation of Hippocampal Subfields: A Review of Methods and Applications, with a Novel Quality Control Procedure for ENIGMA Studies and Other Collaborative Efforts. Hum. Brain Mapp. 2020, 43, 207–233. [Google Scholar] [CrossRef]
- Yeh, F. DSI Studio. Zenodo. 2021. Available online: https://zenodo.org/record/4978980 (accessed on 15 January 2022).
- Yeh, F.-C.; Tseng, W.-Y.I. NTU-90: A High Angular Resolution Brain Atlas Constructed by q-Space Diffeomorphic Reconstruction. Neuroimage 2011, 58, 91–99. [Google Scholar] [CrossRef]
- Yeh, F.-C.; Wedeen, V.J.; Tseng, W.-Y.I. Estimation of Fiber Orientation and Spin Density Distribution by Diffusion Deconvolution. Neuroimage 2011, 55, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C.; Badre, D.; Verstynen, T. Connectometry: A Statistical Approach Harnessing the Analytical Potential of the Local Connectome. Neuroimage 2016, 125, 162–171. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Liu, M.; Ma, L.; Yu, S. Lower Hippocampal Subfields Volume in Relation to Anxiety in Medication-Overuse Headache. Mol. Pain 2018, 14, 1744806918761257. [Google Scholar] [CrossRef] [PubMed]
- Szymkowicz, S.M.; McLaren, M.E.; O’Shea, A.; Woods, A.J.; Anton, S.D.; Dotson, V.M. Depressive Symptoms Modify Age Effects on Hippocampal Subfields in Older Adults. Geriatr. Gerontol. Int. 2017, 17, 1494–1500. [Google Scholar] [CrossRef] [Green Version]
- Conrad, C.D. Chronic Stress-Induced Hippocampal Vulnerability: The Glucocorticoid Vulnerability Hypothesis. Rev. Neurosci. 2008, 19, 395–412. [Google Scholar] [CrossRef] [Green Version]
- Edlow, B.L.; McNab, J.A.; Witzel, T.; Kinney, H.C. The Structural Connectome of the Human Central Homeostatic Network. Brain Connect. 2016, 6, 187–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyszka, J.M.; Readhead, C.; Bearer, E.L.; Pautler, R.G.; Jacobs, R.E. Statistical Diffusion Tensor Histology Reveals Regional Dysmyelination Effects in the Shiverer Mouse Mutant. Neuroimage 2006, 29, 1058–1065. [Google Scholar] [CrossRef] [Green Version]
- Boretius, S.; Escher, A.; Dallenga, T.; Wrzos, C.; Tammer, R.; Brück, W.; Nessler, S.; Frahm, J.; Stadelmann, C. Assessment of Lesion Pathology in a New Animal Model of MS by Multiparametric MRI and DTI. Neuroimage 2011, 59, 2678–2688. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Lee, E.J.; Lee, J.E. The Fornix: Functional Anatomy, Normal Neuroimaging, and Various Pathological Conditions. Investig. Magn. Reson. Imag. 2021, 25, 59–75. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Eales, B.A.; Biddle, F.G. Short-Term and Long-Term Memory Deficits in Handedness Learning in Mice with Absent Corpus Callosum and Reduced Hippocampal Commissure. Behav. Brain Res. 2013, 245, 145–151. [Google Scholar] [CrossRef]

| Subclinical Depression (n = 19) | Control (n = 19) | t or u | p | |
|---|---|---|---|---|
| Age | 72.37 ± 4.60 | 69.58 ± 4.51 | 1.89 | 0.067 |
| Sex | Male: 6, Female: 13 | Male: 6, Female: 13 | ||
| Years of education | 10.21 ± 4.08 | 10.89 ± 3.93 | −0.63 | 0.628 |
| GDS-K | 10.26 ± 2.00 | 2.21 ± 2.27 | 11.60 | <0.001 |
| MMSE-KC | 27.89 ± 1.37 | 28.58 ± 0.77 | −1.90 | 0.068 |
| Word-List Test (z-score) | ||||
| Learning | 0.37 ± 0.70 | 0.73 ± 0.84 | −1.46 | 0.153 |
| Recall | −0.45 ± 0.73 | 0.23 ± 0.88 | −2.60 | 0.014 |
| Recognition | −0.61 ± 0.83 | 0.48 ± 0.33 | −4.13 | <0.001 |
| eTIV (mm3) | 1.52 × 106 ± 1.22 × 105 | 1.52 × 106 ± 1.61 × 105 | −0.05 | 0.964 |
| Left Hippocampus | Right Hippocampus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclinical Depression | Control | Fgroup | pgroup | ES | FDR | Subclinical Depression | Control | Fgroup | pgroup | ES | FDR | |
| Parasubiculum | 59.44 ± 15.12 | 56.59 ± 13.17 | 0.41 | 0.526 | 0.011 | 0.570 | 51.91 ± 8.75 | 53.02 ± 7.06 | 0.05 | 0.827 | 0.006 | 0.827 |
| Presubiculum | 279.25 ± 40.09 | 297.26 ± 36.2 | 1.16 | 0.290 | 0.057 | 0.377 | 261.79 ± 31.84 | 284.42 ± 25.67 | 3.21 | 0.082 | 0.153 | 0.124 |
| Subiculum | 388.32 ± 46.81 | 435.28 ± 49.72 | 6.21 | 0.018 | 0.208 | 0.039 | 399.61 ± 55 | 444.59 ± 38.01 | 5.27 | 0.028 | 0.212 | 0.061 |
| CA1 | 545.73 ± 62.86 | 614.26 ± 72.41 | 6.41 | 0.016 | 0.227 | 0.039 | 589.8 ± 68.66 | 662.84 ± 74.75 | 6.36 | 0.016 | 0.233 | 0.042 |
| CA3 | 179.19 ± 24.03 | 208.56 ± 26.05 | 9.44 | 0.004 | 0.277 | 0.020 | 204.64 ± 26.82 | 226.4 ± 30.16 | 3.12 | 0.086 | 0.144 | 0.124 |
| CA4 | 217.84 ± 25.93 | 241.02 ± 22.35 | 5.16 | 0.029 | 0.22 | 0.054 | 229.15 ± 24.38 | 257.3 ± 25.09 | 7.78 | 0.008 | 0.308 | 0.035 |
| GC-ML-DG | 248.06 ± 32.73 | 277.14 ± 30.52 | 4.37 | 0.044 | 0.218 | 0.064 | 261.91 ± 30.42 | 295.28 ± 32.66 | 6.35 | 0.016 | 0.279 | 0.042 |
| Molecular layer | 475.11 ± 52.46 | 535.01 ± 50.56 | 8.77 | 0.005 | 0.284 | 0.020 | 503.02 ± 59.13 | 564.59 ± 47.84 | 8.08 | 0.007 | 0.293 | 0.035 |
| HATA | 49.35 ± 12.17 | 52.72 ± 10.62 | 0.02 | 0.888 | 0.027 | 0.888 | 52.18 ± 9.39 | 55.98 ± 10.04 | 0.08 | 0.779 | 0.051 | 0.827 |
| Fimbria | 56.72 ± 24.1 | 67.7 ± 18.85 | 1.02 | 0.321 | 0.069 | 0.379 | 50.75 ± 24.61 | 63.95 ± 17.7 | 1.42 | 0.242 | 0.105 | 0.315 |
| Hippocampal tail | 478.7 ± 64.32 | 564.33 ± 55.91 | 15.10 | <0.001 | 0.353 | <0.001 | 526.88 ± 62.62 | 575.37 ± 49.44 | 4.11 | 0.050 | 0.179 | 0.093 |
| Hippocampal fissure | 146.91 ± 19.75 | 170.35 ± 35.74 | 4.93 | 0.033 | 0.149 | 0.054 | 166.39 ± 28.5 | 179.03 ± 37.85 | 1.11 | 0.300 | 0.036 | 0.355 |
| Whole hippocampus | 2976.78 ± 323.44 | 3350.84 ± 314.1 | 8.52 | 0.006 | 0.310 | 0.020 | 3130.97 ± 343.42 | 3484.41 ± 271.9 | 7.79 | 0.008 | 0.324 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Ju, G.; Park, H.; Chung, S.; Son, J.-W.; Shin, C.-J.; Lee, S.I.; Kim, S. Hippocampal Subfields and White Matter Connectivity in Patients with Subclinical Geriatric Depression. Brain Sci. 2022, 12, 329. https://doi.org/10.3390/brainsci12030329
Lee J, Ju G, Park H, Chung S, Son J-W, Shin C-J, Lee SI, Kim S. Hippocampal Subfields and White Matter Connectivity in Patients with Subclinical Geriatric Depression. Brain Sciences. 2022; 12(3):329. https://doi.org/10.3390/brainsci12030329
Chicago/Turabian StyleLee, Jeonghwan, Gawon Ju, Hyemi Park, Seungwon Chung, Jung-Woo Son, Chul-Jin Shin, Sang Ick Lee, and Siekyeong Kim. 2022. "Hippocampal Subfields and White Matter Connectivity in Patients with Subclinical Geriatric Depression" Brain Sciences 12, no. 3: 329. https://doi.org/10.3390/brainsci12030329
APA StyleLee, J., Ju, G., Park, H., Chung, S., Son, J.-W., Shin, C.-J., Lee, S. I., & Kim, S. (2022). Hippocampal Subfields and White Matter Connectivity in Patients with Subclinical Geriatric Depression. Brain Sciences, 12(3), 329. https://doi.org/10.3390/brainsci12030329







